 Found 38 hits with Last Name = 'mohan' and Initial = 'cg'
Found 38 hits with Last Name = 'mohan' and Initial = 'cg' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM11639

(4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...)Show SMILES Cc1ccc(cc1)-c1cc(nn1-c1ccc(cc1)S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C17H14F3N3O2S/c1-11-2-4-12(5-3-11)15-10-16(17(18,19)20)22-23(15)13-6-8-14(9-7-13)26(21,24)25/h2-10H,1H3,(H2,21,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM17638

(2-{1-[(4-chlorophenyl)carbonyl]-5-methoxy-2-methyl...)Show SMILES COc1ccc2n(C(=O)c3ccc(Cl)cc3)c(C)c(CC(O)=O)c2c1 Show InChI InChI=1S/C19H16ClNO4/c1-11-15(10-18(22)23)16-9-14(25-2)7-8-17(16)21(11)19(24)12-3-5-13(20)6-4-12/h3-9H,10H2,1-2H3,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50336472
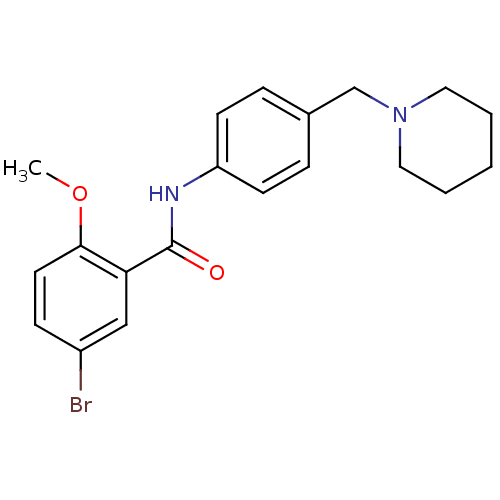
(5-bromo-2-methoxy-N-(4-(piperidin-1-ylmethyl)pheny...)Show InChI InChI=1S/C20H23BrN2O2/c1-25-19-10-7-16(21)13-18(19)20(24)22-17-8-5-15(6-9-17)14-23-11-3-2-4-12-23/h5-10,13H,2-4,11-12,14H2,1H3,(H,22,24) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem Lett 21: 1105-12 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.131
BindingDB Entry DOI: 10.7270/Q2V988B9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50336473
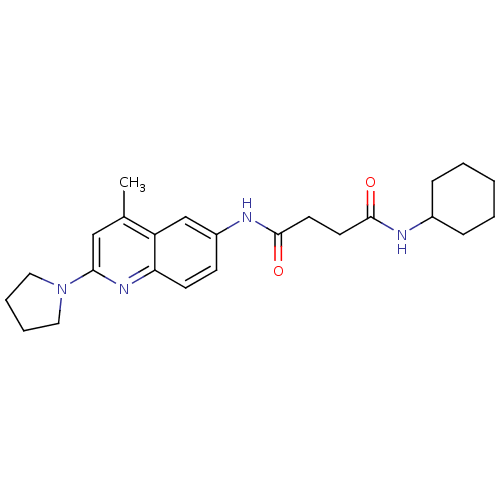
(CHEMBL1668494 | N1-cyclohexyl-N4-(4-methyl-2-(pyrr...)Show SMILES Cc1cc(nc2ccc(NC(=O)CCC(=O)NC3CCCCC3)cc12)N1CCCC1 Show InChI InChI=1S/C24H32N4O2/c1-17-15-22(28-13-5-6-14-28)27-21-10-9-19(16-20(17)21)26-24(30)12-11-23(29)25-18-7-3-2-4-8-18/h9-10,15-16,18H,2-8,11-14H2,1H3,(H,25,29)(H,26,30) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem Lett 21: 1105-12 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.131
BindingDB Entry DOI: 10.7270/Q2V988B9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM51607
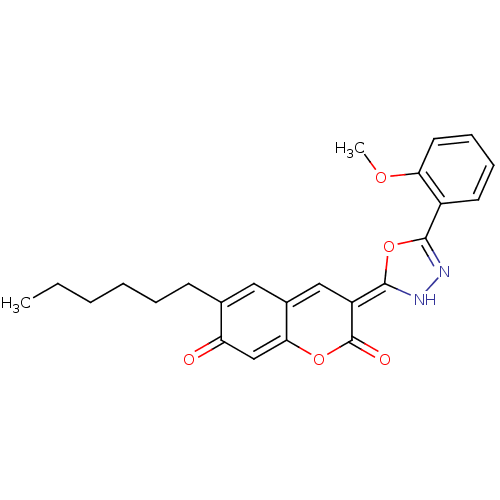
((3E)-6-hexyl-3-[5-(2-methoxyphenyl)-3H-1,3,4-oxadi...)Show SMILES CCCCCCC1=Cc2c\c(=C3\NN=C(O3)c3ccccc3OC)c(=O)oc2=CC1=O |c:13,30,t:6| Show InChI InChI=1S/C24H24N2O5/c1-3-4-5-6-9-15-12-16-13-18(24(28)30-21(16)14-19(15)27)23-26-25-22(31-23)17-10-7-8-11-20(17)29-2/h7-8,10-14,26H,3-6,9H2,1-2H3/b23-18+ | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem Lett 21: 1105-12 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.131
BindingDB Entry DOI: 10.7270/Q2V988B9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50336471
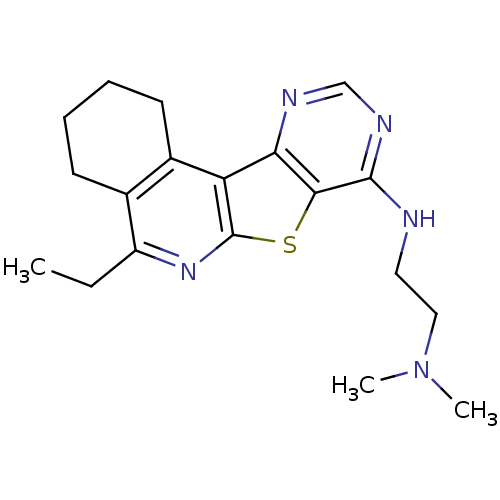
