

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Integrase (Human immunodeficiency virus 1) | BDBM50480466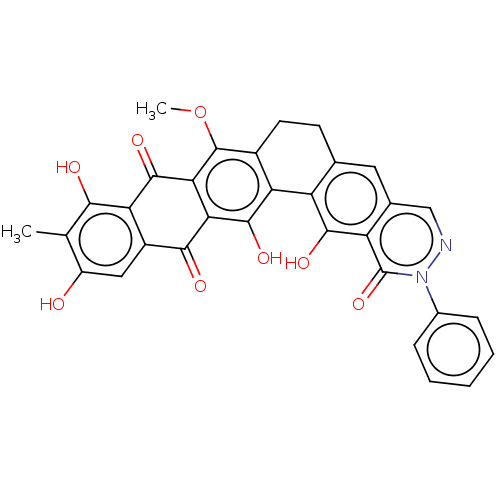 (CHEMBL541369) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480455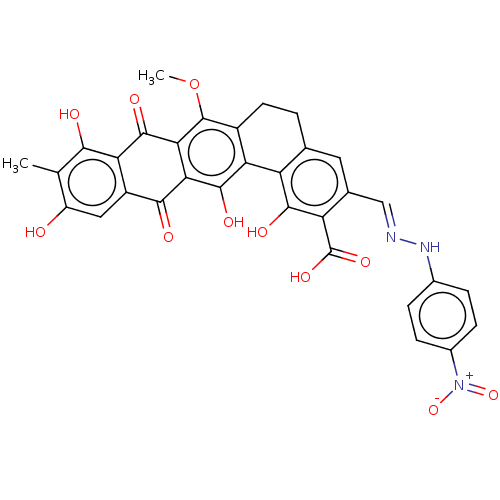 (CHEMBL555736) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480471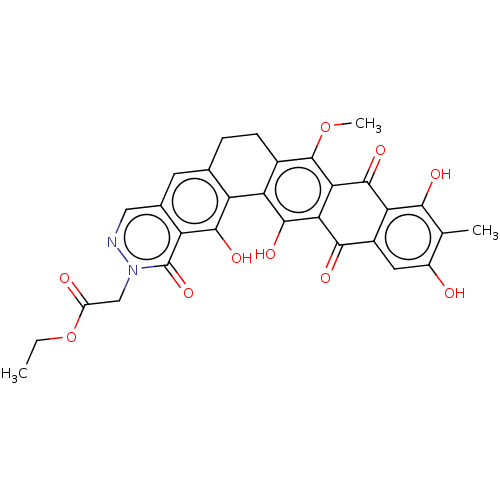 (CHEMBL555734) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480461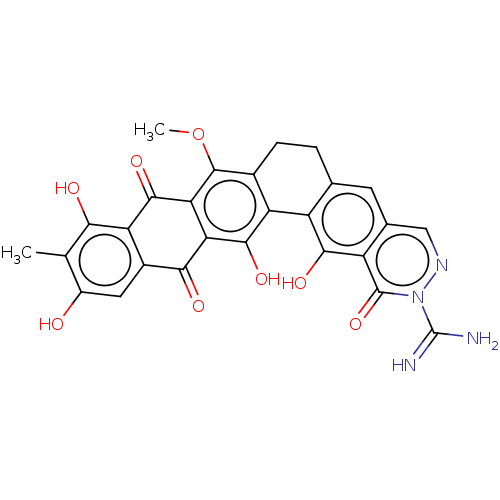 (CHEMBL562425) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50192289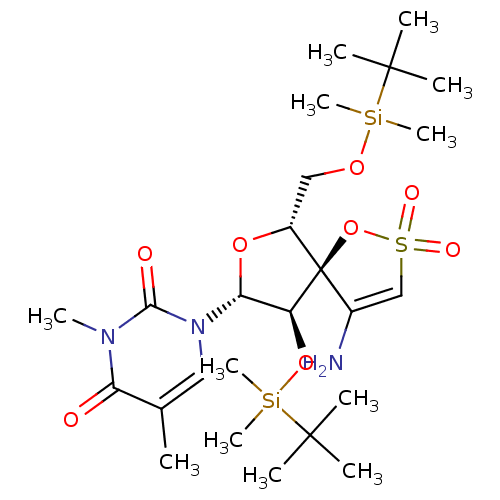 (1-[(5R,6R,8R,9R)-4-amino-9-(tert-butyl-dimethyl-si...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration against wild type HIV-1 reverse transcriptase (RT) using poly rC.dG as the template or primer | J Med Chem 47: 3418-26 (2004) Article DOI: 10.1021/jm031045o BindingDB Entry DOI: 10.7270/Q2B857KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480469 (CHEMBL562741) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480460 (CHEMBL540405) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50148763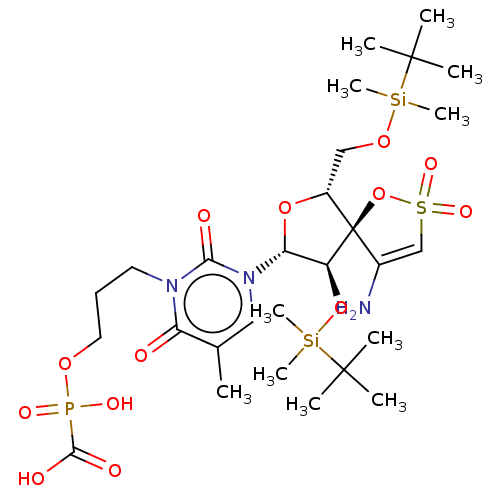 (CHEMBL3142918 | [1-[2',5'-Bis-O-(tert-butyldimethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration against wild type HIV-1 reverse transcriptase (RT) using poly rC.dG as the template or primer | J Med Chem 47: 3418-26 (2004) Article DOI: 10.1021/jm031045o BindingDB Entry DOI: 10.7270/Q2B857KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50148764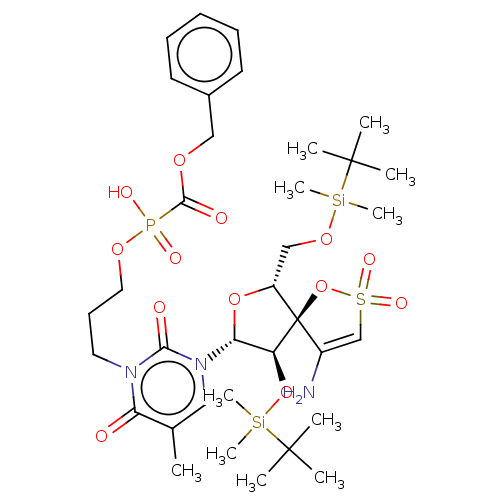 (CHEMBL3142919 | [1-[2',5'-Bis-O-(tert-butyldimethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration against wild type HIV-1 reverse transcriptase (RT) using poly rC.dG as the template or primer | J Med Chem 47: 3418-26 (2004) Article DOI: 10.1021/jm031045o BindingDB Entry DOI: 10.7270/Q2B857KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480459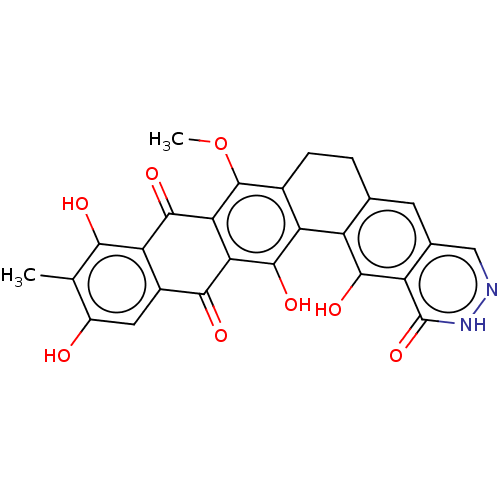 (CHEMBL552100) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480470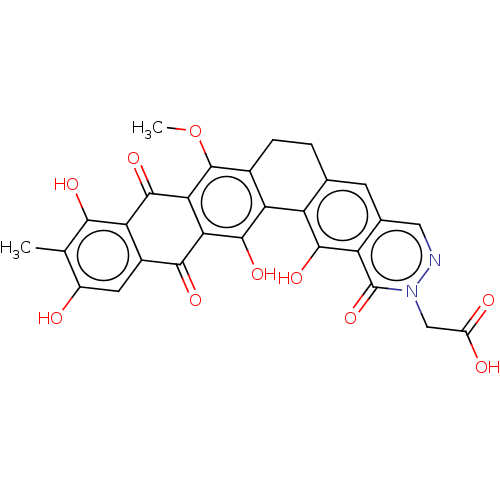 (CHEMBL564339) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50148762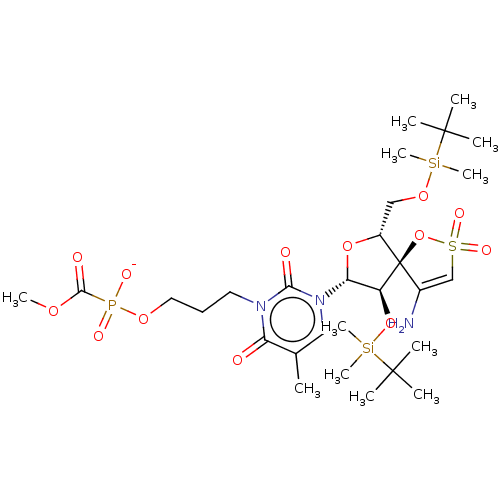 (CHEMBL3142917 | [1-[2',5'-Bis-O-(tert-butyldimethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration against wild type HIV-1 reverse transcriptase (RT) using poly rC.dG as the template or primer | J Med Chem 47: 3418-26 (2004) Article DOI: 10.1021/jm031045o BindingDB Entry DOI: 10.7270/Q2B857KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480454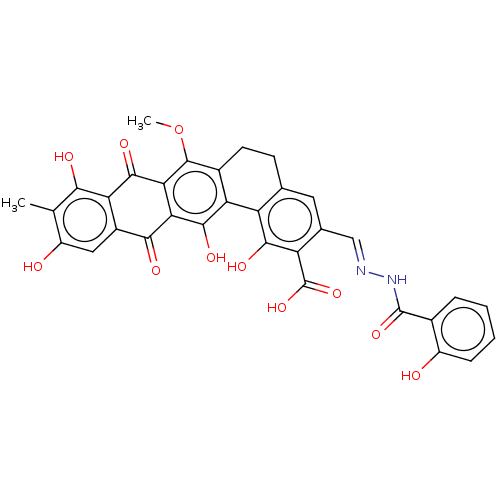 (CHEMBL538448) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480458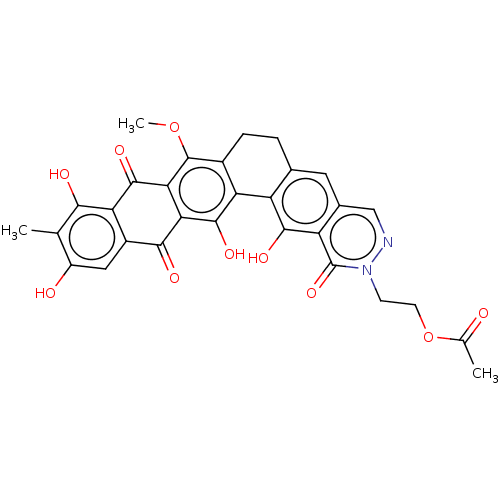 (CHEMBL554095) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50148760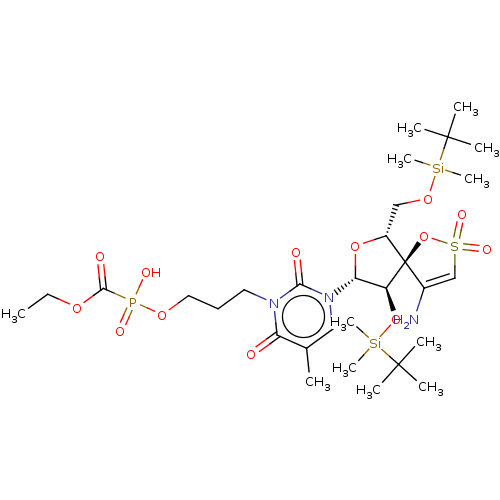 (CHEMBL3142914 | [1-[2',5'-Bis-O-(tert-butyldimethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration against wild type HIV-1 reverse transcriptase (RT) using poly rC.dG as the template or primer | J Med Chem 47: 3418-26 (2004) Article DOI: 10.1021/jm031045o BindingDB Entry DOI: 10.7270/Q2B857KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480457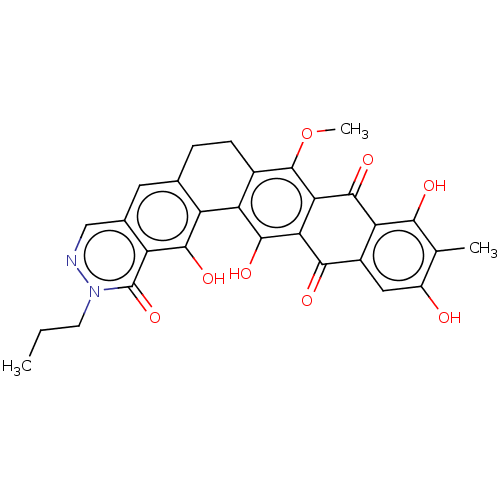 (CHEMBL557286) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480464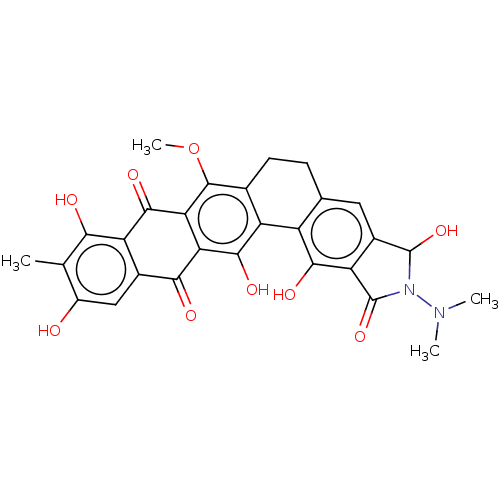 (CHEMBL550839) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50100110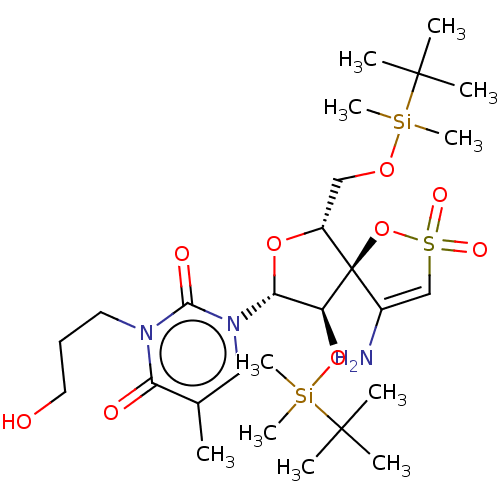 (1-[4-Amino-9-(tert-butyl-dimethyl-silanyloxy)-6-(t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration against wild type HIV-1 reverse transcriptase (RT) using poly rC.dG as the template or primer | J Med Chem 47: 3418-26 (2004) Article DOI: 10.1021/jm031045o BindingDB Entry DOI: 10.7270/Q2B857KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50148761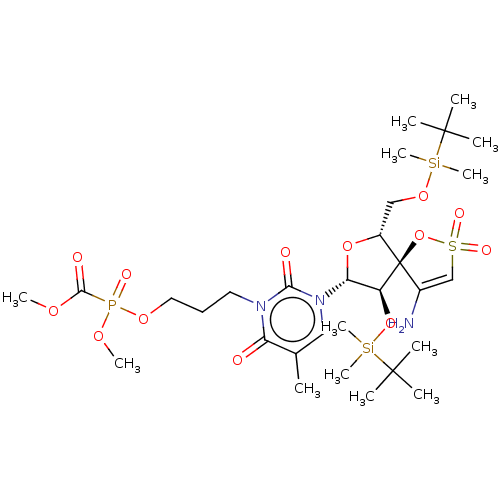 (CHEMBL3142920 | [1-[2',5'-Bis-O-(tert-butyldimethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration against wild type HIV-1 reverse transcriptase (RT) using poly rC.dG as the template or primer | J Med Chem 47: 3418-26 (2004) Article DOI: 10.1021/jm031045o BindingDB Entry DOI: 10.7270/Q2B857KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480462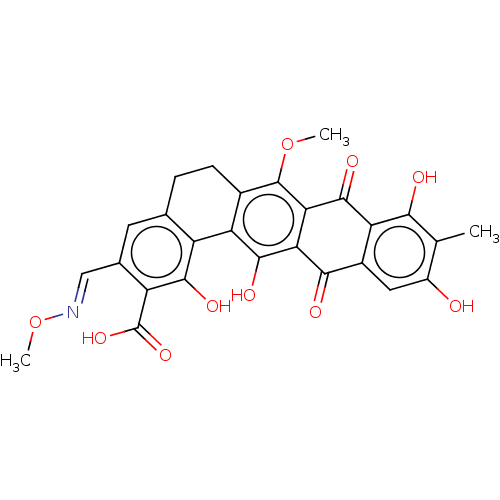 (CHEMBL561566) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480453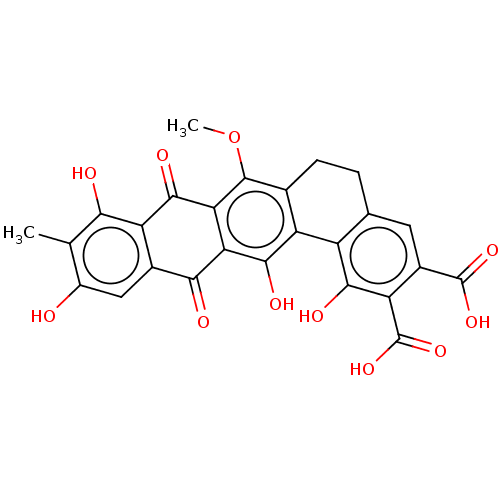 (CHEMBL554301) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480452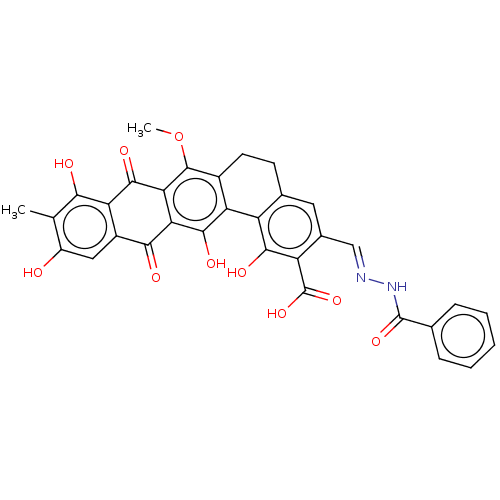 (CHEMBL538878) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.46E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480449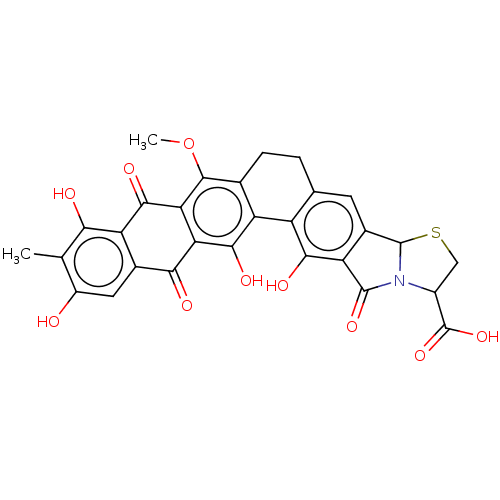 (CHEMBL554975) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480468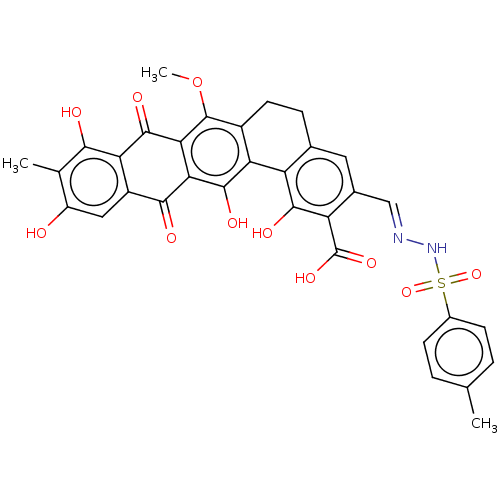 (CHEMBL538947) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50011181 ((PFA)dihydroxyphosphinecarboxylic acid oxide | CHE...