 Found 37 hits with Last Name = 'miura' and Initial = 'n'
Found 37 hits with Last Name = 'miura' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50131385

((2R,3R,4R,5S)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15+,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 9 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50143729

((2R,3R,4R,5R)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15-,16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0970 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 9 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50143729

((2R,3R,4R,5R)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15-,16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 3 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50131385

((2R,3R,4R,5S)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15+,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.510 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 3 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50141577
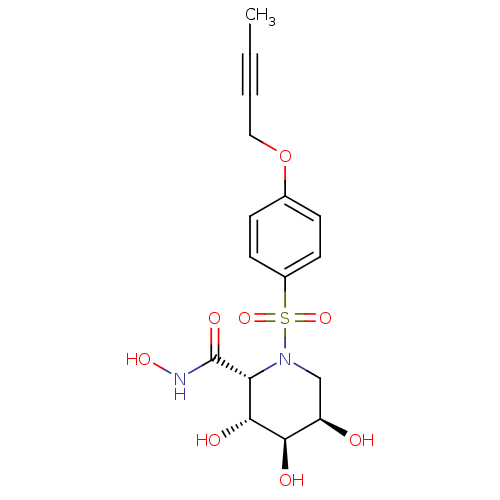
((2R,3R,4R,5R)-1-(4-But-2-ynyloxy-benzenesulfonyl)-...)Show SMILES CC#CCOc1ccc(cc1)S(=O)(=O)N1C[C@@H](O)[C@@H](O)[C@H](O)[C@@H]1C(=O)NO Show InChI InChI=1S/C16H20N2O8S/c1-2-3-8-26-10-4-6-11(7-5-10)27(24,25)18-9-12(19)14(20)15(21)13(18)16(22)17-23/h4-7,12-15,19-21,23H,8-9H2,1H3,(H,17,22)/t12-,13-,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human tumor necrosis factor alpha converting enzyme |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50143732

((2R,3R,4R,5R)-1-(4-Benzyloxy-benzenesulfonyl)-3,4,...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@H](O)CN1S(=O)(=O)c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C19H22N2O8S/c22-15-10-21(16(19(25)20-26)18(24)17(15)23)30(27,28)14-8-6-13(7-9-14)29-11-12-4-2-1-3-5-12/h1-9,15-18,22-24,26H,10-11H2,(H,20,25)/t15-,16-,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human tumor necrosis factor alpha converting enzyme |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50143731
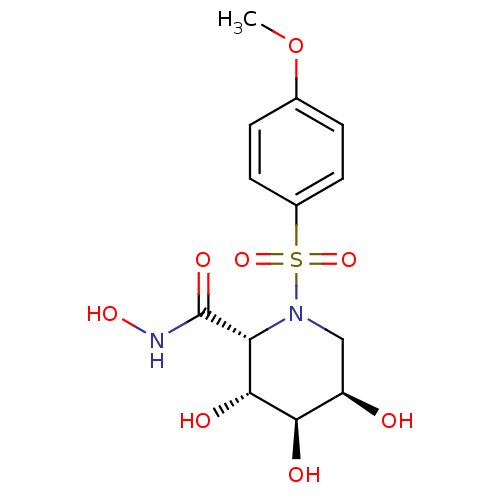
((2R,3R,4R,5R)-3,4,5-Trihydroxy-1-(4-methoxy-benzen...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1C[C@@H](O)[C@@H](O)[C@H](O)[C@@H]1C(=O)NO Show InChI InChI=1S/C13H18N2O8S/c1-23-7-2-4-8(5-3-7)24(21,22)15-6-9(16)11(17)12(18)10(15)13(19)14-20/h2-5,9-12,16-18,20H,6H2,1H3,(H,14,19)/t9-,10-,11-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 3 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50143731

((2R,3R,4R,5R)-3,4,5-Trihydroxy-1-(4-methoxy-benzen...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1C[C@@H](O)[C@@H](O)[C@H](O)[C@@H]1C(=O)NO Show InChI InChI=1S/C13H18N2O8S/c1-23-7-2-4-8(5-3-7)24(21,22)15-6-9(16)11(17)12(18)10(15)13(19)14-20/h2-5,9-12,16-18,20H,6H2,1H3,(H,14,19)/t9-,10-,11-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 9 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50143732