(CHEMBL1668492 | N'-(5-Ethyl-1,2,3,4-tetrahydro-7-t...)Show InChI InChI=1S/C19H25N5S/c1-4-14-12-7-5-6-8-13(12)15-16-17(25-19(15)23-14)18(22-11-21-16)20-9-10-24(2)3/h11H,4-10H2,1-3H3,(H,20,21,22) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem Lett 21: 1105-12 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.131
BindingDB Entry DOI: 10.7270/Q2V988B9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50336470
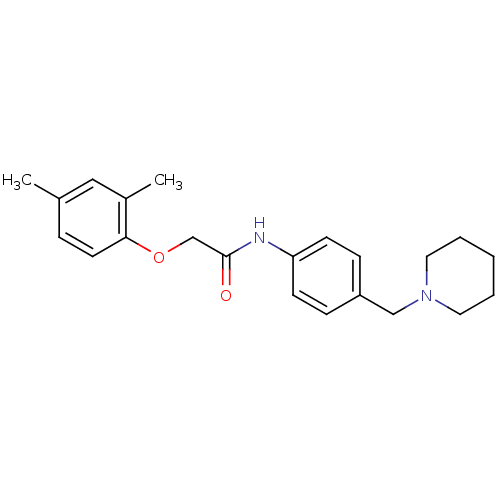
(2-(2,4-dimethylphenoxy)-N-(4-(piperidin-1-ylmethyl...)Show InChI InChI=1S/C22H28N2O2/c1-17-6-11-21(18(2)14-17)26-16-22(25)23-20-9-7-19(8-10-20)15-24-12-4-3-5-13-24/h6-11,14H,3-5,12-13,15-16H2,1-2H3,(H,23,25) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem Lett 21: 1105-12 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.131
BindingDB Entry DOI: 10.7270/Q2V988B9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50336469
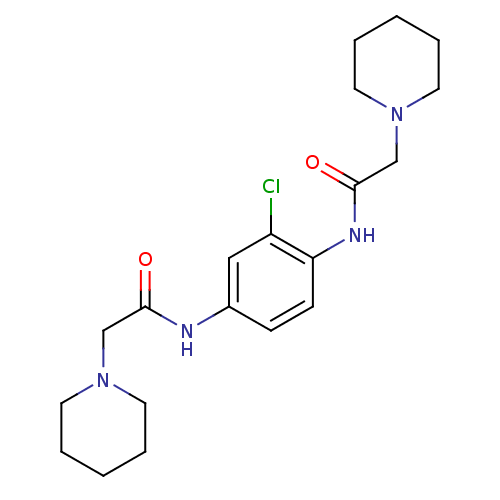
(CHEMBL1668491 | N,N'-(2-chloro-1,4-phenylene)bis(2...)Show InChI InChI=1S/C20H29ClN4O2/c21-17-13-16(22-19(26)14-24-9-3-1-4-10-24)7-8-18(17)23-20(27)15-25-11-5-2-6-12-25/h7-8,13H,1-6,9-12,14-15H2,(H,22,26)(H,23,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem Lett 21: 1105-12 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.131
BindingDB Entry DOI: 10.7270/Q2V988B9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50336468
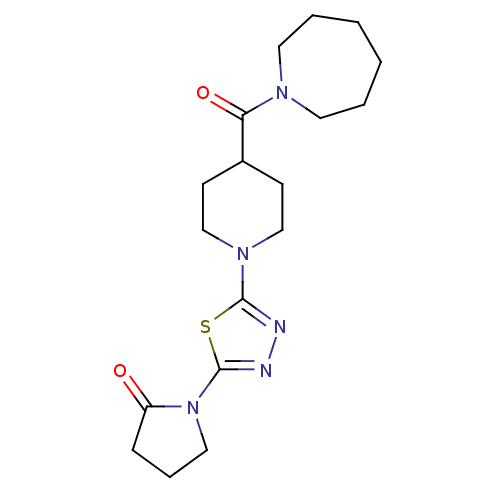
(1-(5-(4-(azepane-1-carbonyl)piperidin-1-yl)-1,3,4-...)Show InChI InChI=1S/C18H27N5O2S/c24-15-6-5-11-23(15)18-20-19-17(26-18)22-12-7-14(8-13-22)16(25)21-9-3-1-2-4-10-21/h14H,1-13H2 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem Lett 21: 1105-12 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.131
BindingDB Entry DOI: 10.7270/Q2V988B9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50336467
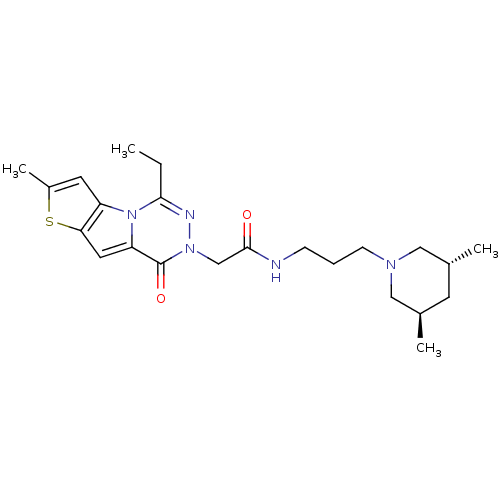
(CHEMBL1668489 | N-[3-((3R,5R)-3,5-Dimethyl-piperid...)Show SMILES CCc1nn(CC(=O)NCCCN2C[C@H](C)C[C@@H](C)C2)c(=O)c2cc3sc(C)cc3n12 |r| Show InChI InChI=1S/C23H33N5O2S/c1-5-21-25-27(23(30)19-11-20-18(28(19)21)10-17(4)31-20)14-22(29)24-7-6-8-26-12-15(2)9-16(3)13-26/h10-11,15-16H,5-9,12-14H2,1-4H3,(H,24,29)/t15-,16-/m1/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem Lett 21: 1105-12 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.131
BindingDB Entry DOI: 10.7270/Q2V988B9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50336474