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration against wild type HIV-1 reverse transcriptase (RT) using poly rC.dG as the template or primer | J Med Chem 47: 3418-26 (2004) Article DOI: 10.1021/jm031045o BindingDB Entry DOI: 10.7270/Q2B857KW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480451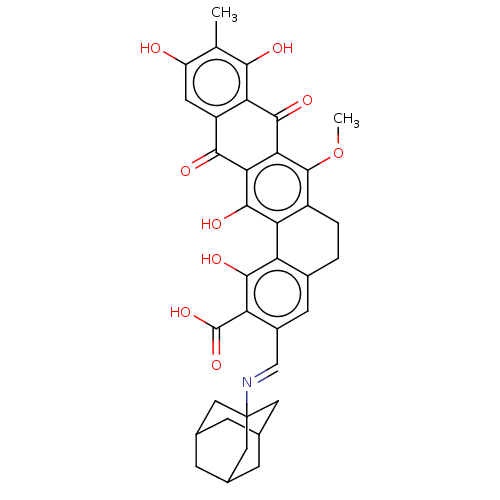 (CHEMBL552987) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480450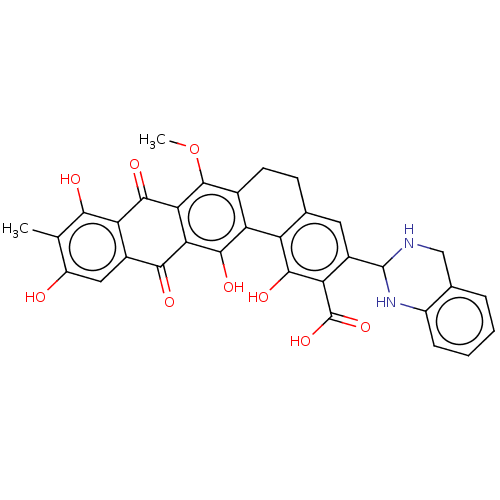 (CHEMBL538879) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50011181 ((PFA)dihydroxyphosphinecarboxylic acid oxide | CHE...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration against wild type HIV-1/138Lys reverse transcriptase (RT) using [3H]dGTP as a radioligand | J Med Chem 47: 3418-26 (2004) Article DOI: 10.1021/jm031045o BindingDB Entry DOI: 10.7270/Q2B857KW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480448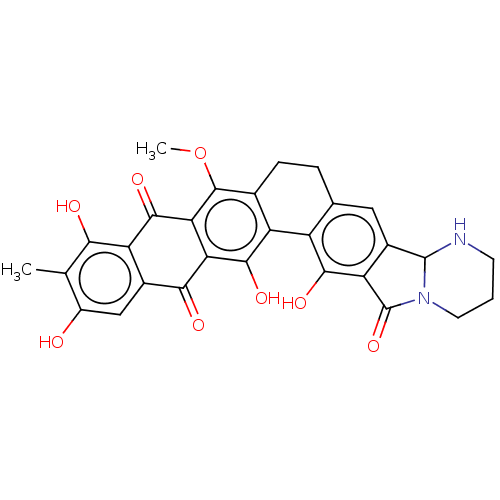 (CHEMBL549679) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480447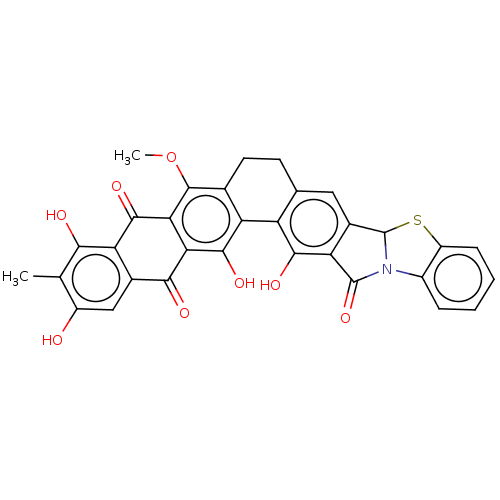 (CHEMBL541701) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50192289 (1-[(5R,6R,8R,9R)-4-amino-9-(tert-butyl-dimethyl-si...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration against wild type HIV-1/138Lys reverse transcriptase (RT) using [3H]dGTP as a radioligand | J Med Chem 47: 3418-26 (2004) Article DOI: 10.1021/jm031045o BindingDB Entry DOI: 10.7270/Q2B857KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50100110 (1-[4-Amino-9-(tert-butyl-dimethyl-silanyloxy)-6-(t...