

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sialidase-2 (Homo sapiens (Human)) | BDBM50295841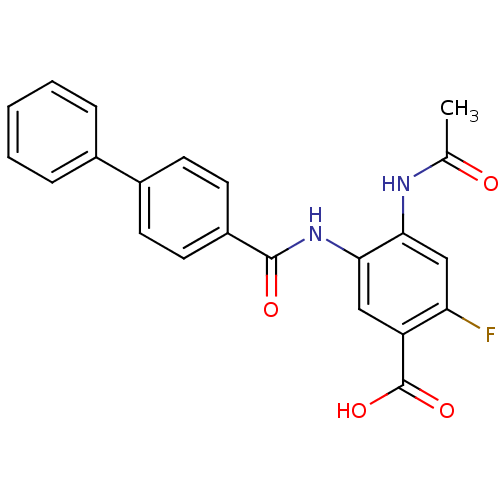 (4-Acetamido-5-(biphenyl-4-ylcarboxamido)-2-fluorob...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.07E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gifu University Curated by ChEMBL | Assay Description Inhibition of human NEU2 transiently transfected in HEK293 cells by Line-Weaver Burk plotting | Bioorg Med Chem 17: 4595-603 (2009) Article DOI: 10.1016/j.bmc.2009.04.065 BindingDB Entry DOI: 10.7270/Q2TX3FD8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/28/2005(H6N2)) neuraminidase N2 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/67/2005(H1N1)) neuraminidase N1 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/394/2005(H5N3)) neuraminidase N3 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins foll... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/20/2007(H8N4)) neuraminidase N4 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Beta-secretase 1 (Homo sapiens (Human)) | BDBM50452874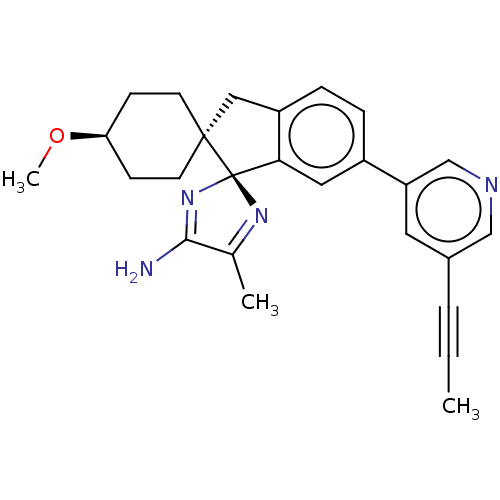 (CHEMBL4217620) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Binding affinity to human recombinant BACE-1 (1 to 460 residue) using CEVNLDAEFK as substrate preincubated for 10 mins followed by substrate addition... | Citation and Details Article DOI: 10.1016/j.bmc.2021.116459 BindingDB Entry DOI: 10.7270/Q2057KSB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/700/2007(H7N7)) neuraminidase N7 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins foll... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261482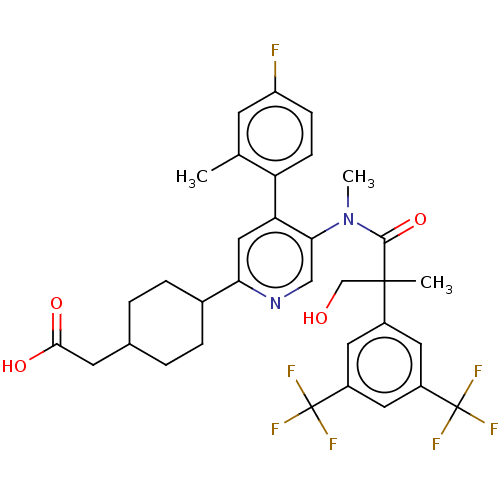 (US10011568, Ex. No. 5 | US9708266, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | US Patent US9708266 (2017) BindingDB Entry DOI: 10.7270/Q2HM5BGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261482 (US10011568, Ex. No. 5 | US9708266, 5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.980 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | J Med Chem 52: 5013-6 (2009) BindingDB Entry DOI: 10.7270/Q2HT2RQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Shiga/8/2004(H4N6)) neuraminidase N6 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261483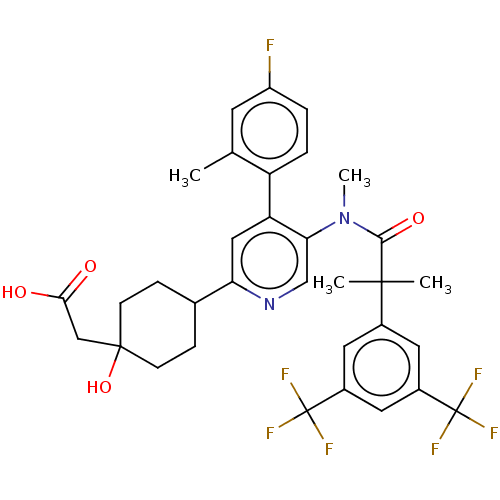 (US10011568, Ex. No. 6 | US9708266, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | J Med Chem 52: 5013-6 (2009) BindingDB Entry DOI: 10.7270/Q2HT2RQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261483 (US10011568, Ex. No. 6 | US9708266, 6) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | US Patent US9708266 (2017) BindingDB Entry DOI: 10.7270/Q2HM5BGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50362779 (CHEMBL1940181 | US9126931, 346) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of ALK using Bio-Gastrintide as substrate by TR-FRET assay in presence of 30 uM of ATP | Bioorg Med Chem 20: 1271-80 (2012) Article DOI: 10.1016/j.bmc.2011.12.021 BindingDB Entry DOI: 10.7270/Q23B60KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50362778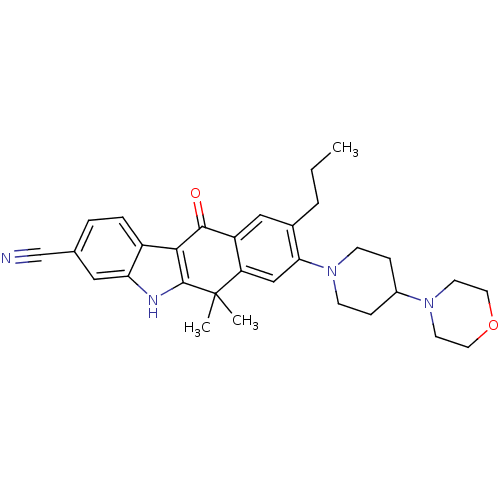 (CHEMBL1940182) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of ALK using Bio-Gastrintide as substrate by TR-FRET assay in presence of 30 uM of ATP | Bioorg Med Chem 20: 1271-80 (2012) Article DOI: 10.1016/j.bmc.2011.12.021 BindingDB Entry DOI: 10.7270/Q23B60KW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/duck/Tsukuba/441/05(H11N9) neuraminidase N9 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followe... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/Yamaguchi/20/06(H1N1) neuraminidase N1 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed by ... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM292016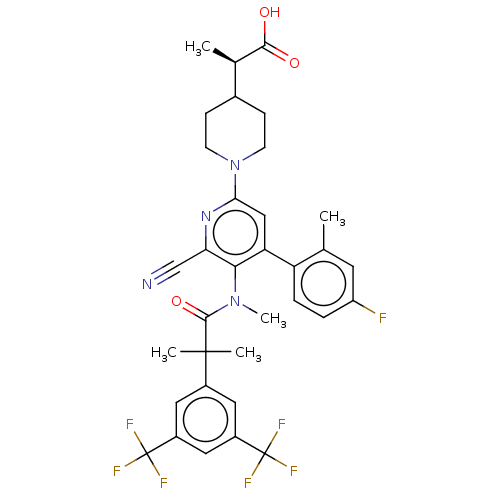 (US10100030, Example 11) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.32 | n/a | n/a | n/a | n/a | 7.4 | 60 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 uL/well. DMSO solutions of a ... | US Patent US10100030 (2018) BindingDB Entry DOI: 10.7270/Q2W95C7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261478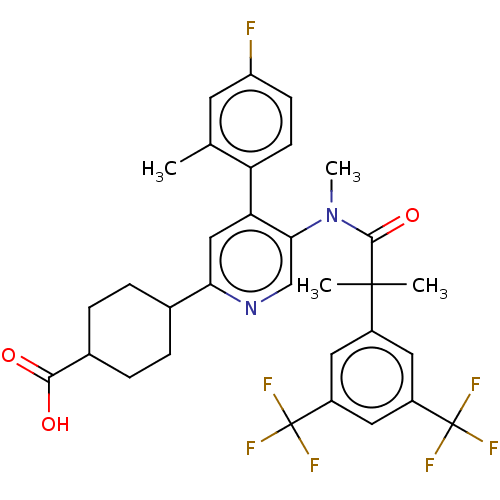 (US10011568, Ex. No. 1 | US9708266, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.45 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | US Patent US9708266 (2017) BindingDB Entry DOI: 10.7270/Q2HM5BGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261478 (US10011568, Ex. No. 1 | US9708266, 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.45 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | J Med Chem 52: 5013-6 (2009) BindingDB Entry DOI: 10.7270/Q2HT2RQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50344664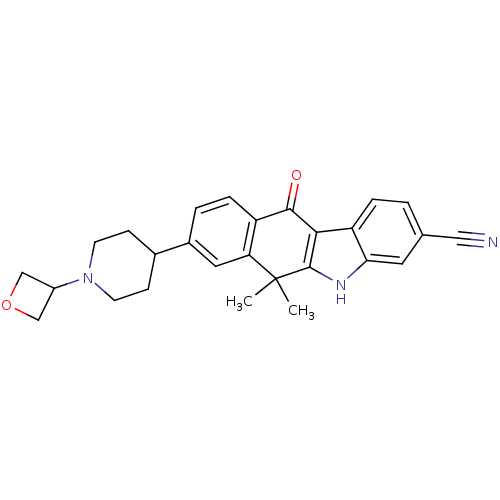 (6,6-dimethyl-8-(1-(oxetan-3-yl)piperidin-4-yl)-11-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd. Curated by ChEMBL | Assay Description Inhibition of ALK activity by TR-FRET assay | J Med Chem 54: 6286-94 (2011) Article DOI: 10.1021/jm200652u BindingDB Entry DOI: 10.7270/Q2P55NWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus (A/Puerto Rico/8/34/Mount Sinai(...) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.56 | n/a | n/a | n/a | n/a | n/a | n/a |
Miyagi Cancer Center Research Institute Curated by ChEMBL | Assay Description Inhibition of human Influenza A virus A/PR/8/34(H1N1) neuraminidase by fluorometric method using 4MU-NeuAc substrate | Antimicrob Agents Chemother 52: 3484-91 (2008) Article DOI: 10.1128/AAC.00344-08 BindingDB Entry DOI: 10.7270/Q2BR8T42 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus (strain A/Aichi/2/1968 H3N2)) | BDBM4994 ((3R,4R,5S)-5-amino-4-acetamido-3-(pentan-3-yloxy)c...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Miyagi Cancer Center Research Institute Curated by ChEMBL | Assay Description Inhibition of human influenza A virus A/Aichi/2/1968(H3N2) neuraminidase by fluorometric method using 4MU-NeuAc substrate | Antimicrob Agents Chemother 52: 3484-91 (2008) Article DOI: 10.1128/AAC.00344-08 BindingDB Entry DOI: 10.7270/Q2BR8T42 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/California/04/2009(H1N1)) neuraminidase N1 V149I mutant activity using 4MU-Neu5Ac as substrate preincubated for 15... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261490 (US10011568, Ex. No. 13 | US9708266, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.62 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | J Med Chem 52: 5013-6 (2009) BindingDB Entry DOI: 10.7270/Q2HT2RQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261490 (US10011568, Ex. No. 13 | US9708266, 13) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.62 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | US Patent US9708266 (2017) BindingDB Entry DOI: 10.7270/Q2HM5BGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261484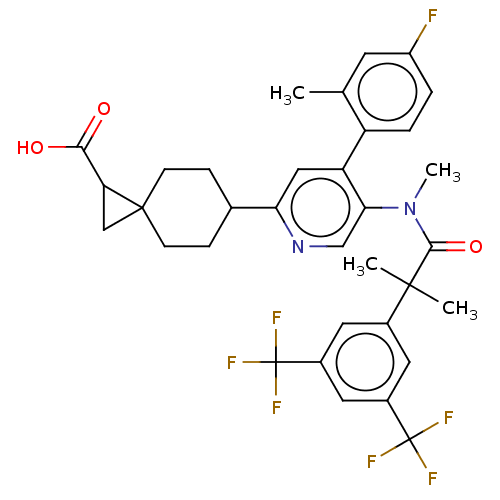 (US10011568, Ex. No. 7 | US9708266, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | US Patent US9708266 (2017) BindingDB Entry DOI: 10.7270/Q2HM5BGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261484 (US10011568, Ex. No. 7 | US9708266, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.63 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | J Med Chem 52: 5013-6 (2009) BindingDB Entry DOI: 10.7270/Q2HT2RQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/mallard/Hokkaido/24/2009(H5N1)) neuraminidase N1 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins f... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261479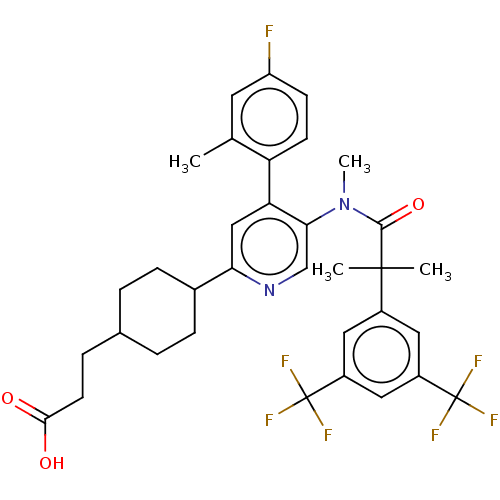 (US10011568, Ex. No. 2 | US9708266, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.78 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | J Med Chem 52: 5013-6 (2009) BindingDB Entry DOI: 10.7270/Q2HT2RQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261479 (US10011568, Ex. No. 2 | US9708266, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.78 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | US Patent US9708266 (2017) BindingDB Entry DOI: 10.7270/Q2HM5BGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Aichi/102/2008(H3N2)) neuraminidase N2 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed by... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/Narita/1/2009(H1N1)) neuraminidase N1 V149I mutant activity using 4MU-Neu5Ac as substrate preincubated for 15 mins... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| ALK tyrosine kinase receptor (Homo sapiens (Human)) | BDBM50362781 (1256580-46-7 | AF802 | Alecensa | Alectinib | CH54...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Chugai Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of ALK using Bio-Gastrintide as substrate by TR-FRET assay in presence of 30 uM of ATP | Bioorg Med Chem 20: 1271-80 (2012) Article DOI: 10.1016/j.bmc.2011.12.021 BindingDB Entry DOI: 10.7270/Q23B60KW | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Gonadotropin-releasing hormone receptor (Homo sapiens (Human)) | BDBM160321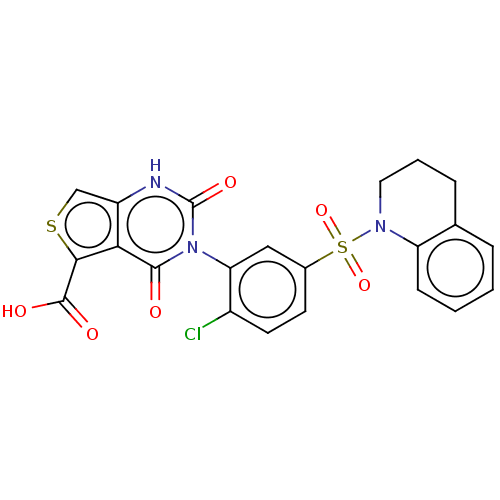 (US9040693, 22) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | 37 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description Antagonizing effect of compounds for human GnRHR1 was evaluated by change of calcium levels in GnRH-stimulated cells. After removing the culture medi... | US Patent US9040693 (2015) BindingDB Entry DOI: 10.7270/Q21R6P8N | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50182249 (CHEMBL3818159) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/20/2007(H8N4)) neuraminidase N4 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261480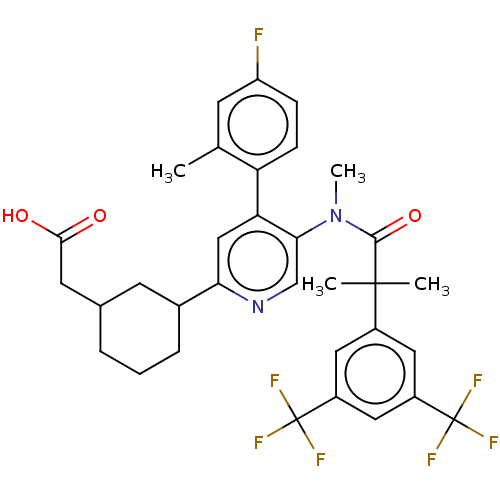 (US10011568, Ex. No. 3 | US9708266, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | US Patent US9708266 (2017) BindingDB Entry DOI: 10.7270/Q2HM5BGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261480 (US10011568, Ex. No. 3 | US9708266, 3) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | J Med Chem 52: 5013-6 (2009) BindingDB Entry DOI: 10.7270/Q2HT2RQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM292006 (US10100030, Example 1) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.11 | n/a | n/a | n/a | n/a | 7.4 | 60 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 uL/well. DMSO solutions of a ... | US Patent US10100030 (2018) BindingDB Entry DOI: 10.7270/Q2W95C7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus A/duck/Chiba/13/06(H12N5) neuraminidase N5 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins followed b... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261491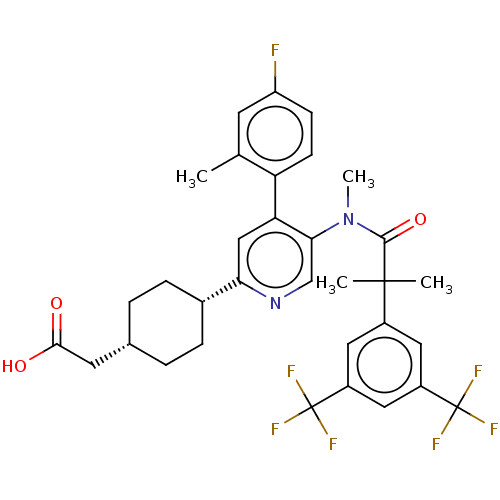 (US10011568, Ex. No. 14 | US9708266, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.21 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | US Patent US9708266 (2017) BindingDB Entry DOI: 10.7270/Q2HM5BGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261491 (US10011568, Ex. No. 14 | US9708266, 14) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.21 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | J Med Chem 52: 5013-6 (2009) BindingDB Entry DOI: 10.7270/Q2HT2RQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM292013 (US10100030, Example 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.25 | n/a | n/a | n/a | n/a | 7.4 | 60 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 uL/well. DMSO solutions of a ... | US Patent US10100030 (2018) BindingDB Entry DOI: 10.7270/Q2W95C7C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/394/2005(H5N3)) neuraminidase N3 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins foll... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261481 (US10011568, Ex. No. 4 | US9708266, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.35 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | US Patent US9708266 (2017) BindingDB Entry DOI: 10.7270/Q2HM5BGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261486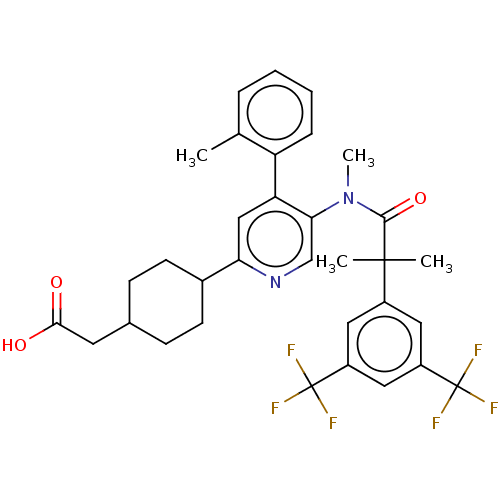 (US10011568, Ex. No. 9 | US9708266, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.35 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | US Patent US9708266 (2017) BindingDB Entry DOI: 10.7270/Q2HM5BGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261481 (US10011568, Ex. No. 4 | US9708266, 4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | J Med Chem 52: 5013-6 (2009) BindingDB Entry DOI: 10.7270/Q2HT2RQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261486 (US10011568, Ex. No. 9 | US9708266, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.35 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | J Med Chem 52: 5013-6 (2009) BindingDB Entry DOI: 10.7270/Q2HT2RQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261497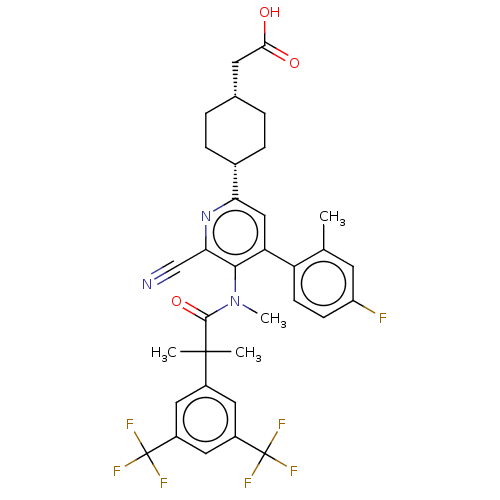 (US10011568, Ex. No. 21 | US9708266, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.36 | n/a | n/a | n/a | n/a | 7.4 | 25 |
KISSEI PHARMACEUTICAL CO., LTD. US Patent | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | US Patent US9708266 (2017) BindingDB Entry DOI: 10.7270/Q2HM5BGS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Substance-P receptor (Homo sapiens (Human)) | BDBM261497 (US10011568, Ex. No. 21 | US9708266, 21) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.36 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description The buffer solution for the receptor binding test was dispensed to the wells of a 96-well assay plate (Greiner) at 22.5 μL/well. DMSO solutions ... | J Med Chem 52: 5013-6 (2009) BindingDB Entry DOI: 10.7270/Q2HT2RQT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neuraminidase (Influenza A virus) | BDBM50330326 ((4S,5R,6R)-5-Acetylamino-4-guanidino-6-((1R,3R)-1,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Thammasat University Curated by ChEMBL | Assay Description Inhibition of Influenza A virus (A/duck/Tsukuba/28/2005(H6N2)) neuraminidase N2 activity using 4MU-Neu5Ac as substrate preincubated for 15 mins follo... | J Med Chem 59: 4563-77 (2016) Article DOI: 10.1021/acs.jmedchem.5b01863 BindingDB Entry DOI: 10.7270/Q2QN68QP | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Displayed 1 to 50 (of 601 total ) | Next | Last >> |