(CHEMBL1668495 | ethyl 4-(3,4-dimethoxyphenyl)-2-(3...)Show SMILES CCOC(=O)c1c(NC(=O)CCN2CCC(C)CC2)scc1-c1ccc(OC)c(OC)c1 Show InChI InChI=1S/C24H32N2O5S/c1-5-31-24(28)22-18(17-6-7-19(29-3)20(14-17)30-4)15-32-23(22)25-21(27)10-13-26-11-8-16(2)9-12-26/h6-7,14-16H,5,8-13H2,1-4H3,(H,25,27) | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.28E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem Lett 21: 1105-12 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.131
BindingDB Entry DOI: 10.7270/Q2V988B9 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Electrophorus electricus (Electric eel)) | BDBM50336475
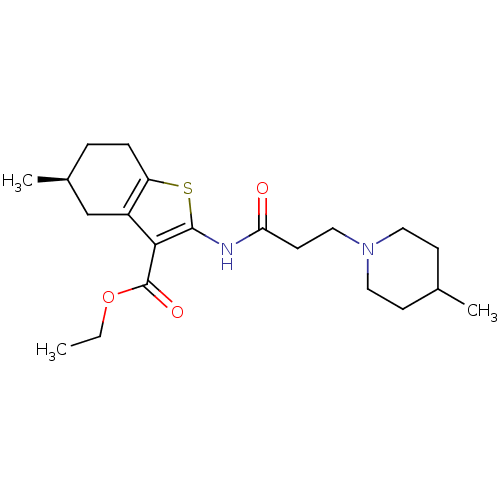
((S)-ethyl 5-methyl-2-(3-(4-methylpiperidin-1-yl)pr...)Show SMILES CCOC(=O)c1c(NC(=O)CCN2CCC(C)CC2)sc2CC[C@H](C)Cc12 |r| Show InChI InChI=1S/C21H32N2O3S/c1-4-26-21(25)19-16-13-15(3)5-6-17(16)27-20(19)22-18(24)9-12-23-10-7-14(2)8-11-23/h14-15H,4-13H2,1-3H3,(H,22,24)/t15-/m0/s1 | UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of electric eel AChE by Ellman's method |
Bioorg Med Chem Lett 21: 1105-12 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.131
BindingDB Entry DOI: 10.7270/Q2V988B9 |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338666