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration against wild type HIV-1/138Lys reverse transcriptase (RT) using [3H]dGTP as a radioligand | J Med Chem 47: 3418-26 (2004) Article DOI: 10.1021/jm031045o BindingDB Entry DOI: 10.7270/Q2B857KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480456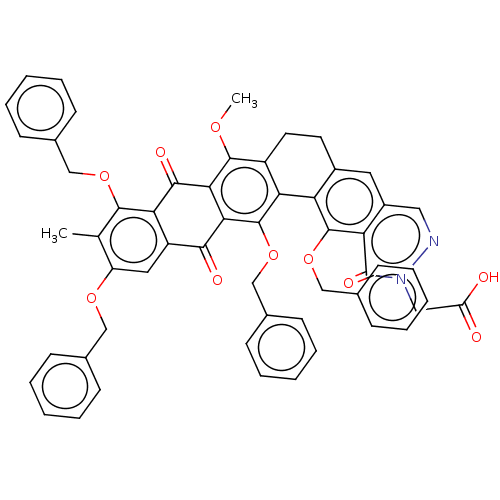 (CHEMBL540406) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.06E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480472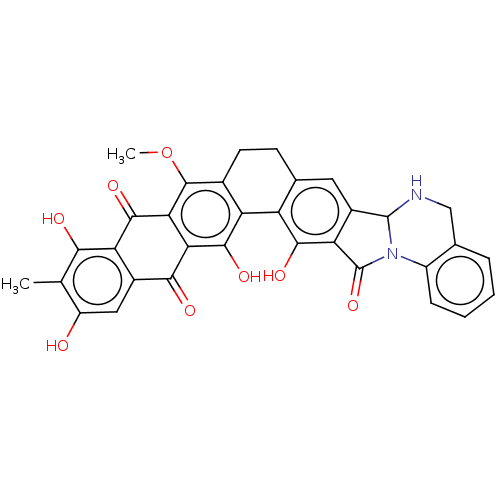 (CHEMBL538450) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.08E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480467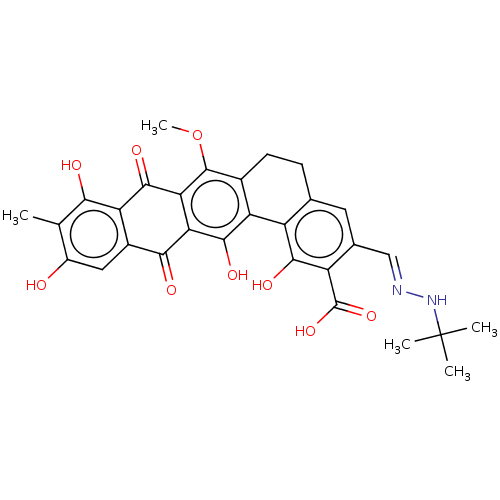 (CHEMBL538880) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.22E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480474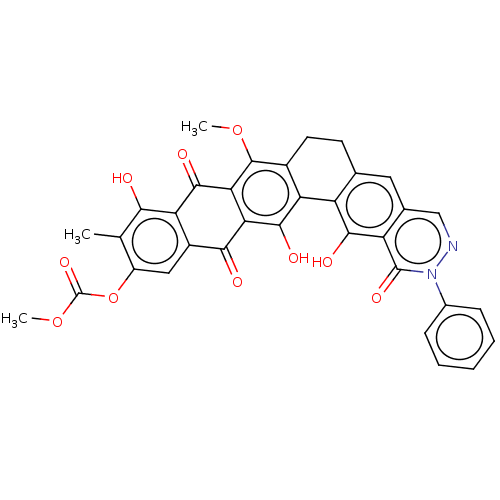 (CHEMBL539709) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50148760 (CHEMBL3142914 | [1-[2',5'-Bis-O-(tert-butyldimethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.93E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration against wild type HIV-1/138Lys reverse transcriptase (RT) using [3H]dGTP as a radioligand | J Med Chem 47: 3418-26 (2004) Article DOI: 10.1021/jm031045o BindingDB Entry DOI: 10.7270/Q2B857KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50148764 (CHEMBL3142919 | [1-[2',5'-Bis-O-(tert-butyldimethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration against wild type HIV-1/138Lys reverse transcriptase (RT) using [3H]dGTP as a radioligand | J Med Chem 47: 3418-26 (2004) Article DOI: 