 Found 1494 hits with Last Name = 'moore' and Initial = 's'
Found 1494 hits with Last Name = 'moore' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413698
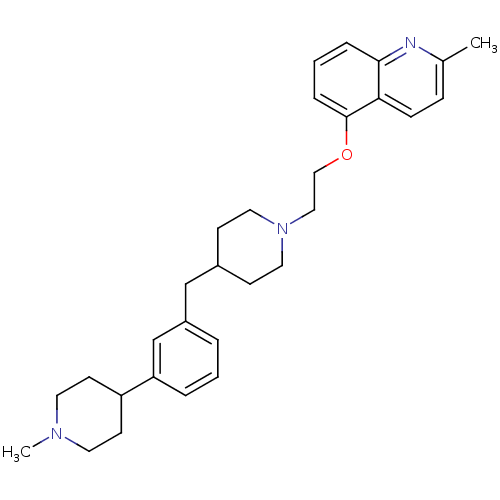
(CHEMBL459282)Show SMILES CN1CCC(CC1)c1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C30H39N3O/c1-23-9-10-28-29(31-23)7-4-8-30(28)34-20-19-33-17-11-24(12-18-33)21-25-5-3-6-27(22-25)26-13-15-32(2)16-14-26/h3-10,22,24,26H,11-21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412126
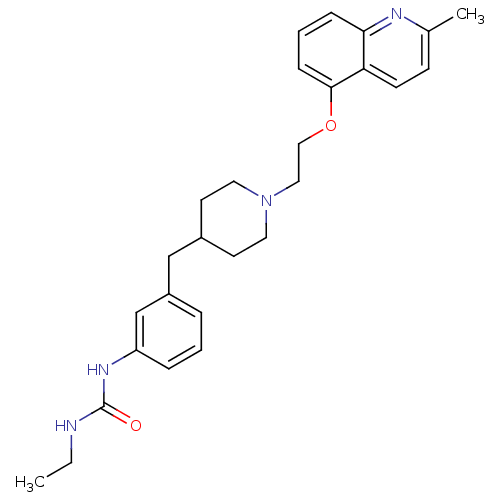
(CHEMBL525362)Show SMILES CCNC(=O)Nc1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C27H34N4O2/c1-3-28-27(32)30-23-7-4-6-22(19-23)18-21-12-14-31(15-13-21)16-17-33-26-9-5-8-25-24(26)11-10-20(2)29-25/h4-11,19,21H,3,12-18H2,1-2H3,(H2,28,30,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.251 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50075926
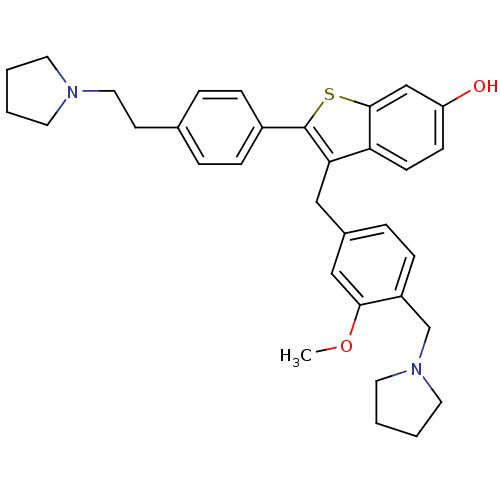
(3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-...)Show SMILES COc1cc(Cc2c(sc3cc(O)ccc23)-c2ccc(CCN3CCCC3)cc2)ccc1CN1CCCC1 Show InChI InChI=1S/C33H38N2O2S/c1-37-31-21-25(8-11-27(31)23-35-17-4-5-18-35)20-30-29-13-12-28(36)22-32(29)38-33(30)26-9-6-24(7-10-26)14-19-34-15-2-3-16-34/h6-13,21-22,36H,2-5,14-20,23H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition |
Bioorg Med Chem Lett 9: 775-80 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V46 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50412114
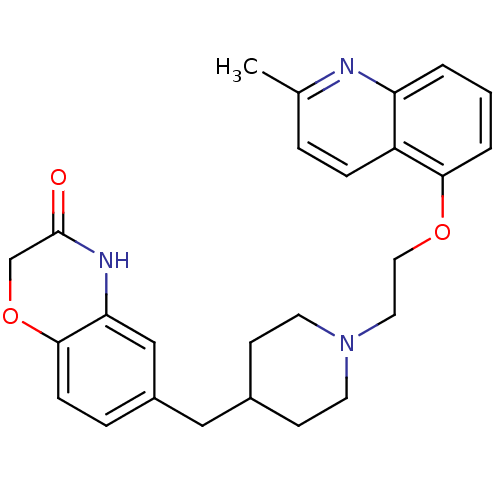
(CHEMBL183460 | SB-649915)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4ccc5OCC(=O)Nc5c4)CC3)cccc2n1 Show InChI InChI=1S/C26H29N3O3/c1-18-5-7-21-22(27-18)3-2-4-24(21)31-14-13-29-11-9-19(10-12-29)15-20-6-8-25-23(16-20)28-26(30)17-32-25/h2-8,16,19H,9-15,17H2,1H3,(H,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412121
(CHEMBL506942)Show SMILES CN(C(C)=O)c1cccc(CN2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C26H32N4O2/c1-20-10-11-24-25(27-20)8-5-9-26(24)32-17-16-29-12-14-30(15-13-29)19-22-6-4-7-23(18-22)28(3)21(2)31/h4-11,18H,12-17,19H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412124
(CHEMBL495213)Show SMILES CC(C)S(=O)(=O)Nc1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C27H35N3O3S/c1-20(2)34(31,32)29-24-7-4-6-23(19-24)18-22-12-14-30(15-13-22)16-17-33-27-9-5-8-26-25(27)11-10-21(3)28-26/h4-11,19-20,22,29H,12-18H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50388878
(CHEMBL1999931 | US9163007, 73)Show SMILES O=C(Nc1n[nH]c2ccc(cc12)-c1cn(Cc2ccccc2)nn1)c1ccccc1 Show InChI InChI=1S/C23H18N6O/c30-23(17-9-5-2-6-10-17)24-22-19-13-18(11-12-20(19)25-27-22)21-15-29(28-26-21)14-16-7-3-1-4-8-16/h1-13,15H,14H2,(H2,24,25,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc.