(5,6,7-trihydroxy-2-methyl-4H-chromen-4-one | CHEMB...)Show InChI InChI=1S/C10H8O5/c1-4-2-5(11)8-7(15-4)3-6(12)9(13)10(8)14/h2-3,12-14H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338658
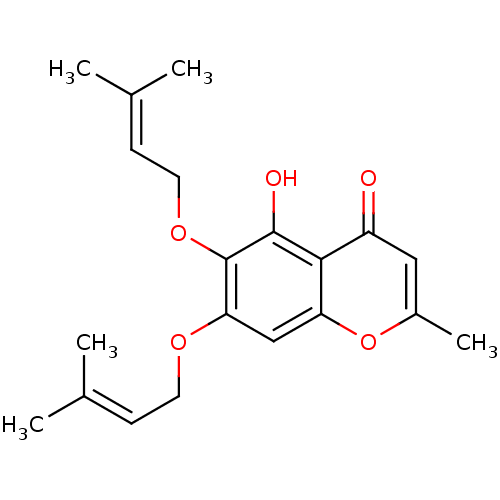
(5-hydroxy-2-methyl-6,7-bis[(3-methylbut-2-en-1-yl)...)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#8]-c1cc2oc(-[#6])cc(=O)c2c(-[#8])c1-[#8]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C20H24O5/c1-12(2)6-8-23-17-11-16-18(15(21)10-14(5)25-16)19(22)20(17)24-9-7-13(3)4/h6-7,10-11,22H,8-9H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338657
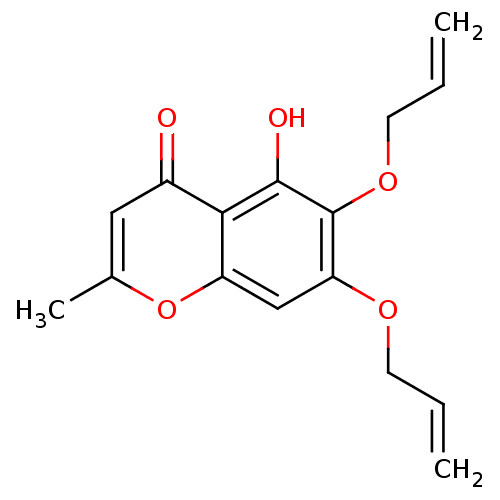
(5-hydroxy-2-methyl-6,7-bis(prop-2-en-1-yloxy)-4H-c...)Show InChI InChI=1S/C16H16O5/c1-4-6-19-13-9-12-14(11(17)8-10(3)21-12)15(18)16(13)20-7-5-2/h4-5,8-9,18H,1-2,6-7H2,3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338666