((2R,3R,4R,5R)-1-(4-Benzyloxy-benzenesulfonyl)-3,4,...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@H](O)CN1S(=O)(=O)c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C19H22N2O8S/c22-15-10-21(16(19(25)20-26)18(24)17(15)23)30(27,28)14-8-6-13(7-9-14)29-11-12-4-2-1-3-5-12/h1-9,15-18,22-24,26H,10-11H2,(H,20,25)/t15-,16-,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 3 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50131385

((2R,3R,4R,5S)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15+,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human tumor necrosis factor alpha converting enzyme |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50141577

((2R,3R,4R,5R)-1-(4-But-2-ynyloxy-benzenesulfonyl)-...)Show SMILES CC#CCOc1ccc(cc1)S(=O)(=O)N1C[C@@H](O)[C@@H](O)[C@H](O)[C@@H]1C(=O)NO Show InChI InChI=1S/C16H20N2O8S/c1-2-3-8-26-10-4-6-11(7-5-10)27(24,25)18-9-12(19)14(20)15(21)13(18)16(22)17-23/h4-7,12-15,19-21,23H,8-9H2,1H3,(H,17,22)/t12-,13-,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 3 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50143729

((2R,3R,4R,5R)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15-,16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 1 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50143729

((2R,3R,4R,5R)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15-,16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human tumor necrosis factor alpha converting enzyme |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50143732

((2R,3R,4R,5R)-1-(4-Benzyloxy-benzenesulfonyl)-3,4,...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@H](O)CN1S(=O)(=O)c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C19H22N2O8S/c22-15-10-21(16(19(25)20-26)18(24)17(15)23)30(27,28)14-8-6-13(7-9-14)29-11-12-4-2-1-3-5-12/h1-9,15-18,22-24,26H,10-11H2,(H,20,25)/t15-,16-,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 9 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50131385

((2R,3R,4R,5S)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15+,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 1 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50141577

((2R,3R,4R,5R)-1-(4-But-2-ynyloxy-benzenesulfonyl)-...)Show SMILES CC#CCOc1ccc(cc1)S(=O)(=O)N1C[C@@H](O)[C@@H](O)[C@H](O)[C@@H]1C(=O)NO Show InChI InChI=1S/C16H20N2O8S/c1-2-3-8-26-10-4-6-11(7-5-10)27(24,25)18-9-12(19)14(20)15(21)13(18)16(22)17-23/h4-7,12-15,19-21,23H,8-9H2,1H3,(H,17,22)/t12-,13-,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 9 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50143731

((2R,3R,4R,5R)-3,4,5-Trihydroxy-1-(4-methoxy-benzen...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1C[C@@H](O)[C@@H](O)[C@H](O)[C@@H]1C(=O)NO Show InChI InChI=1S/C13H18N2O8S/c1-23-7-2-4-8(5-3-7)24(21,22)15-6-9(16)11(17)12(18)10(15)13(19)14-20/h2-5,9-12,16-18,20H,6H2,1H3,(H,14,19)/t9-,10-,11-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human tumor necrosis factor alpha converting enzyme |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50143730
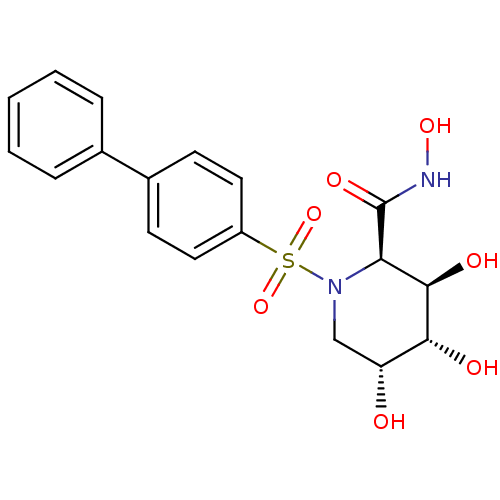
((2R,3R,4R,5R)-1-(Biphenyl-4-sulfonyl)-3,4,5-trihyd...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@H](O)CN1S(=O)(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C18H20N2O7S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)28(26,27)13-8-6-12(7-9-13)11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15-,16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human tumor necrosis factor alpha converting enzyme |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50143731

