

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50045775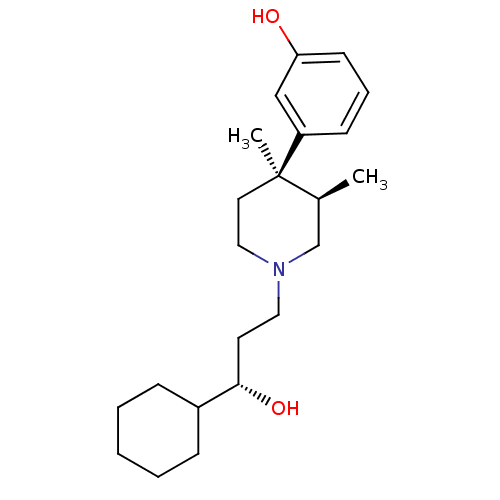 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50130563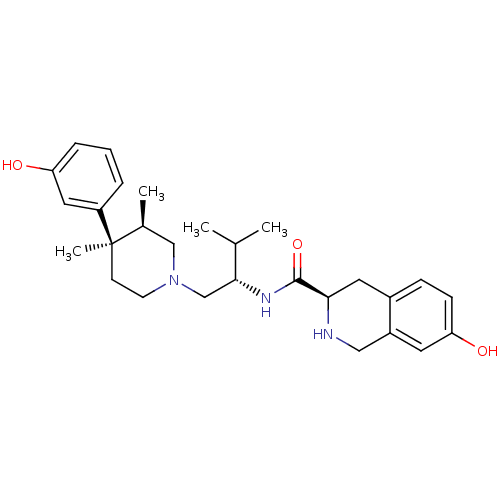 ((3R)-7-Hydroxy-N-[(1S)-1-{[(3R,4R)-4-(3-hydroxyphe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 0.0591 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219919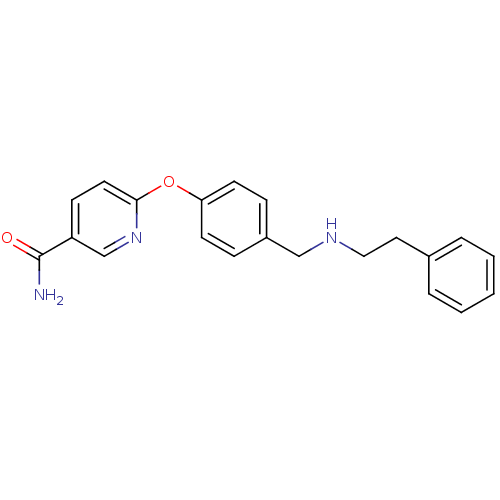 (6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from cloned human mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 6841-6 (2007) Article DOI: 10.1016/j.bmcl.2007.10.025 BindingDB Entry DOI: 10.7270/Q2125TGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219921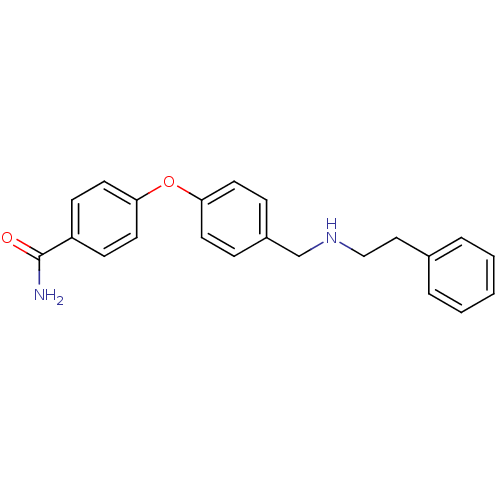 (4-(4-((phenethylamino)methyl)phenoxy)benzamide | C...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM82551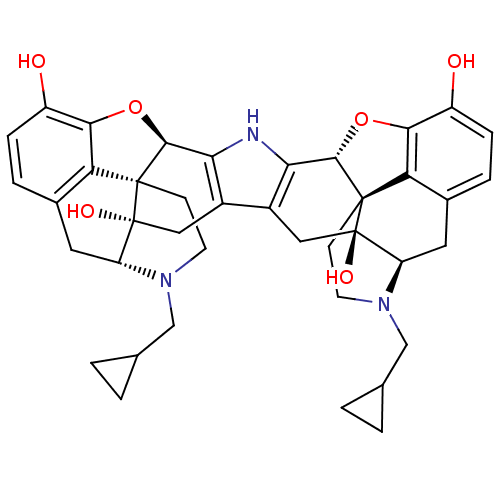 (C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid UniChem Similars | Article PubMed | 0.153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50026614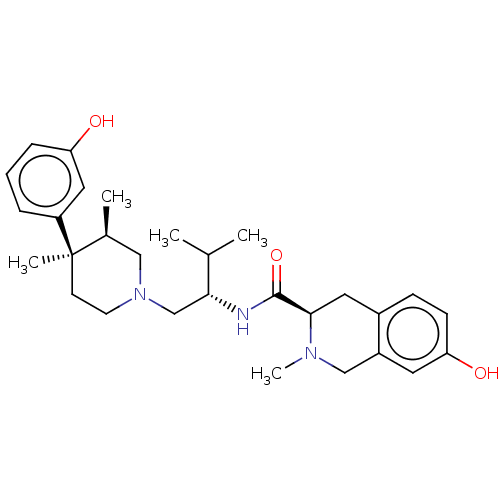 (CHEMBL575508) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.153 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045792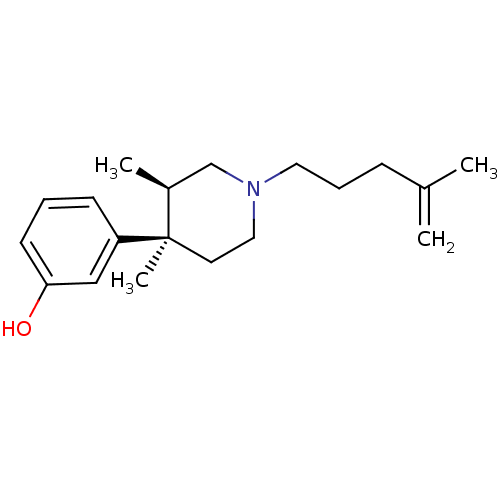 (3-[3,4-Dimethyl-1-(4-methyl-pent-4-enyl)-piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219919 (6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human mu opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219919 (6-(4-((phenethylamino)methyl)phenoxy)nicotinamide ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from cloned human mu opioid receptor expressed in CHO cells | Bioorg Med Chem Lett 17: 6841-6 (2007) Article DOI: 10.1016/j.bmcl.2007.10.025 BindingDB Entry DOI: 10.7270/Q2125TGQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045733 (3-[3,4-Dimethyl-1-(3-thiophen-2-yl-propyl)-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu opioid receptor by displacement of [3H]-NAL from rat brain homogenates | J Med Chem 36: 2833-41 (1993) BindingDB Entry DOI: 10.7270/Q2W0951M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045733 (3-[3,4-Dimethyl-1-(3-thiophen-2-yl-propyl)-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by displacement of [3H]-NAL at 10 nM from rat brain homogenates | J Med Chem 36: 2833-41 (1993) BindingDB Entry DOI: 10.7270/Q2W0951M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471498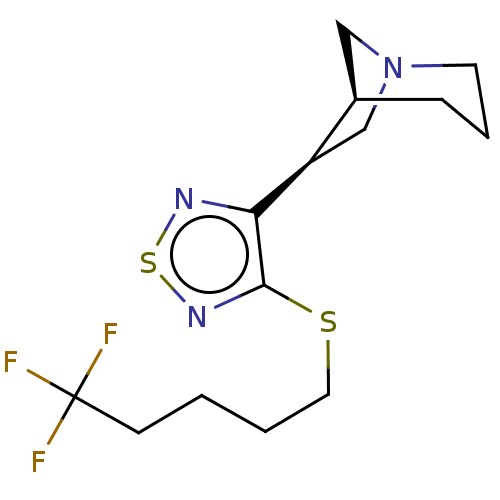 (CHEMBL150450) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045742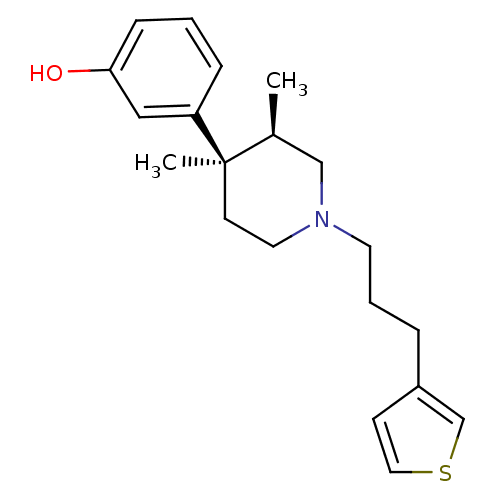 (3-[3,4-Dimethyl-1-(3-thiophen-3-yl-propyl)-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045742 (3-[3,4-Dimethyl-1-(3-thiophen-3-yl-propyl)-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by displacement of [3H]-NAL at 10 nM from rat brain homogenates | J Med Chem 36: 2833-41 (1993) BindingDB Entry DOI: 10.7270/Q2W0951M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045738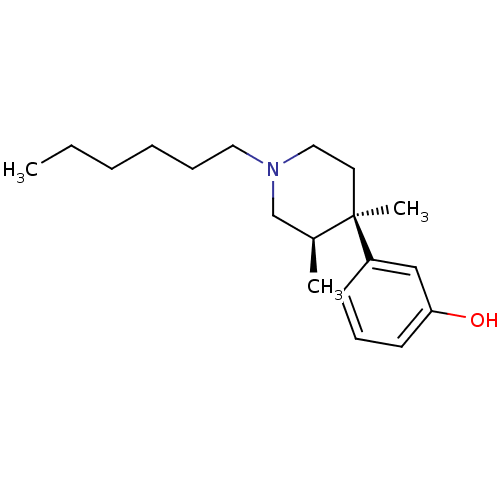 ((3R,4R)3-(1-Hexyl-3,4-dimethyl-piperidin-4-yl)-phe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu opioid receptor by displacement of [3H]-NAL from rat brain homogenates | J Med Chem 36: 2833-41 (1993) BindingDB Entry DOI: 10.7270/Q2W0951M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045779 (3-[3,4-Dimethyl-1-(4-methyl-pentyl)-piperidin-4-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219921 (4-(4-((phenethylamino)methyl)phenoxy)benzamide | C...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human mu opioid receptor expressed in CHO cells in presence of high sodium by SPA | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045776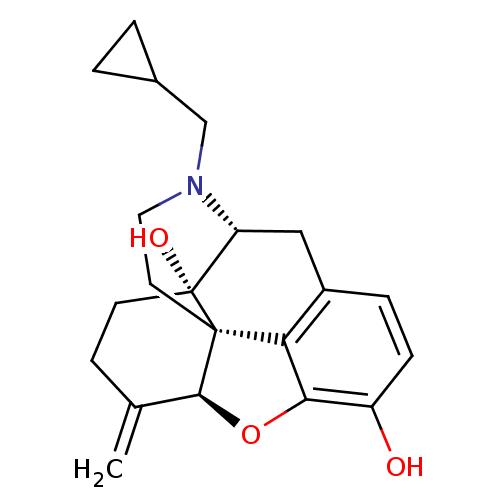 (CHEMBL982 | JF-1 | NALMEFENE | Nalmetrene | ORF-11...