(5,6,7-trihydroxy-2-methyl-4H-chromen-4-one | CHEMB...)Show InChI InChI=1S/C10H8O5/c1-4-2-5(11)8-7(15-4)3-6(12)9(13)10(8)14/h2-3,12-14H,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338665
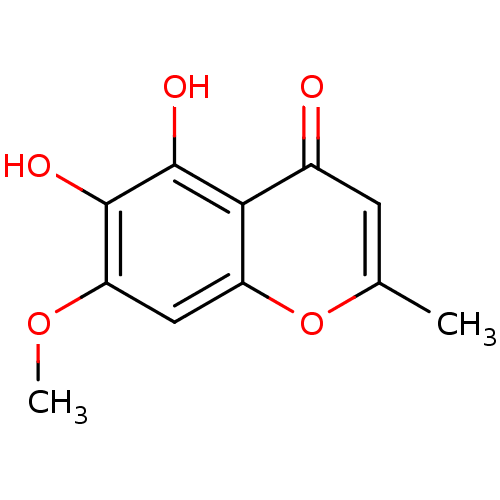
(5,6-dihydroxy-7-methoxy-2-methyl-4H-chromen-4-one ...)Show InChI InChI=1S/C11H10O5/c1-5-3-6(12)9-7(16-5)4-8(15-2)10(13)11(9)14/h3-4,13-14H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338668
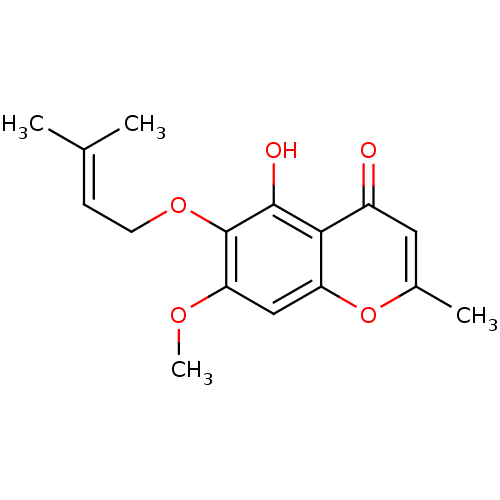
(5-hydroxy-7-methoxy-2-methyl-6-(3-methylbut-2-enyl...)Show SMILES [#6]-[#8]-c1cc2oc(-[#6])cc(=O)c2c(-[#8])c1-[#8]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C16H18O5/c1-9(2)5-6-20-16-13(19-4)8-12-14(15(16)18)11(17)7-10(3)21-12/h5,7-8,18H,6H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.55E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338658