US Patent
| Assay Description
6 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 us... |
US Patent US9163007 (2015)
BindingDB Entry DOI: 10.7270/Q2KW5DTG |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM185854
(US9163007, 185)Show SMILES CN(C)c1ccc(cc1)-c1c(nnn1Cc1ccccc1)-c1ccc2[nH]nc(N)c2c1 Show InChI InChI=1S/C24H23N7/c1-30(2)19-11-8-17(9-12-19)23-22(18-10-13-21-20(14-18)24(25)28-26-21)27-29-31(23)15-16-6-4-3-5-7-16/h3-14H,15H2,1-2H3,(H3,25,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc.
US Patent
| Assay Description
11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... |
US Patent US9163007 (2015)
BindingDB Entry DOI: 10.7270/Q2KW5DTG |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50075934
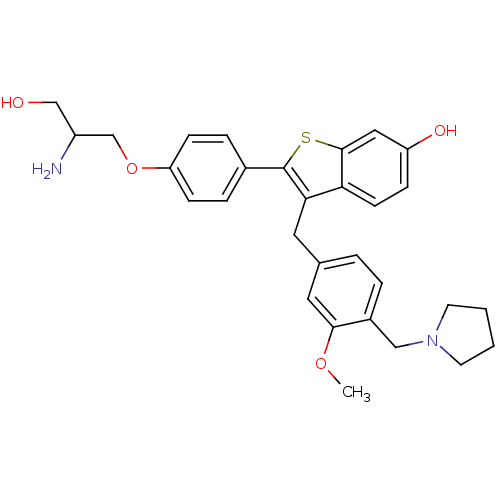
(2-[4-(2-Amino-3-hydroxy-propoxy)-phenyl]-3-(3-meth...)Show SMILES COc1cc(Cc2c(sc3cc(O)ccc23)-c2ccc(OCC(N)CO)cc2)ccc1CN1CCCC1 Show InChI InChI=1S/C30H34N2O4S/c1-35-28-15-20(4-5-22(28)17-32-12-2-3-13-32)14-27-26-11-8-24(34)16-29(26)37-30(27)21-6-9-25(10-7-21)36-19-23(31)18-33/h4-11,15-16,23,33-34H,2-3,12-14,17-19,31H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition |
Bioorg Med Chem Lett 9: 775-80 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V46 |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Sus scrofa) | BDBM50201438
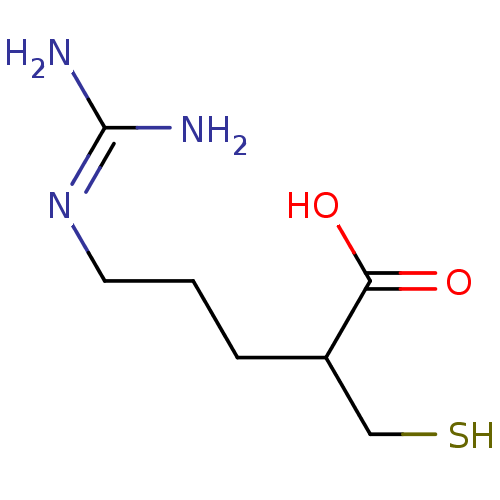
((+/-)-5-guanidino-2-(mercaptomethyl)pentanoic acid...)Show SMILES [#7]\[#6](-[#7])=[#7]\[#6]-[#6]-[#6]-[#6](-[#6]-[#16])-[#6](-[#8])=O Show InChI InChI=1S/C7H15N3O2S/c8-7(9)10-3-1-2-5(4-13)6(11)12/h5,13H,1-4H2,(H,11,12)(H4,8,9,10) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of porcine pancreatic carboxypeptidase B |
J Med Chem 50: 6095-103 (2007)
Article DOI: 10.1021/jm0702433
BindingDB Entry DOI: 10.7270/Q2T153CG |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM185901
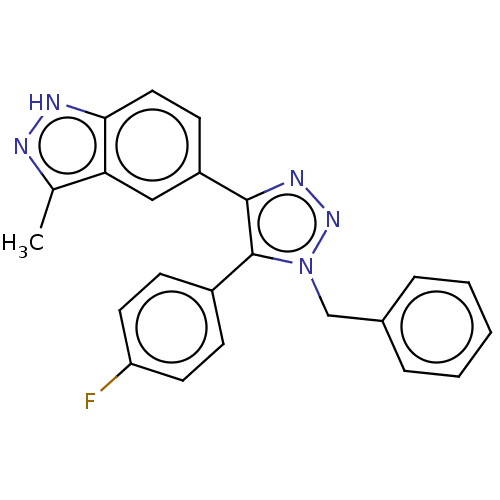
(US9163007, 408)Show SMILES Cc1n[nH]c2ccc(cc12)-c1nnn(Cc2ccccc2)c1-c1ccc(F)cc1 Show InChI InChI=1S/C23H18FN5/c1-15-20-13-18(9-12-21(20)26-25-15)22-23(17-7-10-19(24)11-8-17)29(28-27-22)14-16-5-3-2-4-6-16/h2-13H,14H2,1H3,(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.428 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc.
US Patent
| Assay Description
11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... |
US Patent US9163007 (2015)
BindingDB Entry DOI: 10.7270/Q2KW5DTG |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM185888
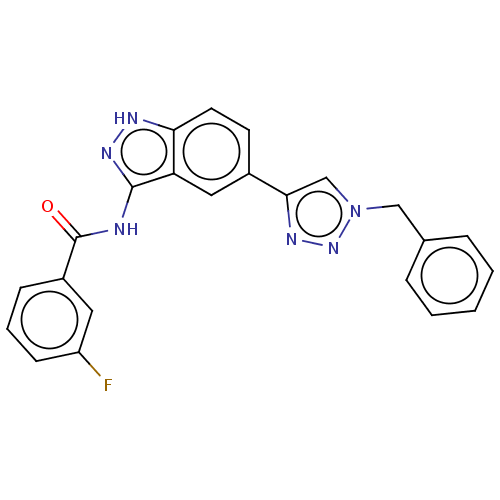
(US9163007, 395)Show SMILES Fc1cccc(c1)C(=O)Nc1n[nH]c2ccc(cc12)-c1cn(Cc2ccccc2)nn1 Show InChI InChI=1S/C23H17FN6O/c24-18-8-4-7-17(11-18)23(31)25-22-19-12-16(9-10-20(19)26-28-22)21-14-30(29-27-21)13-15-5-2-1-3-6-15/h1-12,14H,13H2,(H2,25,26,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc.