10.1021/jm031045o BindingDB Entry DOI: 10.7270/Q2B857KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480465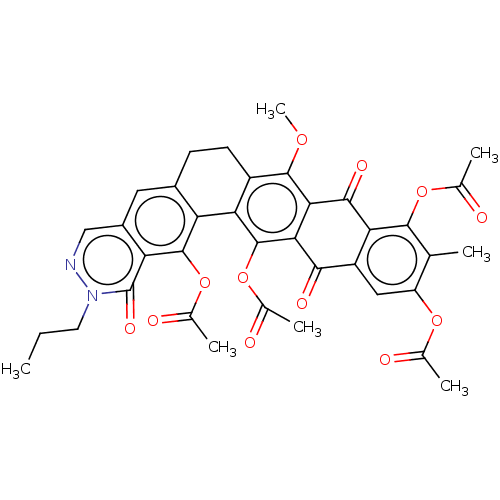 (CHEMBL554982) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480473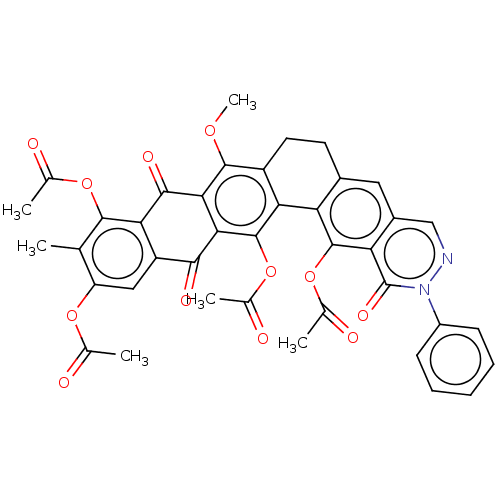 (CHEMBL540407) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrase (Human immunodeficiency virus 1) | BDBM50480463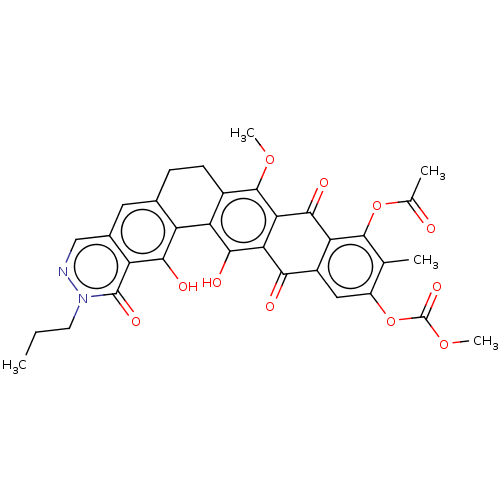 (CHEMBL552765) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >3.33E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Inhibition of HIV1 recombinant integrase by electrochemiluminescent-based high-throughput strand transfer assay | Antimicrob Agents Chemother 52: 361-4 (2008) Article DOI: 10.1128/aac.00883-07 BindingDB Entry DOI: 10.7270/Q2C2507R | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50148762 (CHEMBL3142917 | [1-[2',5'-Bis-O-(tert-butyldimethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.94E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration against wild type HIV-1/138Lys reverse transcriptase (RT) using [3H]dGTP as a radioligand | J Med Chem 47: 3418-26 (2004) Article DOI: 10.1021/jm031045o BindingDB Entry DOI: 10.7270/Q2B857KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50148763 (CHEMBL3142918 | [1-[2',5'-Bis-O-(tert-butyldimethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.37E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration against wild type HIV-1/138Lys reverse transcriptase (RT) using [3H]dGTP as a radioligand | J Med Chem 47: 3418-26 (2004) Article DOI: 10.1021/jm031045o BindingDB Entry DOI: 10.7270/Q2B857KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50148761 (CHEMBL3142920 | [1-[2',5'-Bis-O-(tert-butyldimethy...