((2R,3R,4R,5R)-3,4,5-Trihydroxy-1-(4-methoxy-benzen...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1C[C@@H](O)[C@@H](O)[C@H](O)[C@@H]1C(=O)NO Show InChI InChI=1S/C13H18N2O8S/c1-23-7-2-4-8(5-3-7)24(21,22)15-6-9(16)11(17)12(18)10(15)13(19)14-20/h2-5,9-12,16-18,20H,6H2,1H3,(H,14,19)/t9-,10-,11-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 1 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50143730

((2R,3R,4R,5R)-1-(Biphenyl-4-sulfonyl)-3,4,5-trihyd...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@H](O)CN1S(=O)(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C18H20N2O7S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)28(26,27)13-8-6-12(7-9-13)11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15-,16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 9 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50143730

((2R,3R,4R,5R)-1-(Biphenyl-4-sulfonyl)-3,4,5-trihyd...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@H](O)CN1S(=O)(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C18H20N2O7S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)28(26,27)13-8-6-12(7-9-13)11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15-,16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 3 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50141577

((2R,3R,4R,5R)-1-(4-But-2-ynyloxy-benzenesulfonyl)-...)Show SMILES CC#CCOc1ccc(cc1)S(=O)(=O)N1C[C@@H](O)[C@@H](O)[C@H](O)[C@@H]1C(=O)NO Show InChI InChI=1S/C16H20N2O8S/c1-2-3-8-26-10-4-6-11(7-5-10)27(24,25)18-9-12(19)14(20)15(21)13(18)16(22)17-23/h4-7,12-15,19-21,23H,8-9H2,1H3,(H,17,22)/t12-,13-,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 128 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 1 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50143730

((2R,3R,4R,5R)-1-(Biphenyl-4-sulfonyl)-3,4,5-trihyd...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@H](O)CN1S(=O)(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C18H20N2O7S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)28(26,27)13-8-6-12(7-9-13)11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15-,16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 162 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 1 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50143732

((2R,3R,4R,5R)-1-(4-Benzyloxy-benzenesulfonyl)-3,4,...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@H](O)CN1S(=O)(=O)c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C19H22N2O8S/c22-15-10-21(16(19(25)20-26)18(24)17(15)23)30(27,28)14-8-6-13(7-9-14)29-11-12-4-2-1-3-5-12/h1-9,15-18,22-24,26H,10-11H2,(H,20,25)/t15-,16-,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant matrix metalloprotease 1 |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Beta-1,4-galactosyltransferase 1
(Homo sapiens (Human)) | BDBM50370676