US Patent
| Assay Description
11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... |
US Patent US9163007 (2015)
BindingDB Entry DOI: 10.7270/Q2KW5DTG |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50075937
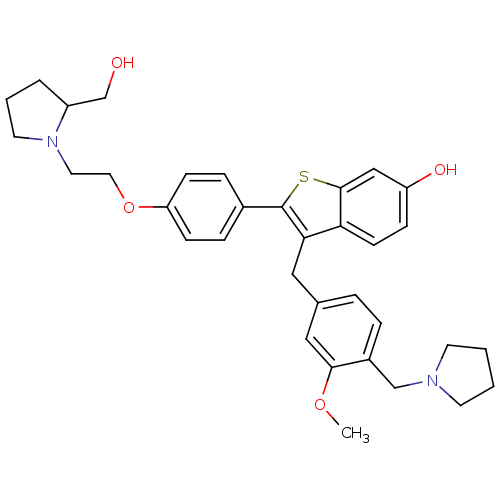
(2-{4-[2-(2-Hydroxymethyl-pyrrolidin-1-yl)-ethoxy]-...)Show SMILES COc1cc(Cc2c(sc3cc(O)ccc23)-c2ccc(OCCN3CCCC3CO)cc2)ccc1CN1CCCC1 Show InChI InChI=1S/C34H40N2O4S/c1-39-32-20-24(6-7-26(32)22-35-14-2-3-15-35)19-31-30-13-10-28(38)21-33(30)41-34(31)25-8-11-29(12-9-25)40-18-17-36-16-4-5-27(36)23-37/h6-13,20-21,27,37-38H,2-5,14-19,22-23H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition |
Bioorg Med Chem Lett 9: 775-80 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V46 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50075928
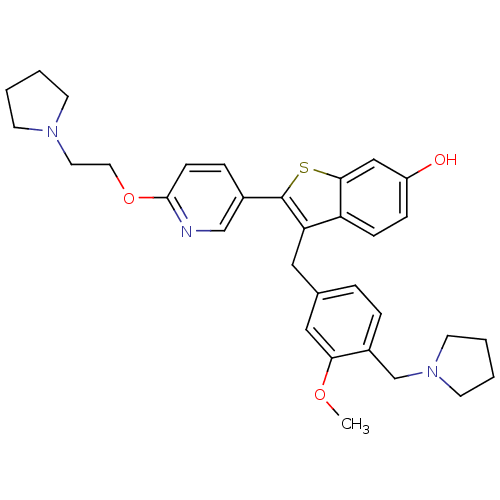
(3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[6-...)Show SMILES COc1cc(Cc2c(sc3cc(O)ccc23)-c2ccc(OCCN3CCCC3)nc2)ccc1CN1CCCC1 Show InChI InChI=1S/C32H37N3O3S/c1-37-29-19-23(6-7-25(29)22-35-14-4-5-15-35)18-28-27-10-9-26(36)20-30(27)39-32(28)24-8-11-31(33-21-24)38-17-16-34-12-2-3-13-34/h6-11,19-21,36H,2-5,12-18,22H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition |
Bioorg Med Chem Lett 9: 775-80 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V46 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM185899
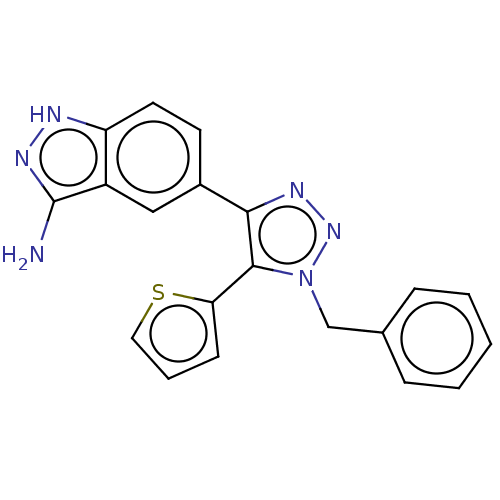
(US9163007, 406)Show SMILES Nc1n[nH]c2ccc(cc12)-c1nnn(Cc2ccccc2)c1-c1cccs1 Show InChI InChI=1S/C20H16N6S/c21-20-15-11-14(8-9-16(15)22-24-20)18-19(17-7-4-10-27-17)26(25-23-18)12-13-5-2-1-3-6-13/h1-11H,12H2,(H3,21,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc.