(5-hydroxy-2-methyl-6,7-bis[(3-methylbut-2-en-1-yl)...)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#8]-c1cc2oc(-[#6])cc(=O)c2c(-[#8])c1-[#8]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C20H24O5/c1-12(2)6-8-23-17-11-16-18(15(21)10-14(5)25-16)19(22)20(17)24-9-7-13(3)4/h6-7,10-11,22H,8-9H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338664
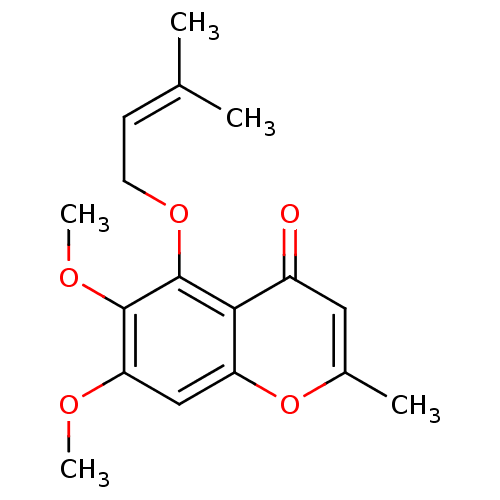
(6,7-dimethoxy-2-methyl-5-[(3-methylbut-2-en-1-yl)o...)Show SMILES [#6]-[#8]-c1cc2oc(-[#6])cc(=O)c2c(-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])c1-[#8]-[#6] Show InChI InChI=1S/C17H20O5/c1-10(2)6-7-21-17-15-12(18)8-11(3)22-13(15)9-14(19-4)16(17)20-5/h6,8-9H,7H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338662
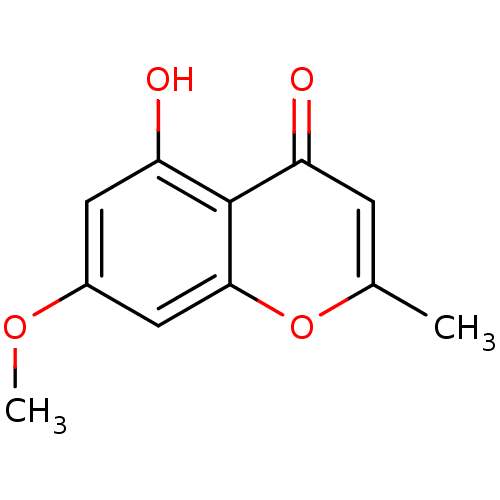
(5-hydroxy-7-methoxy-2-methyl-4H-chromen-4-one | CH...)Show InChI InChI=1S/C11H10O4/c1-6-3-8(12)11-9(13)4-7(14-2)5-10(11)15-6/h3-5,13H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.78E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338667
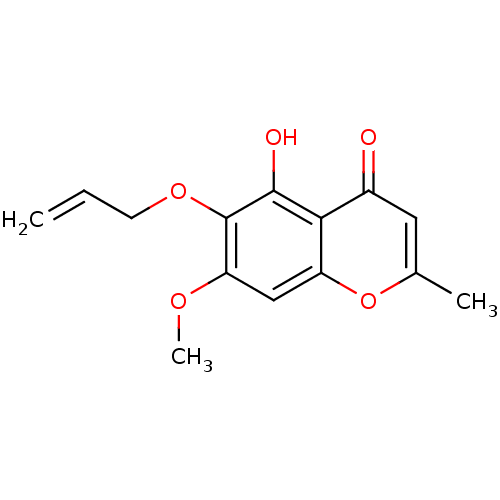
(5-hydroxy-7-methoxy-2-methyl-6-(prop-2-en-1-yloxy)...)Show InChI InChI=1S/C14H14O5/c1-4-5-18-14-11(17-3)7-10-12(13(14)16)9(15)6-8(2)19-10/h4,6-7,16H,1,5H2,2-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338668

(5-hydroxy-7-methoxy-2-methyl-6-(3-methylbut-2-enyl...)Show SMILES [#6]-[#8]-c1cc2oc(-[#6])cc(=O)c2c(-[#8])c1-[#8]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C16H18O5/c1-9(2)5-6-20-16-13(19-4)8-12-14(15(16)18)11(17)7-10(3)21-12/h5,7-8,18H,6H2,1-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.87E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338661
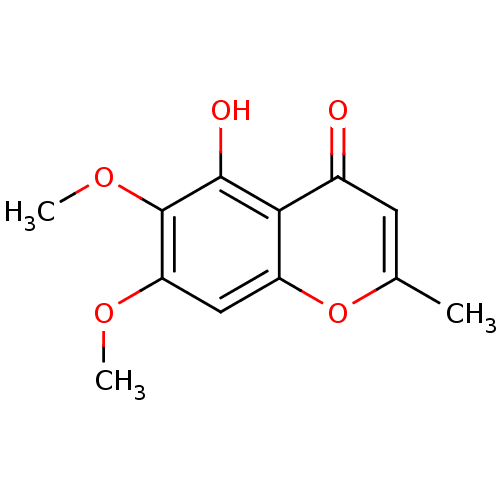
(CHEMBL1684136 | Stellatin)Show InChI InChI=1S/C12H12O5/c1-6-4-7(13)10-8(17-6)5-9(15-2)12(16-3)11(10)14/h4-5,14H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.97E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338665