(CHEMBL607907)Show SMILES O[C@@H]1[C@@H](COP(O)(=O)OP(O)(=O)O[C@H]2O[C@@H](COCCOCCOCCOCc3ccc4ccccc4c3)[C@H](O)[C@H](O)[C@@H]2O)OC([C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C32H44N2O20P2/c35-24-7-8-34(32(41)33-24)30-28(39)26(37)23(51-30)18-50-55(42,43)54-56(44,45)53-31-29(40)27(38)25(36)22(52-31)17-49-14-12-47-10-9-46-11-13-48-16-19-5-6-20-3-1-2-4-21(20)15-19/h1-8,15,22-23,25-31,36-40H,9-14,16-18H2,(H,42,43)(H,44,45)(H,33,35,41)/t22-,23+,25-,26+,27-,28+,29-,30?,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.86E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibitory constant against human galactosyltransferase using UDP-Gal |
J Med Chem 48: 6054-65 (2005)
Article DOI: 10.1021/jm0504297
BindingDB Entry DOI: 10.7270/Q2JW8FPX |
More data for this
Ligand-Target Pair | |
Beta-1,4-galactosyltransferase 1
(Homo sapiens (Human)) | BDBM50370674
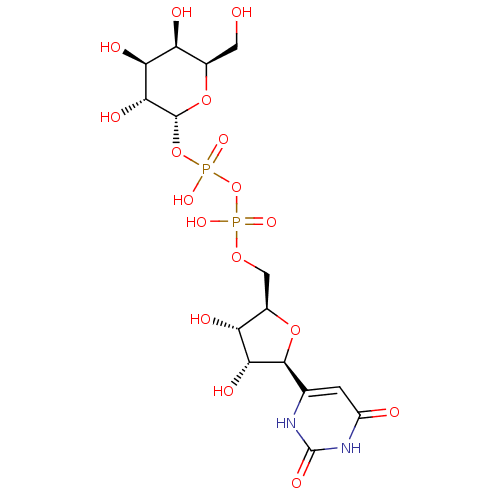
(UDP-GALACTOSE)Show SMILES OC[C@H]1O[C@H](OP(O)(=O)OP(O)(=O)OC[C@H]2O[C@H]([C@H](O)[C@@H]2O)c2cc(=O)[nH]c(=O)[nH]2)[C@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C15H24N2O17P2/c18-2-5-8(20)10(22)12(24)14(32-5)33-36(28,29)34-35(26,27)30-3-6-9(21)11(23)13(31-6)4-1-7(19)17-15(25)16-4/h1,5-6,8-14,18,20-24H,2-3H2,(H,26,27)(H,28,29)(H2,16,17,19,25)/t5-,6-,8+,9-,10+,11-,12-,13+,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4.91E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibitory constant against human recombinant Beta-1,4-galactosyltransferase I |
J Med Chem 48: 6054-65 (2005)
Article DOI: 10.1021/jm0504297
BindingDB Entry DOI: 10.7270/Q2JW8FPX |
More data for this
Ligand-Target Pair | |
Beta-1,4-galactosyltransferase 1
(Homo sapiens (Human)) | BDBM50370675
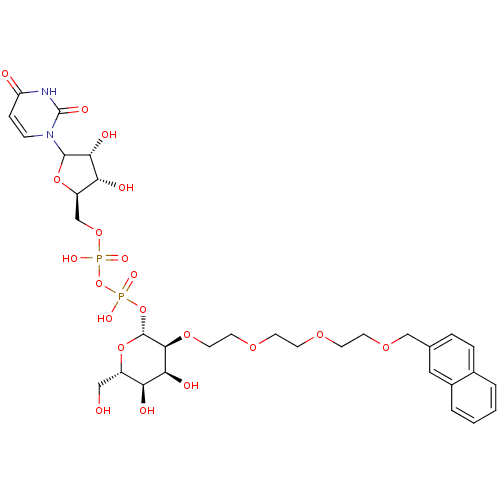
(CHEMBL607908)Show SMILES OC[C@@H]1O[C@H](OP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)[C@@H](OCCOCCOCCOCc2ccc3ccccc3c2)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C32H44N2O20P2/c35-16-22-25(37)27(39)29(49-14-13-47-10-9-46-11-12-48-17-19-5-6-20-3-1-2-4-21(20)15-19)31(52-22)53-56(44,45)54-55(42,43)50-18-23-26(38)28(40)30(51-23)34-8-7-24(36)33-32(34)41/h1-8,15,22-23,25-31,35,37-40H,9-14,16-18H2,(H,42,43)(H,44,45)(H,33,36,41)/t22-,23+,25-,26+,27-,28+,29-,30?,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibitory constant against human recombinant Beta-1,4-galactosyltransferase I |
J Med Chem 48: 6054-65 (2005)
Article DOI: 10.1021/jm0504297
BindingDB Entry DOI: 10.7270/Q2JW8FPX |
More data for this
Ligand-Target Pair | |
Beta-1,4-galactosyltransferase 1
(Homo sapiens (Human)) | BDBM50370678