US Patent
| Assay Description
11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... |
US Patent US9163007 (2015)
BindingDB Entry DOI: 10.7270/Q2KW5DTG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413691
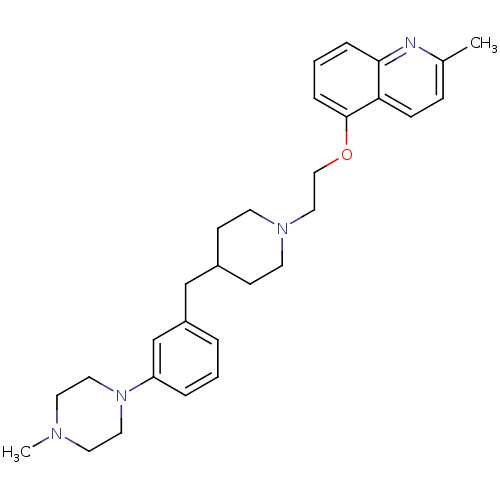
(CHEMBL458199)Show SMILES CN1CCN(CC1)c1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C29H38N4O/c1-23-9-10-27-28(30-23)7-4-8-29(27)34-20-19-32-13-11-24(12-14-32)21-25-5-3-6-26(22-25)33-17-15-31(2)16-18-33/h3-10,22,24H,11-21H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412132
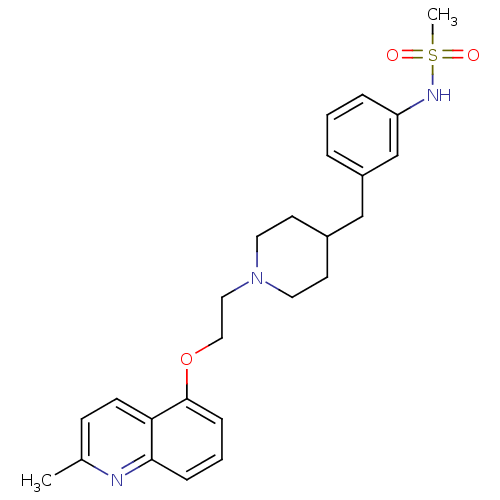
(CHEMBL497963)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4cccc(NS(C)(=O)=O)c4)CC3)cccc2n1 Show InChI InChI=1S/C25H31N3O3S/c1-19-9-10-23-24(26-19)7-4-8-25(23)31-16-15-28-13-11-20(12-14-28)17-21-5-3-6-22(18-21)27-32(2,29)30/h3-10,18,20,27H,11-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412118
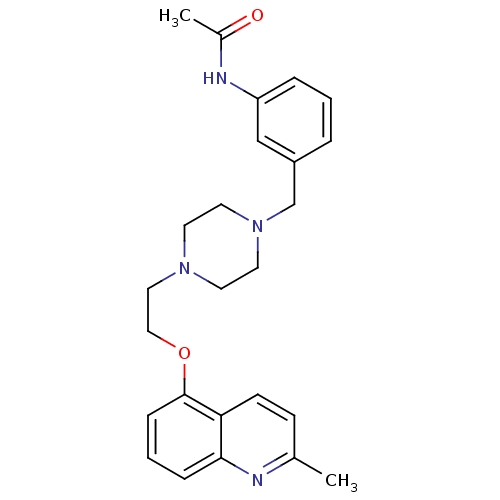
(CHEMBL494806)Show SMILES CC(=O)Nc1cccc(CN2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C25H30N4O2/c1-19-9-10-23-24(26-19)7-4-8-25(23)31-16-15-28-11-13-29(14-12-28)18-21-5-3-6-22(17-21)27-20(2)30/h3-10,17H,11-16,18H2,1-2H3,(H,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412134

(CHEMBL525712)Show SMILES CC(=O)Nc1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C26H31N3O2/c1-19-9-10-24-25(27-19)7-4-8-26(24)31-16-15-29-13-11-21(12-14-29)17-22-5-3-6-23(18-22)28-20(2)30/h3-10,18,21H,11-17H2,1-2H3,(H,28,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412132

(CHEMBL497963)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4cccc(NS(C)(=O)=O)c4)CC3)cccc2n1 Show InChI InChI=1S/C25H31N3O3S/c1-19-9-10-23-24(26-19)7-4-8-25(23)31-16-15-28-13-11-20(12-14-28)17-21-5-3-6-22(18-21)27-32(2,29)30/h3-10,18,20,27H,11-17H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 2
(Homo sapiens (Human)) | BDBM50272772

(10-(4-Amino-butyl)-19-(2-amino-3-phenyl-propionyla...)Show SMILES C[C@@H](O)[C@@H](CO)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@H](N)Cc2ccccc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H]([C@@H](C)O)C(=O)N1 |r| Show InChI InChI=1S/C49H66N10O10S2/c1-28(61)39(25-60)56-48(68)41-27-71-70-26-40(57-43(63)34(51)21-30-13-5-3-6-14-30)47(67)54-37(22-31-15-7-4-8-16-31)45(65)55-38(23-32-24-52-35-18-10-9-17-33(32)35)46(66)53-36(19-11-12-20-50)44(64)59-42(29(2)62)49(69)58-41/h3-10,13-18,24,28-29,34,36-42,52,60-62H,11-12,19-23,25-27,50-51H2,1-2H3,(H,53,66)(H,54,67)(H,55,65)(H,56,68)(H,57,63)(H,58,69)(H,59,64)/t28-,29-,34-,36+,37+,38-,39-,40+,41+,42+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California-San Diego
Curated by ChEMBL
| Assay Description
Binding affinity towards somatostatin receptor type 2 |
J Med Chem 48: 6643-52 (2005)
Article DOI: 10.1021/jm050376t
BindingDB Entry DOI: 10.7270/Q2D50NRS |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM50388878

(CHEMBL1999931 | US9163007, 73)Show SMILES O=C(Nc1n[nH]c2ccc(cc12)-c1cn(Cc2ccccc2)nn1)c1ccccc1 Show InChI InChI=1S/C23H18N6O/c30-23(17-9-5-2-6-10-17)24-22-19-13-18(11-12-20(19)25-27-22)21-15-29(28-26-21)14-16-7-3-1-4-8-16/h1-13,15H,14H2,(H2,24,25,27,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc.
US Patent
| Assay Description
11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... |
US Patent US9163007 (2015)
BindingDB Entry DOI: 10.7270/Q2KW5DTG |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM185839
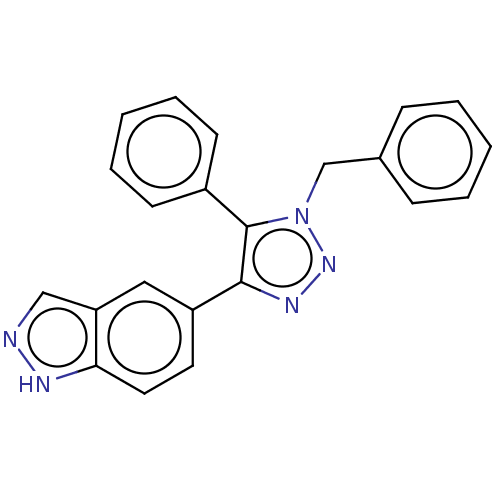
(US9163007, 87)Show SMILES C(c1ccccc1)n1nnc(c1-c1ccccc1)-c1ccc2[nH]ncc2c1 Show InChI InChI=1S/C22H17N5/c1-3-7-16(8-4-1)15-27-22(17-9-5-2-6-10-17)21(25-26-27)18-11-12-20-19(13-18)14-23-24-20/h1-14H,15H2,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.601 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc.