(5,6-dihydroxy-7-methoxy-2-methyl-4H-chromen-4-one ...)Show InChI InChI=1S/C11H10O5/c1-5-3-6(12)9-7(16-5)4-8(15-2)10(13)11(9)14/h3-4,13-14H,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.98E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338662

(5-hydroxy-7-methoxy-2-methyl-4H-chromen-4-one | CH...)Show InChI InChI=1S/C11H10O4/c1-6-3-8(12)11-9(13)4-7(14-2)5-10(11)15-6/h3-5,13H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338663
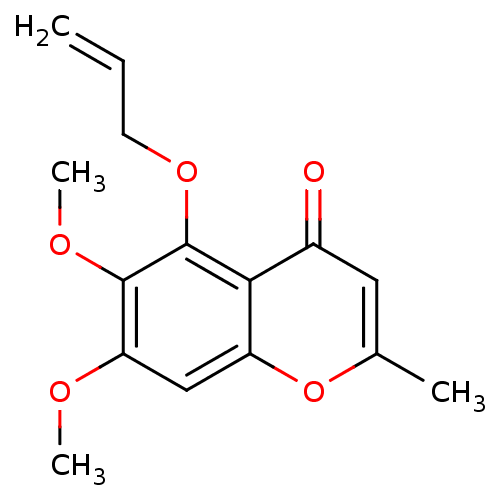
(6,7-dimethoxy-2-methyl-5-(prop-2-en-1-yloxy)-4H-ch...)Show InChI InChI=1S/C15H16O5/c1-5-6-19-15-13-10(16)7-9(2)20-11(13)8-12(17-3)14(15)18-4/h5,7-8H,1,6H2,2-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338657

(5-hydroxy-2-methyl-6,7-bis(prop-2-en-1-yloxy)-4H-c...)Show InChI InChI=1S/C16H16O5/c1-4-6-19-13-9-12-14(11(17)8-10(3)21-12)15(18)16(13)20-7-5-2/h4-5,8-9,18H,1-2,6-7H2,3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338661

(CHEMBL1684136 | Stellatin)Show InChI InChI=1S/C12H12O5/c1-6-4-7(13)10-8(17-6)5-9(15-2)12(16-3)11(10)14/h4-5,14H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338664

(6,7-dimethoxy-2-methyl-5-[(3-methylbut-2-en-1-yl)o...)Show SMILES [#6]-[#8]-c1cc2oc(-[#6])cc(=O)c2c(-[#8]-[#6]\[#6]=[#6](\[#6])-[#6])c1-[#8]-[#6] Show InChI InChI=1S/C17H20O5/c1-10(2)6-7-21-17-15-12(18)8-11(3)22-13(15)9-14(19-4)16(17)20-5/h6,8-9H,7H2,1-5H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338660
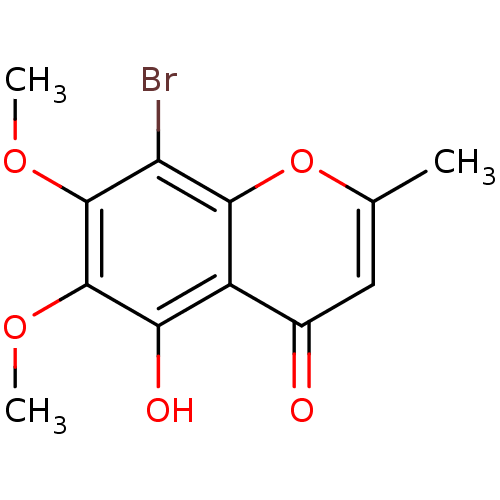
(8-bromo-5-hydroxy-6,7-dimethoxy-2-methyl-4H-chrome...)Show InChI InChI=1S/C12H11BrO5/c1-5-4-6(14)7-9(15)12(17-3)11(16-2)8(13)10(7)18-5/h4,15H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.54E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338667