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045780 (3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-dimethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045748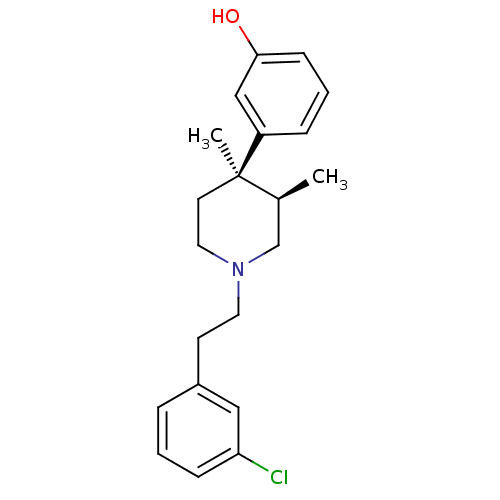 (3-{1-[2-(3-Chloro-phenyl)-ethyl]-3,4-dimethyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu opioid receptor by displacement of [3H]-NAL from rat brain homogenates | J Med Chem 36: 2833-41 (1993) BindingDB Entry DOI: 10.7270/Q2W0951M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human kappa opioid receptor expressed in CHO cells by [35S]GTP-gamma-S binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471487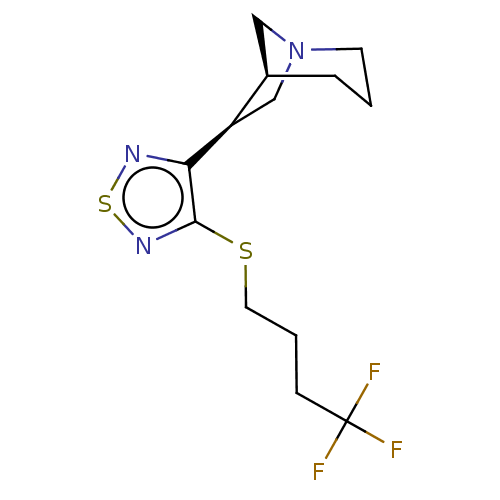 (CHEMBL436075) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045781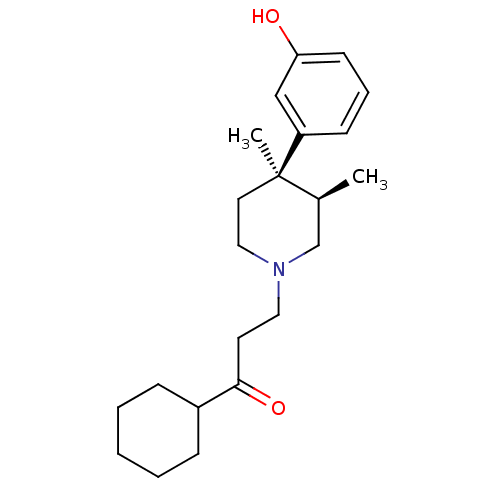 (1-Cyclohexyl-3-[4-(3-hydroxy-phenyl)-3,4-dimethyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471494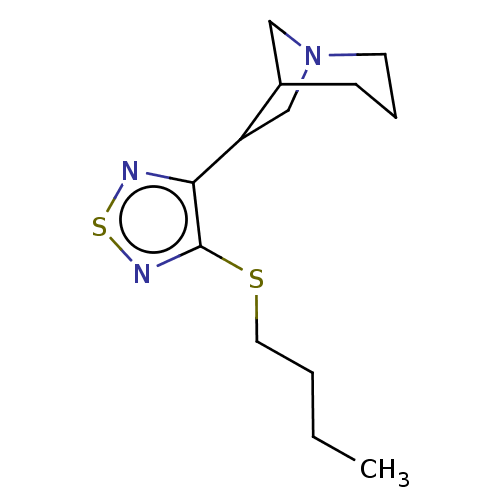 (CHEMBL153723) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045791 (3-[1-(3-Cyclopentyl-propyl)-3,4-dimethyl-piperidin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471499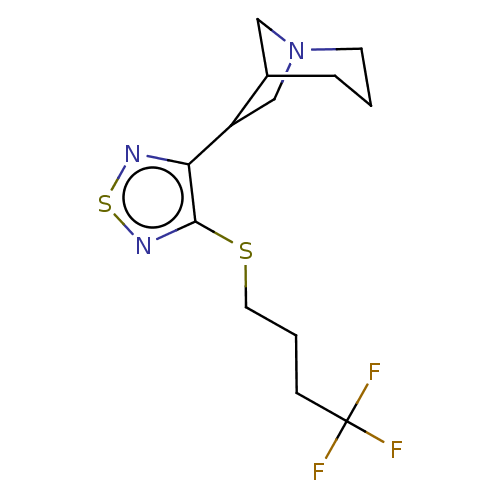 (CHEMBL345774) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptor using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471485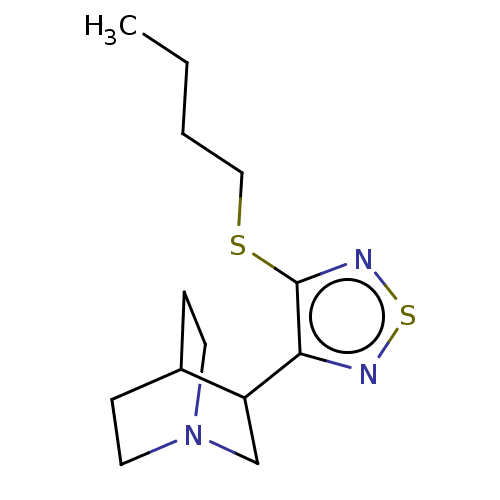 (Vedaclidine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045775 ((3R,4R)3-[1-(3-Cyclohexyl-3-hydroxy-propyl)-3,4-di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045786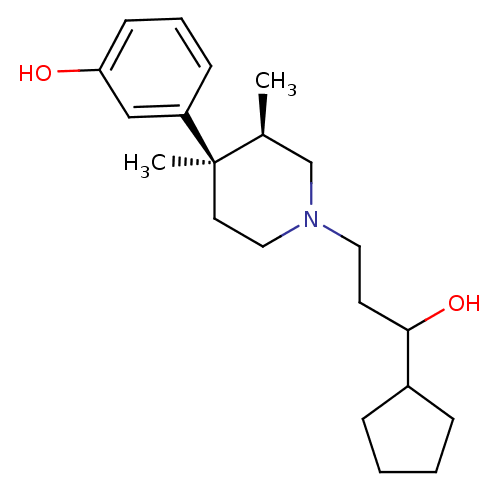 (3-[1-(3-Cyclopentyl-3-hydroxy-propyl)-3,4-dimethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471492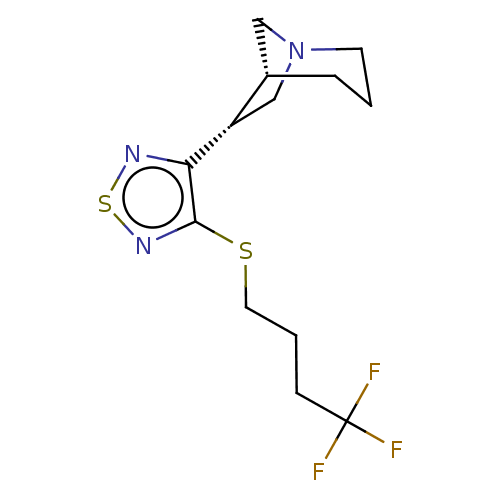 (CHEMBL150293) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50219918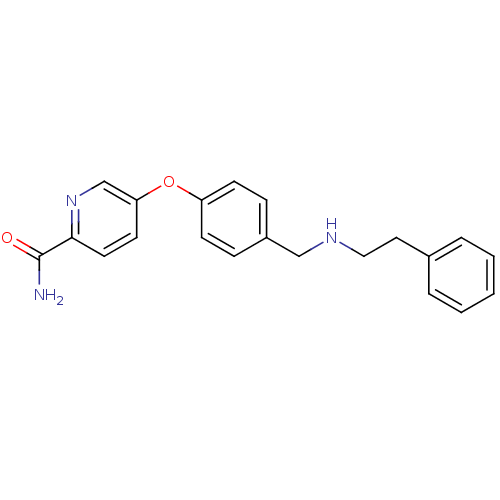 (5-(4-((phenethylamino)methyl)phenoxy)picolinamide ...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471495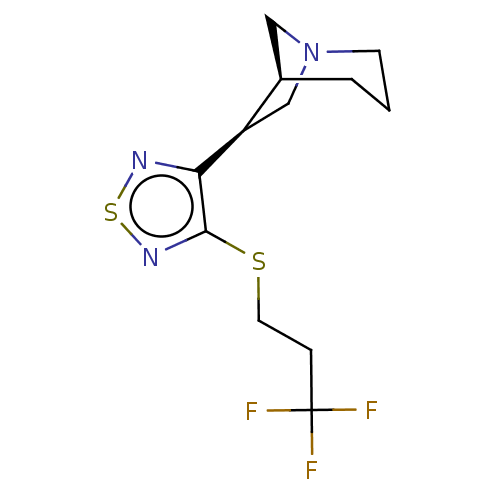 (CHEMBL153478) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50471486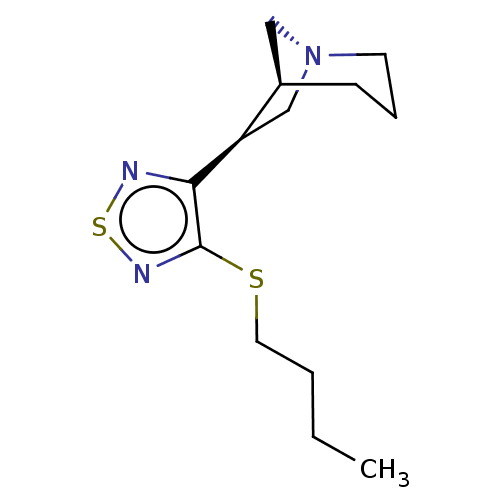 (CHEMBL343731) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045785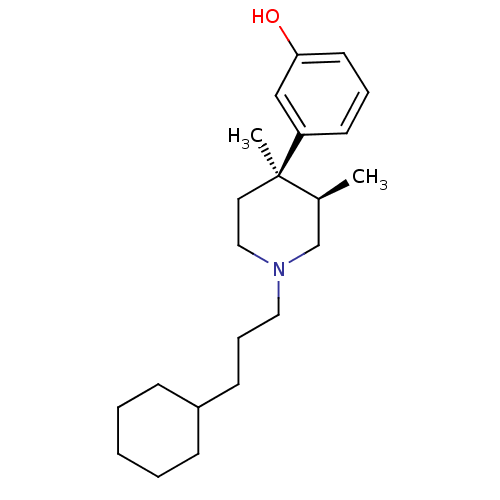 (3-[1-(3-Cyclohexyl-propyl)-3,4-dimethyl-piperidin-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M1/M2/M3/M4/M5 (RAT) | BDBM50033155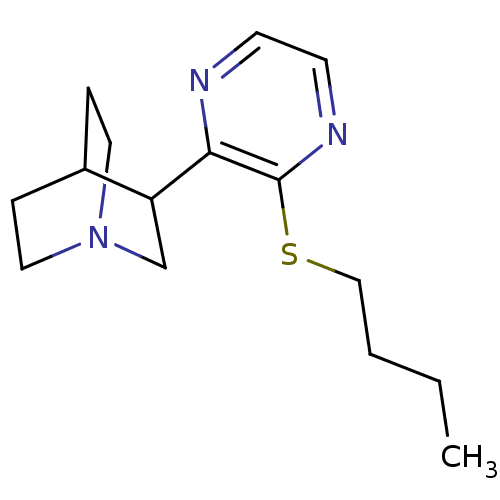 (3-(3-Butylsulfanyl-pyrazin-2-yl)-1-aza-bicyclo[2.2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity against Muscarinic acetylcholine receptors using [3H]oxotremorine-M as radioligand in rat cortex | J Med Chem 40: 538-46 (1997) Article DOI: 10.1021/jm9602470 BindingDB Entry DOI: 10.7270/Q2SQ934F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045735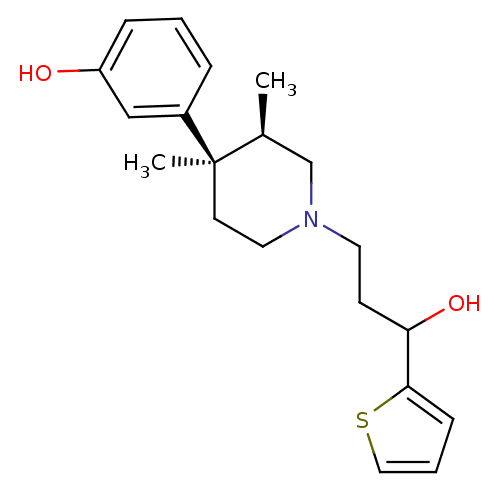 (3-[1-(3-Hydroxy-3-thiophen-2-yl-propyl)-3,4-dimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045735 (3-[1-(3-Hydroxy-3-thiophen-2-yl-propyl)-3,4-dimeth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu opioid receptor by displacement of [3H]-NAL from rat brain homogenates | J Med Chem 36: 2833-41 (1993) BindingDB Entry DOI: 10.7270/Q2W0951M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50346474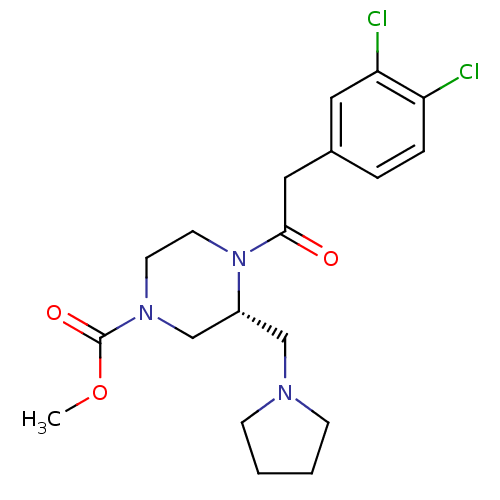 ((R)-4-[2-(3,4-Dichloro-phenyl)-acetyl]-3-pyrrolidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.502 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045733 (3-[3,4-Dimethyl-1-(3-thiophen-2-yl-propyl)-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045733 (3-[3,4-Dimethyl-1-(3-thiophen-2-yl-propyl)-piperid...