US Patent
| Assay Description
11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... |
US Patent US9163007 (2015)
BindingDB Entry DOI: 10.7270/Q2KW5DTG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412130

(CHEMBL498354)Show SMILES CCC(=O)Nc1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C27H33N3O2/c1-3-27(31)29-23-7-4-6-22(19-23)18-21-12-14-30(15-13-21)16-17-32-26-9-5-8-25-24(26)11-10-20(2)28-25/h4-11,19,21H,3,12-18H2,1-2H3,(H,29,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413696
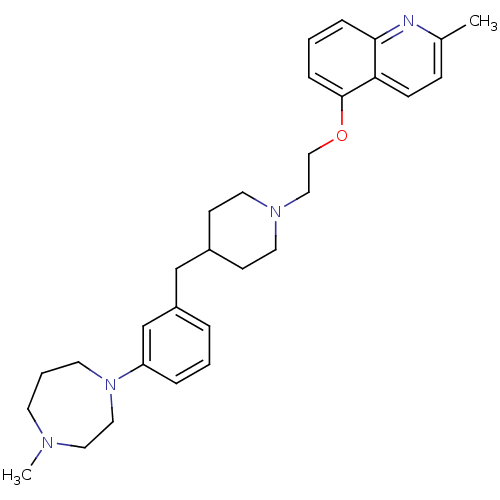
(CHEMBL514429)Show SMILES CN1CCCN(CC1)c1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C30H40N4O/c1-24-10-11-28-29(31-24)8-4-9-30(28)35-21-20-33-16-12-25(13-17-33)22-26-6-3-7-27(23-26)34-15-5-14-32(2)18-19-34/h3-4,6-11,23,25H,5,12-22H2,1-2H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413688
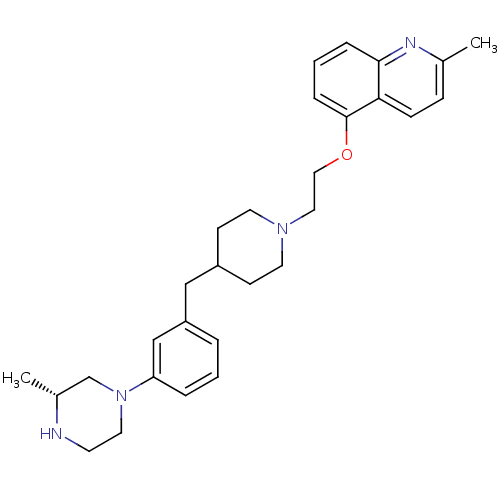
(CHEMBL517170)Show SMILES C[C@@H]1CN(CCN1)c1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 |r| Show InChI InChI=1S/C29H38N4O/c1-22-9-10-27-28(31-22)7-4-8-29(27)34-18-17-32-14-11-24(12-15-32)19-25-5-3-6-26(20-25)33-16-13-30-23(2)21-33/h3-10,20,23-24,30H,11-19,21H2,1-2H3/t23-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413687
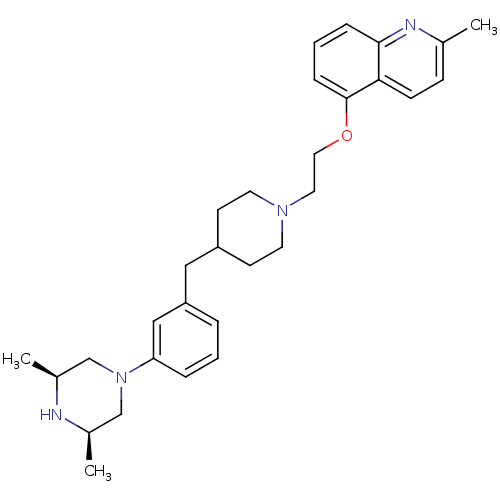
(CHEMBL461670)Show SMILES C[C@H]1CN(C[C@@H](C)N1)c1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 |r| Show InChI InChI=1S/C30H40N4O/c1-22-10-11-28-29(32-22)8-5-9-30(28)35-17-16-33-14-12-25(13-15-33)18-26-6-4-7-27(19-26)34-20-23(2)31-24(3)21-34/h4-11,19,23-25,31H,12-18,20-21H2,1-3H3/t23-,24+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM86708

(CAS_146714-97-8 | CHEMBL31354 | CHEMBL514874 | CHE...)Show SMILES COc1ccccc1N1CCN(CCN(C(=O)C2CCCCC2)c2ccccn2)CC1 Show InChI InChI=1S/C25H34N4O2/c1-31-23-12-6-5-11-22(23)28-18-15-27(16-19-28)17-20-29(24-13-7-8-14-26-24)25(30)21-9-3-2-4-10-21/h5-8,11-14,21H,2-4,9-10,15-20H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]WAY100635 from human recombinant 5HT1A receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM21393

(7-(dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-o...)Show InChI InChI=1S/C16H25NO/c1-3-10-17(11-4-2)14-9-8-13-6-5-7-16(18)15(13)12-14/h5-7,14,18H,3-4,8-12H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]8-OH-DPAT from human recombinant 5HT1A receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412120
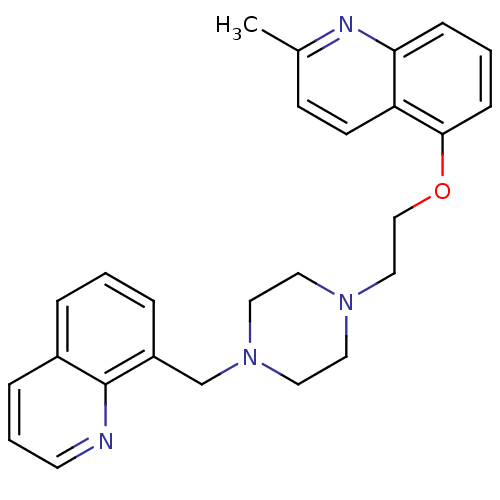
(CHEMBL425190 | SB-714786)Show SMILES Cc1ccc2c(OCCN3CCN(Cc4cccc5cccnc45)CC3)cccc2n1 Show InChI InChI=1S/C26H28N4O/c1-20-10-11-23-24(28-20)8-3-9-25(23)31-18-17-29-13-15-30(16-14-29)19-22-6-2-5-21-7-4-12-27-26(21)22/h2-12H,13-19H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM185846
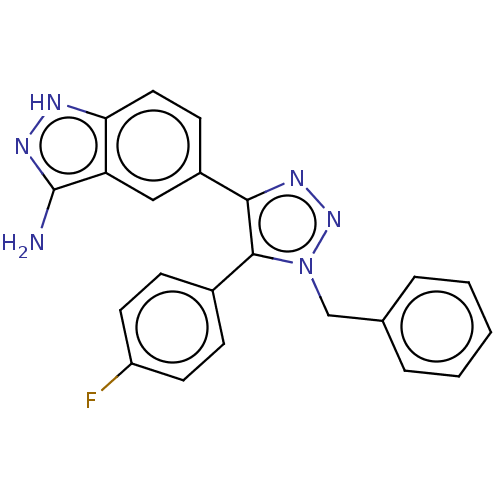
(US9163007, 151)Show SMILES Nc1n[nH]c2ccc(cc12)-c1nnn(Cc2ccccc2)c1-c1ccc(F)cc1 Show InChI InChI=1S/C22H17FN6/c23-17-9-6-15(7-10-17)21-20(16-8-11-19-18(12-16)22(24)27-25-19)26-28-29(21)13-14-4-2-1-3-5-14/h1-12H,13H2,(H3,24,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc.