(CHEMBL611116)Show SMILES O[C@@H]1[C@@H](COP(O)(=O)OP(O)(=O)O[C@H]2O[C@@H](CNNC(=O)COCCOCCOCc3ccc4ccccc4c3)[C@H](O)[C@H](O)[C@@H]2O)OC([C@@H]1O)n1ccc(=O)[nH]c1=O |r| Show InChI InChI=1S/C32H44N4O20P2/c37-23-7-8-36(32(44)34-23)30-28(42)26(40)22(53-30)16-52-57(45,46)56-58(47,48)55-31-29(43)27(41)25(39)21(54-31)14-33-35-24(38)17-51-12-10-49-9-11-50-15-18-5-6-19-3-1-2-4-20(19)13-18/h1-8,13,21-22,25-31,33,39-43H,9-12,14-17H2,(H,35,38)(H,45,46)(H,47,48)(H,34,37,44)/t21-,22+,25-,26+,27-,28+,29-,30?,31+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibitory constant against human recombinant Beta-1,4-galactosyltransferase I |
J Med Chem 48: 6054-65 (2005)
Article DOI: 10.1021/jm0504297
BindingDB Entry DOI: 10.7270/Q2JW8FPX |
More data for this
Ligand-Target Pair | |
Beta-1,4-galactosyltransferase 1
(Homo sapiens (Human)) | BDBM50370677

(CHEMBL609634)Show SMILES Cc1ccc2ccc(COC(=O)COCCOCC(=O)NC[C@@H]3O[C@H](OP(O)(=O)OP(O)(=O)OC[C@H]4OC([C@H](O)[C@@H]4O)n4ccc(=O)[nH]c4=O)[C@@H](O)[C@@H](O)[C@H]3O)cc2c1 |r| Show InChI InChI=1S/C33H43N3O21P2/c1-17-2-4-19-5-3-18(11-20(19)10-17)13-52-25(39)16-51-9-8-50-15-24(38)34-12-21-26(40)28(42)30(44)32(55-21)56-59(48,49)57-58(46,47)53-14-22-27(41)29(43)31(54-22)36-7-6-23(37)35-33(36)45/h2-7,10-11,21-22,26-32,40-44H,8-9,12-16H2,1H3,(H,34,38)(H,46,47)(H,48,49)(H,35,37,45)/t21-,22+,26-,27+,28-,29+,30-,31?,32+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibitory constant against human recombinant Beta-1,4-galactosyltransferase I |
J Med Chem 48: 6054-65 (2005)
Article DOI: 10.1021/jm0504297
BindingDB Entry DOI: 10.7270/Q2JW8FPX |
More data for this
Ligand-Target Pair | |
Beta-1,4-galactosyltransferase 1
(Homo sapiens (Human)) | BDBM50173721
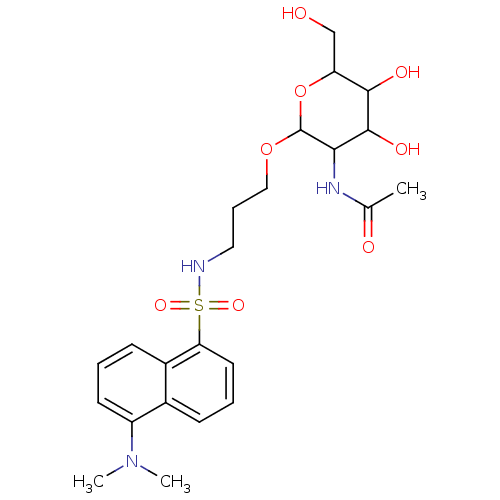
(CHEMBL196432 | Uridine-5'-diphosphogalactose deriv...)Show SMILES CN(C)c1cccc2c(cccc12)S(=O)(=O)NCCCOC1OC(CO)C(O)C(O)C1NC(C)=O Show InChI InChI=1S/C23H33N3O8S/c1-14(28)25-20-22(30)21(29)18(13-27)34-23(20)33-12-6-11-24-35(31,32)19-10-5-7-15-16(19)8-4-9-17(15)26(2)3/h4-5,7-10,18,20-24,27,29-30H,6,11-13H2,1-3H3,(H,25,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 7.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibitory constant against human Beta-1,4-galactosyltransferase I using UDP-Gal (0-160 uM) |
J Med Chem 48: 6054-65 (2005)
Article DOI: 10.1021/jm0504297
BindingDB Entry DOI: 10.7270/Q2JW8FPX |
More data for this
Ligand-Target Pair | |
Beta-1,4-galactosyltransferase 1
(Homo sapiens (Human)) | BDBM50370679