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM60212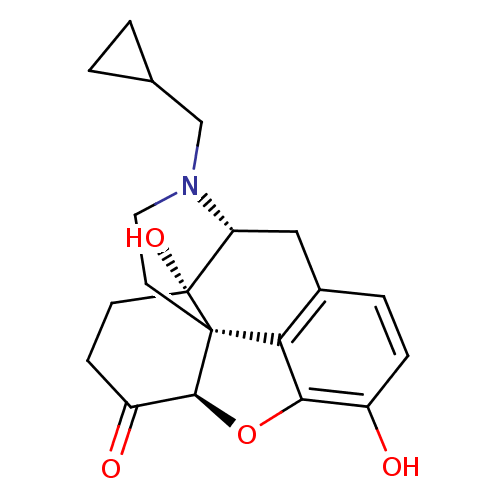 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu opioid receptor by displacement of [3H]-NAL from rat brain homogenates | J Med Chem 36: 2833-41 (1993) BindingDB Entry DOI: 10.7270/Q2W0951M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | PubMed | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50358169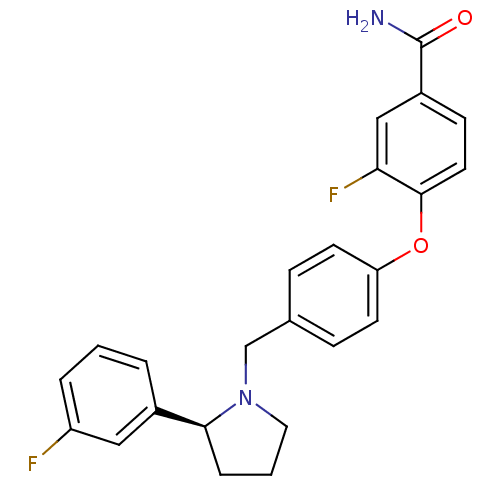 (CHEMBL1921845) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.565 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM60212 ((4R,4aS,7aR,12bS)-3-(cyclopropylmethyl)-4a,9-bis(o...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid UniChem Similars | DrugBank Article PubMed | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Antagonist activity at human mu opioid receptor expressed in CHO cells by [35S]GTPgammaS binding assay | Bioorg Med Chem Lett 17: 5349-52 (2007) Article DOI: 10.1016/j.bmcl.2007.08.008 BindingDB Entry DOI: 10.7270/Q2668CX7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50358168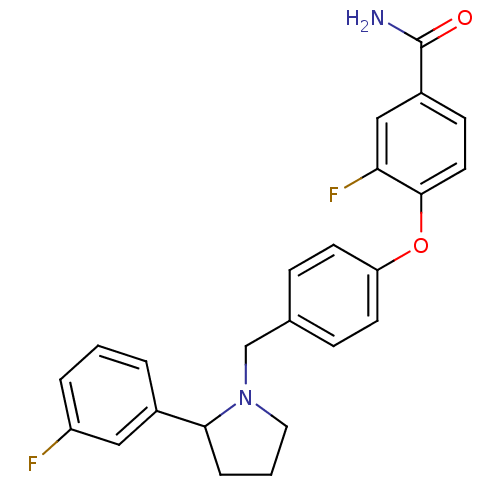 (CHEMBL1921844) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.617 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]diprenorphine from human opioid kappa receptor expressed in CHO cells after 120 mins by scintillation counting | J Med Chem 54: 8000-12 (2011) Article DOI: 10.1021/jm200789r BindingDB Entry DOI: 10.7270/Q29C6XVV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045770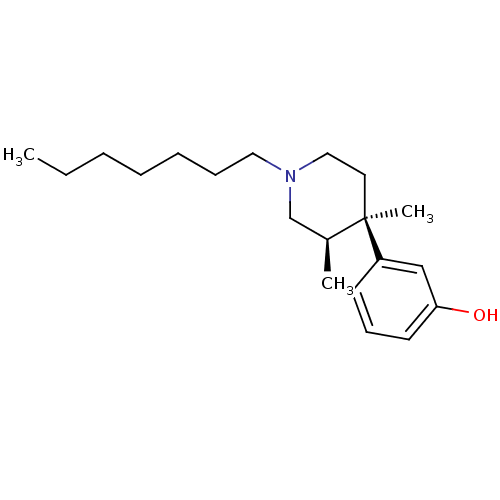 (3-(1-Heptyl-3,4-dimethyl-piperidin-4-yl)-phenol | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu-opioid receptor by the displacement of [3H]-Nal in rat brain homogenates | J Med Chem 36: 2842-50 (1993) BindingDB Entry DOI: 10.7270/Q2R78D9W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045773 (3-[3,4-Dimethyl-1-(3-o-tolyl-propyl)-piperidin-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu opioid receptor by displacement of [3H]-NAL from rat brain homogenates | J Med Chem 36: 2833-41 (1993) BindingDB Entry DOI: 10.7270/Q2W0951M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Rattus norvegicus (rat)) | BDBM50045770 (3-(1-Heptyl-3,4-dimethyl-piperidin-4-yl)-phenol | ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories Curated by ChEMBL | Assay Description Binding affinity towards mu opioid receptor by displacement of [3H]-NAL from rat brain homogenates | J Med Chem 36: 2833-41 (1993) BindingDB Entry DOI: 10.7270/Q2W0951M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 930 total ) | Next | Last >> |