US Patent
| Assay Description
11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... |
US Patent US9163007 (2015)
BindingDB Entry DOI: 10.7270/Q2KW5DTG |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM185858
(US9163007, 198)Show SMILES O=C(Nc1n[nH]c2ccc(cc12)-c1nnn(Cc2ccccc2)c1-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H22N6O/c36-29(22-14-8-3-9-15-22)30-28-24-18-23(16-17-25(24)31-33-28)26-27(21-12-6-2-7-13-21)35(34-32-26)19-20-10-4-1-5-11-20/h1-18H,19H2,(H2,30,31,33,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc.
US Patent
| Assay Description
6 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 us... |
US Patent US9163007 (2015)
BindingDB Entry DOI: 10.7270/Q2KW5DTG |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM185858

(US9163007, 198)Show SMILES O=C(Nc1n[nH]c2ccc(cc12)-c1nnn(Cc2ccccc2)c1-c1ccccc1)c1ccccc1 Show InChI InChI=1S/C29H22N6O/c36-29(22-14-8-3-9-15-22)30-28-24-18-23(16-17-25(24)31-33-28)26-27(21-12-6-2-7-13-21)35(34-32-26)19-20-10-4-1-5-11-20/h1-18H,19H2,(H2,30,31,33,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc.
US Patent
| Assay Description
11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... |
US Patent US9163007 (2015)
BindingDB Entry DOI: 10.7270/Q2KW5DTG |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50075932

(2-(1,1-Dioxo-1lambda*6*-thiomorpholin-4-yl)-N-{4-[...)Show SMILES COc1cc(Cc2c(sc3cc(O)ccc23)-c2ccc(NC(=O)CN3CCS(=O)(=O)CC3)cc2)ccc1CN1CCCC1 Show InChI InChI=1S/C33H37N3O5S2/c1-41-30-19-23(4-5-25(30)21-35-12-2-3-13-35)18-29-28-11-10-27(37)20-31(28)42-33(29)24-6-8-26(9-7-24)34-32(38)22-36-14-16-43(39,40)17-15-36/h4-11,19-20,37H,2-3,12-18,21-22H2,1H3,(H,34,38) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition |
Bioorg Med Chem Lett 9: 775-80 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V46 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50075938
(3-(3-Methoxy-4-pyrrolidin-1-ylmethyl-benzyl)-2-[4-...)Show SMILES COc1cc(Cc2c(sc3cc(O)ccc23)-c2ccc(OCCN3CCCC3)cc2)ccc1CN1CCCC1 Show InChI InChI=1S/C33H38N2O3S/c1-37-31-21-24(6-7-26(31)23-35-16-4-5-17-35)20-30-29-13-10-27(36)22-32(29)39-33(30)25-8-11-28(12-9-25)38-19-18-34-14-2-3-15-34/h6-13,21-22,36H,2-5,14-20,23H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Research Laboratories
Curated by ChEMBL
| Assay Description
Binding inhibition against thrombin was measured by nonlinear regression analysis using Morrison's equation for tight-binding inhibition |
Bioorg Med Chem Lett 9: 775-80 (1999)
BindingDB Entry DOI: 10.7270/Q2PR7V46 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM185863

(US9163007, 205)Show SMILES Fc1cccc(c1)C(=O)Nc1n[nH]c2ccc(cc12)-c1nnn(Cc2ccccc2)c1C1CC1 Show InChI InChI=1S/C26H21FN6O/c27-20-8-4-7-19(13-20)26(34)28-25-21-14-18(11-12-22(21)29-31-25)23-24(17-9-10-17)33(32-30-23)15-16-5-2-1-3-6-16/h1-8,11-14,17H,9-10,15H2,(H2,28,29,31,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.900 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc.
US Patent
| Assay Description
6 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 us... |
US Patent US9163007 (2015)
BindingDB Entry DOI: 10.7270/Q2KW5DTG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413694
(CHEMBL459061)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4cccc(c4)N4CCCCC4)CC3)cccc2n1 Show InChI InChI=1S/C29H37N3O/c1-23-11-12-27-28(30-23)9-6-10-29(27)33-20-19-31-17-13-24(14-18-31)21-25-7-5-8-26(22-25)32-15-3-2-4-16-32/h5-12,22,24H,2-4,13-21H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM185888

(US9163007, 395)Show SMILES Fc1cccc(c1)C(=O)Nc1n[nH]c2ccc(cc12)-c1cn(Cc2ccccc2)nn1 Show InChI InChI=1S/C23H17FN6O/c24-18-8-4-7-17(11-18)23(31)25-22-19-12-16(9-10-20(19)26-28-22)21-14-30(29-27-21)13-15-5-2-1-3-6-15/h1-12,14H,13H2,(H2,25,26,28,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc.