(CHEMBL611112)Show SMILES COCCOCCOCCOC[C@@H]1O[C@H](OP(O)(=O)OP(O)(=O)OC[C@H]2OC([C@H](O)[C@@H]2O)n2ccc(=O)[nH]c2=O)[C@@H](O)[C@@H](O)[C@H]1O |r| Show InChI InChI=1S/C22H38N2O20P2/c1-36-4-5-37-6-7-38-8-9-39-10-12-15(26)17(28)19(30)21(42-12)43-46(34,35)44-45(32,33)40-11-13-16(27)18(29)20(41-13)24-3-2-14(25)23-22(24)31/h2-3,12-13,15-21,26-30H,4-11H2,1H3,(H,32,33)(H,34,35)(H,23,25,31)/t12-,13+,15-,16+,17-,18+,19-,20?,21+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.49E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido University
Curated by ChEMBL
| Assay Description
Inhibitory constant against human recombinant Beta-1,4-galactosyltransferase I |
J Med Chem 48: 6054-65 (2005)
Article DOI: 10.1021/jm0504297
BindingDB Entry DOI: 10.7270/Q2JW8FPX |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50141577

((2R,3R,4R,5R)-1-(4-But-2-ynyloxy-benzenesulfonyl)-...)Show SMILES CC#CCOc1ccc(cc1)S(=O)(=O)N1C[C@@H](O)[C@@H](O)[C@H](O)[C@@H]1C(=O)NO Show InChI InChI=1S/C16H20N2O8S/c1-2-3-8-26-10-4-6-11(7-5-10)27(24,25)18-9-12(19)14(20)15(21)13(18)16(22)17-23/h4-7,12-15,19-21,23H,8-9H2,1H3,(H,17,22)/t12-,13-,14-,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of heparin-binding HB-EGF shedding |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50143732

((2R,3R,4R,5R)-1-(4-Benzyloxy-benzenesulfonyl)-3,4,...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@H](O)CN1S(=O)(=O)c1ccc(OCc2ccccc2)cc1 Show InChI InChI=1S/C19H22N2O8S/c22-15-10-21(16(19(25)20-26)18(24)17(15)23)30(27,28)14-8-6-13(7-9-14)29-11-12-4-2-1-3-5-12/h1-9,15-18,22-24,26H,10-11H2,(H,20,25)/t15-,16-,17-,18-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 84 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of heparin-binding HB-EGF shedding |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50143729

((2R,3R,4R,5R)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15-,16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of heparin-binding HB-EGF shedding |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50131385

((2R,3R,4R,5S)-3,4,5-Trihydroxy-1-(4-phenoxy-benzen...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@@H](O)CN1S(=O)(=O)c1ccc(Oc2ccccc2)cc1 |r| Show InChI InChI=1S/C18H20N2O8S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)29(26,27)13-8-6-12(7-9-13)28-11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15+,16+,17+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of heparin-binding HB-EGF shedding |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50143731

((2R,3R,4R,5R)-3,4,5-Trihydroxy-1-(4-methoxy-benzen...)Show SMILES COc1ccc(cc1)S(=O)(=O)N1C[C@@H](O)[C@@H](O)[C@H](O)[C@@H]1C(=O)NO Show InChI InChI=1S/C13H18N2O8S/c1-23-7-2-4-8(5-3-7)24(21,22)15-6-9(16)11(17)12(18)10(15)13(19)14-20/h2-5,9-12,16-18,20H,6H2,1H3,(H,14,19)/t9-,10-,11-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of heparin-binding HB-EGF shedding |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Homo sapiens (Human)) | BDBM50143730

((2R,3R,4R,5R)-1-(Biphenyl-4-sulfonyl)-3,4,5-trihyd...)Show SMILES ONC(=O)[C@H]1[C@@H](O)[C@H](O)[C@H](O)CN1S(=O)(=O)c1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C18H20N2O7S/c21-14-10-20(15(18(24)19-25)17(23)16(14)22)28(26,27)13-8-6-12(7-9-13)11-4-2-1-3-5-11/h1-9,14-17,21-23,25H,10H2,(H,19,24)/t14-,15-,16-,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Hokkaido Collaboration Center N-21
Curated by ChEMBL
| Assay Description
Inhibition of heparin-binding HB-EGF shedding |
J Med Chem 47: 1930-8 (2004)
Article DOI: 10.1021/jm0304313
BindingDB Entry DOI: 10.7270/Q2ZS2VXF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data