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Química Médica (C.S.I.C.) Curated by ChEMBL | Assay Description Inhibitory concentration against wild type HIV-1/138Lys reverse transcriptase (RT) using [3H]dGTP as a radioligand | J Med Chem 47: 3418-26 (2004) Article DOI: 10.1021/jm031045o BindingDB Entry DOI: 10.7270/Q2B857KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50370476 (Combivir | ZIDOVUDINE TRIPHOSPHATE) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | >6.00E+4 | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Binding affinity to HIV1 reverse transcriptase M184V mutant assessed as L-3'-azido-NTP incorporation in nascent DNA | Eur J Med Chem 46: 3832-44 (2011) Article DOI: 10.1016/j.ejmech.2011.05.051 BindingDB Entry DOI: 10.7270/Q2RN3BQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50205415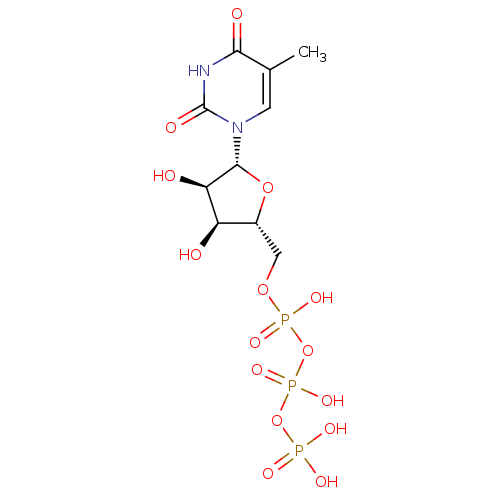 (({[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(5-methyl-2,4-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Binding affinity to HIV1 reverse transcriptase assessed as L-3'-azido-NTP incorporation in nascent DNA | Eur J Med Chem 46: 3832-44 (2011) Article DOI: 10.1016/j.ejmech.2011.05.051 BindingDB Entry DOI: 10.7270/Q2RN3BQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50118233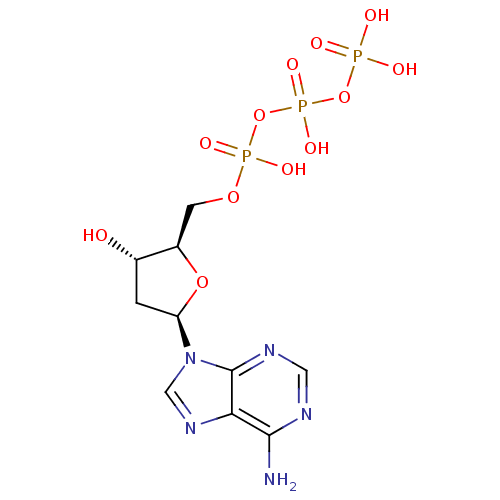 (2'-deoxyadenosine 5'-(tetrahydrogen triphosphate) ...