US Patent
| Assay Description
6 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 us... |
US Patent US9163007 (2015)
BindingDB Entry DOI: 10.7270/Q2KW5DTG |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413690
(CHEMBL457982)Show SMILES C[C@@H]1CN([C@@H](C)CN1)c1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 |r| Show InChI InChI=1S/C30H40N4O/c1-22-10-11-28-29(32-22)8-5-9-30(28)35-17-16-33-14-12-25(13-15-33)18-26-6-4-7-27(19-26)34-21-23(2)31-20-24(34)3/h4-11,19,23-25,31H,12-18,20-21H2,1-3H3/t23-,24+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
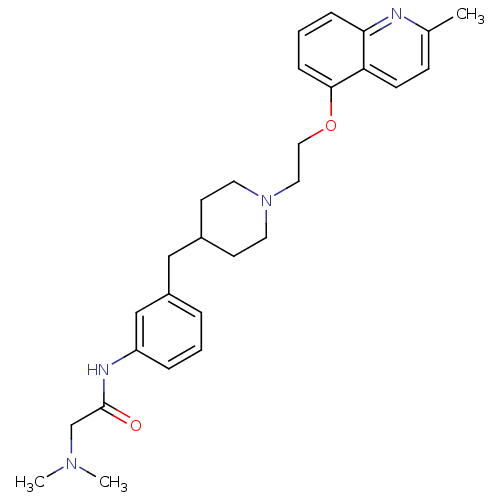
(Homo sapiens (Human)) | BDBM50412127
(CHEMBL496098)Show SMILES CN(C)CC(=O)Nc1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C28H36N4O2/c1-21-10-11-25-26(29-21)8-5-9-27(25)34-17-16-32-14-12-22(13-15-32)18-23-6-4-7-24(19-23)30-28(33)20-31(2)3/h4-11,19,22H,12-18,20H2,1-3H3,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
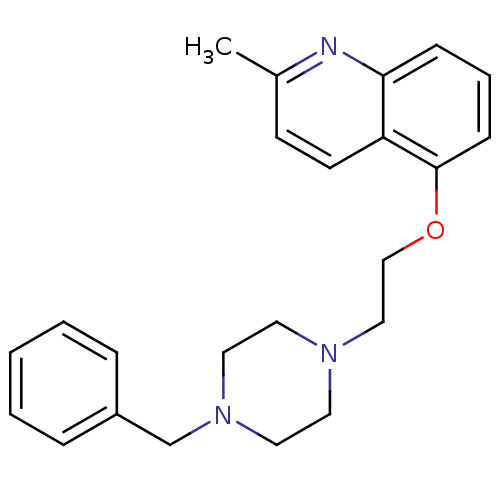
(Homo sapiens (Human)) | BDBM50412119
(CHEMBL494631)Show InChI InChI=1S/C23H27N3O/c1-19-10-11-21-22(24-19)8-5-9-23(21)27-17-16-25-12-14-26(15-13-25)18-20-6-3-2-4-7-20/h2-11H,12-18H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
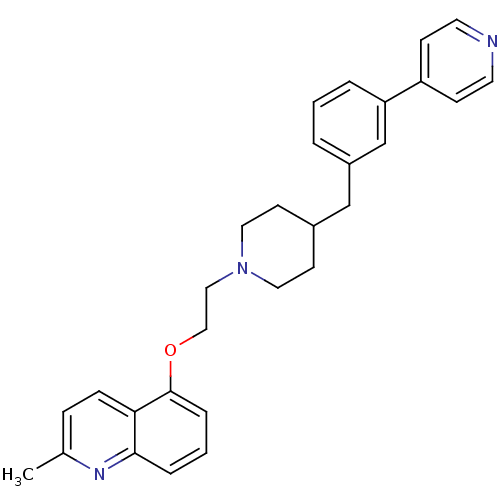
(Homo sapiens (Human)) | BDBM50413702
(CHEMBL462916)Show SMILES Cc1ccc2c(OCCN3CCC(Cc4cccc(c4)-c4ccncc4)CC3)cccc2n1 Show InChI InChI=1S/C29H31N3O/c1-22-8-9-27-28(31-22)6-3-7-29(27)33-19-18-32-16-12-23(13-17-32)20-24-4-2-5-26(21-24)25-10-14-30-15-11-25/h2-11,14-15,21,23H,12-13,16-20H2,1H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50412127

(CHEMBL496098)Show SMILES CN(C)CC(=O)Nc1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C28H36N4O2/c1-21-10-11-25-26(29-21)8-5-9-27(25)34-17-16-32-14-12-22(13-15-32)18-23-6-4-7-24(19-23)30-28(33)20-31(2)3/h4-11,19,22H,12-18,20H2,1-3H3,(H,30,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [3H]5HT from human recombinant 5HT1D receptor |
J Med Chem 51: 2887-90 (2008)
Article DOI: 10.1021/jm8001444
BindingDB Entry DOI: 10.7270/Q2DV1M4K |
More data for this
Ligand-Target Pair | |
Coagulation factor X
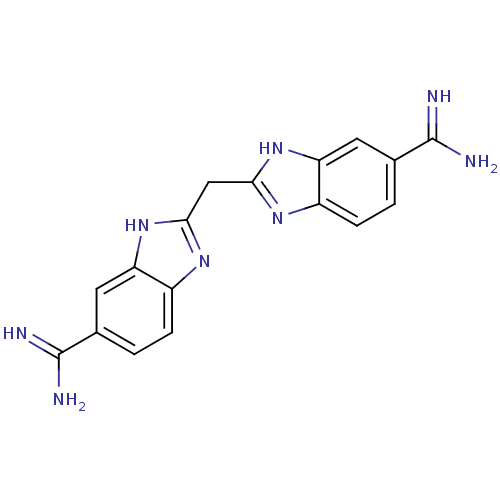
(Homo sapiens (Human)) | BDBM16127
(2,2 -methanediylbis(1H-benzimidazole-6-carboximida...)Show SMILES NC(=N)c1ccc2nc(Cc3nc4ccc(cc4[nH]3)C(N)=N)[nH]c2c1 Show InChI InChI=1S/C17H16N8/c18-16(19)8-1-3-10-12(5-8)24-14(22-10)7-15-23-11-4-2-9(17(20)21)6-13(11)25-15/h1-6H,7H2,(H3,18,19)(H3,20,21)(H,22,24)(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1 | -50.9 | n/a | n/a | n/a | n/a | n/a | 8.2 | 22 |
Arris
| Assay Description
Enzymes were incubated with inhibitors at eight inhibitor concentrations bracketing the Ki, prepared by serial dilution along with control lacking th... |
Nature 391: 608-12 (1998)
Article DOI: 10.1038/35422
BindingDB Entry DOI: 10.7270/Q29P2ZW9 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1A
(Homo sapiens (Human)) | BDBM50413691