(5-hydroxy-7-methoxy-2-methyl-6-(prop-2-en-1-yloxy)...)Show InChI InChI=1S/C14H14O5/c1-4-5-18-14-11(17-3)7-10-12(13(14)16)9(15)6-8(2)19-10/h4,6-7,16H,1,5H2,2-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338659
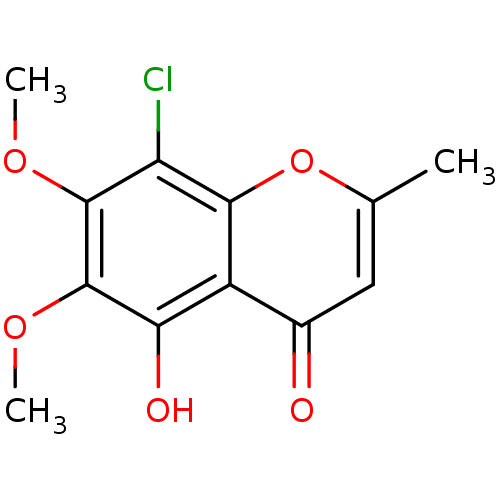
(8-chloro-5-hydroxy-6,7-dimethoxy-2-methyl-4H-chrom...)Show InChI InChI=1S/C12H11ClO5/c1-5-4-6(14)7-9(15)12(17-3)11(16-2)8(13)10(7)18-5/h4,15H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338660

(8-bromo-5-hydroxy-6,7-dimethoxy-2-methyl-4H-chrome...)Show InChI InChI=1S/C12H11BrO5/c1-5-4-6(14)7-9(15)12(17-3)11(16-2)8(13)10(7)18-5/h4,15H,1-3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.93E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50067040
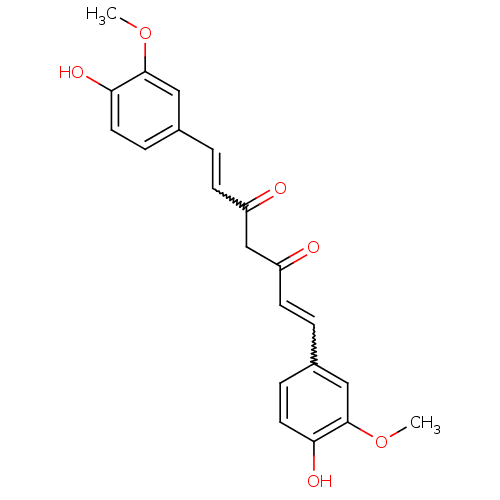
(((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hept...)Show SMILES COc1cc(C=CC(=O)CC(=O)C=Cc2ccc(O)c(OC)c2)ccc1O |w:12.11,5.4| Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 1
(Homo sapiens (Human)) | BDBM50338663

(6,7-dimethoxy-2-methyl-5-(prop-2-en-1-yloxy)-4H-ch...)Show InChI InChI=1S/C15H16O5/c1-5-6-19-15-13-10(16)7-9(2)20-11(13)8-12(17-3)14(15)18-4/h5,7-8H,1,6H2,2-4H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.83E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX1 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50338659

(8-chloro-5-hydroxy-6,7-dimethoxy-2-methyl-4H-chrom...)Show InChI InChI=1S/C12H11ClO5/c1-5-4-6(14)7-9(15)12(17-3)11(16-2)8(13)10(7)18-5/h4,15H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |
Prostaglandin G/H synthase 2
(Homo sapiens (Human)) | BDBM50067040

(((E,E)-1,7-bis(4-hydroxy-3-methoxyphenyl)-1,6-hept...)Show SMILES COc1cc(C=CC(=O)CC(=O)C=Cc2ccc(O)c(OC)c2)ccc1O |w:12.11,5.4| Show InChI InChI=1S/C21H20O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h3-12,24-25H,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
| Assay Description
Inhibition of COX2 |
Bioorg Med Chem Lett 21: 1612-6 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.116
BindingDB Entry DOI: 10.7270/Q2CN746K |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data