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Binding affinity to HIV1 reverse transcriptase M184V mutant assessed as L-3'-azido-NTP incorporation in nascent DNA | Eur J Med Chem 46: 3832-44 (2011) Article DOI: 10.1016/j.ejmech.2011.05.051 BindingDB Entry DOI: 10.7270/Q2RN3BQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase (Human immunodeficiency virus 1) | BDBM50484279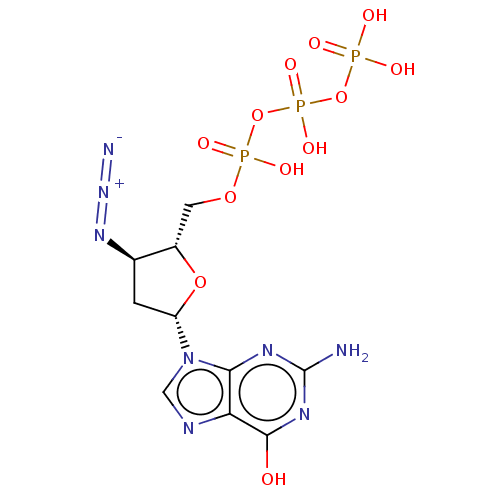 (CHEMBL1830935) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Binding affinity to HIV1 reverse transcriptase assessed as L-3'-azido-NTP incorporation in nascent DNA | Eur J Med Chem 46: 3832-44 (2011) Article DOI: 10.1016/j.ejmech.2011.05.051 BindingDB Entry DOI: 10.7270/Q2RN3BQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50367094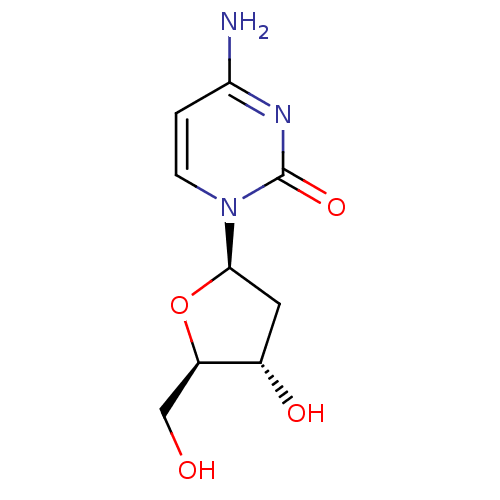 (DEOXYCYTIDINE | EN300-6477283) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Binding affinity to HIV1 reverse transcriptase M184V mutant assessed as L-3'-azido-NTP incorporation in nascent DNA | Eur J Med Chem 46: 3832-44 (2011) Article DOI: 10.1016/j.ejmech.2011.05.051 BindingDB Entry DOI: 10.7270/Q2RN3BQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase protein (Human immunodeficiency virus 1) | BDBM50484280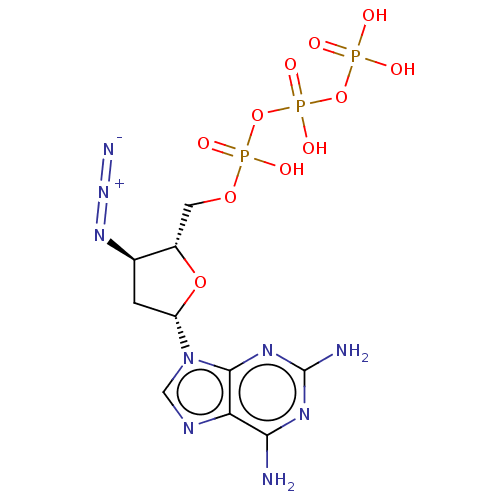 (CHEMBL1830934) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a |
Veterans Affairs Medical Center Curated by ChEMBL | Assay Description Binding affinity to HIV1 reverse transcriptase M184V mutant assessed as L-3'-azido-NTP incorporation in nascent DNA | Eur J Med Chem 46: 3832-44 (2011) Article DOI: 10.1016/j.ejmech.2011.05.051 BindingDB Entry DOI: 10.7270/Q2RN3BQP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 95 total ) | Next | Last >> |