(CHEMBL458199)Show SMILES CN1CCN(CC1)c1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 Show InChI InChI=1S/C29H38N4O/c1-23-9-10-27-28(30-23)7-4-8-29(27)34-20-19-32-13-11-24(12-14-32)21-25-5-3-6-26(22-25)33-17-15-31(2)16-18-33/h3-10,22,24H,11-21H2,1-2H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of [125I]WAY100635 from human 5HT1A receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 1D
(Homo sapiens (Human)) | BDBM50413689
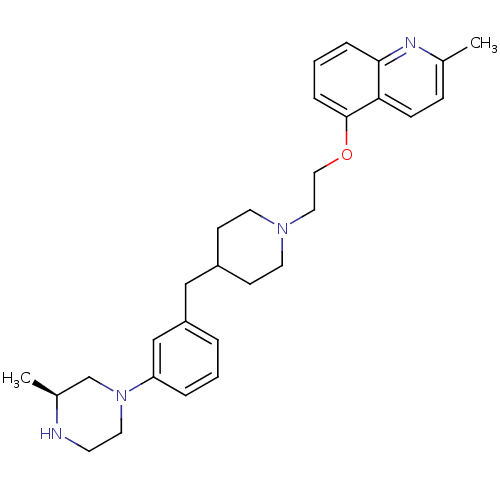
(CHEMBL518545)Show SMILES C[C@H]1CN(CCN1)c1cccc(CC2CCN(CCOc3cccc4nc(C)ccc34)CC2)c1 |r| Show InChI InChI=1S/C29H38N4O/c1-22-9-10-27-28(31-22)7-4-8-29(27)34-18-17-32-14-11-24(12-15-32)19-25-5-3-6-26(20-25)33-16-13-30-23(2)21-33/h3-10,20,23-24,30H,11-19,21H2,1-2H3/t23-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Displacement of 3H]5HT from human 5HT1D receptor expressed in CHO cells |
Bioorg Med Chem Lett 19: 428-32 (2008)
Article DOI: 10.1016/j.bmcl.2008.11.052
BindingDB Entry DOI: 10.7270/Q2RV0PW0 |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM185850
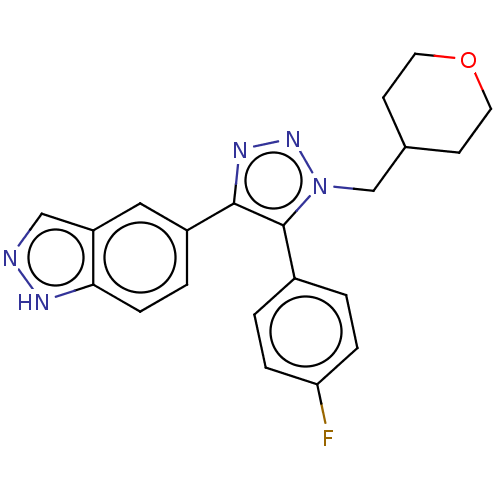
(US9163007, 167)Show SMILES Fc1ccc(cc1)-c1c(nnn1CC1CCOCC1)-c1ccc2[nH]ncc2c1 Show InChI InChI=1S/C21H20FN5O/c22-18-4-1-15(2-5-18)21-20(16-3-6-19-17(11-16)12-23-24-19)25-26-27(21)13-14-7-9-28-10-8-14/h1-6,11-12,14H,7-10,13H2,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.05 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc.
US Patent
| Assay Description
11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... |
US Patent US9163007 (2015)
BindingDB Entry DOI: 10.7270/Q2KW5DTG |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM185898
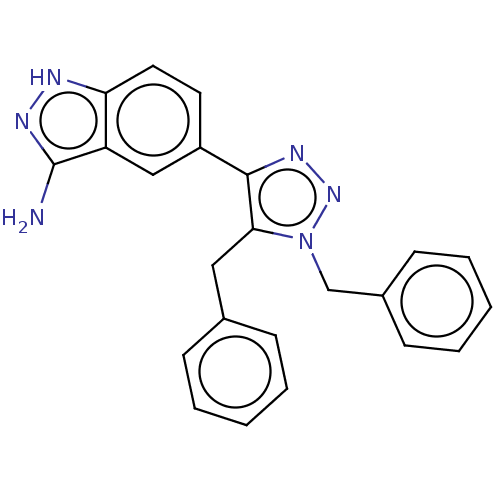
(US9163007, 405)Show SMILES Nc1n[nH]c2ccc(cc12)-c1nnn(Cc2ccccc2)c1Cc1ccccc1 Show InChI InChI=1S/C23H20N6/c24-23-19-14-18(11-12-20(19)25-27-23)22-21(13-16-7-3-1-4-8-16)29(28-26-22)15-17-9-5-2-6-10-17/h1-12,14H,13,15H2,(H3,24,25,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc.
US Patent
| Assay Description
11 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 u... |
US Patent US9163007 (2015)
BindingDB Entry DOI: 10.7270/Q2KW5DTG |
More data for this
Ligand-Target Pair | |
Cell division cycle 7-related protein kinase
(Homo sapiens (Human)) | BDBM185841
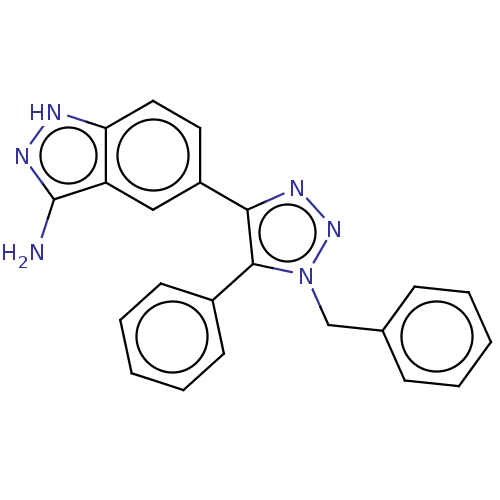
(US9163007, 102)Show SMILES Nc1n[nH]c2ccc(cc12)-c1nnn(Cc2ccccc2)c1-c1ccccc1 Show InChI InChI=1S/C22H18N6/c23-22-18-13-17(11-12-19(18)24-26-22)20-21(16-9-5-2-6-10-16)28(27-25-20)14-15-7-3-1-4-8-15/h1-13H,14H2,(H3,23,24,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | 7.5 | n/a |
AbbVie Inc.
US Patent
| Assay Description
6 data point. Cdc7 kinase assays were carried out in 25 mM HEPES, pH 7.5, 1 mM DTT, 10 mM MgCl2, 100 μM Na3VO4, and 0.075 mg/ml Triton X-100 us... |
US Patent US9163007 (2015)
BindingDB Entry DOI: 10.7270/Q2KW5DTG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data