 Found 232 hits with Last Name = 'muchamuel' and Initial = 't'
Found 232 hits with Last Name = 'muchamuel' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277781
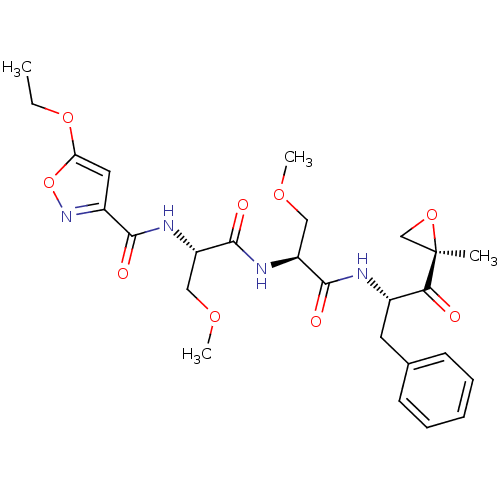
((2S)-2-[(2S)-2-[(5-ethoxy-1,2-oxazol-3-yl)formamid...)Show SMILES CCOc1cc(no1)C(=O)N[C@@H](COC)C(=O)N[C@@H](COC)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C26H34N4O9/c1-5-37-21-12-18(30-39-21)23(32)28-20(14-36-4)25(34)29-19(13-35-3)24(33)27-17(22(31)26(2)15-38-26)11-16-9-7-6-8-10-16/h6-10,12,17,19-20H,5,11,13-15H2,1-4H3,(H,27,33)(H,28,32)(H,29,34)/t17-,19-,20-,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50069989

((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)c1cnccn1)B(O)O |r| Show InChI InChI=1S/C19H25BN4O4/c1-13(2)10-17(20(27)28)24-18(25)15(11-14-6-4-3-5-7-14)23-19(26)16-12-21-8-9-22-16/h3-9,12-13,15,17,27-28H,10-11H2,1-2H3,(H,23,26)(H,24,25)/t15-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277889
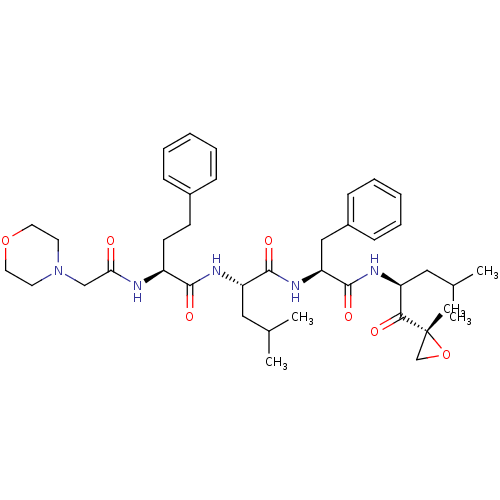
(CARFILZOMIB | CHEMBL451887)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CCc1ccccc1)NC(=O)CN1CCOCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C40H57N5O7/c1-27(2)22-32(36(47)40(5)26-52-40)42-39(50)34(24-30-14-10-7-11-15-30)44-38(49)33(23-28(3)4)43-37(48)31(17-16-29-12-8-6-9-13-29)41-35(46)25-45-18-20-51-21-19-45/h6-15,27-28,31-34H,16-26H2,1-5H3,(H,41,46)(H,42,50)(H,43,48)(H,44,49)/t31-,32-,33-,34-,40+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277779
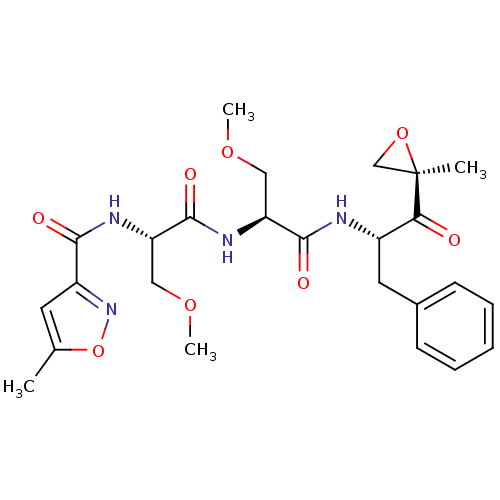
(CHEMBL484003 | N-((S)-3-methoxy-1-((S)-3-methoxy-1...)Show SMILES COC[C@H](NC(=O)[C@H](COC)NC(=O)c1cc(C)on1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C25H32N4O8/c1-15-10-18(29-37-15)22(31)27-20(13-35-4)24(33)28-19(12-34-3)23(32)26-17(21(30)25(2)14-36-25)11-16-8-6-5-7-9-16/h5-10,17,19-20H,11-14H2,1-4H3,(H,26,32)(H,27,31)(H,28,33)/t17-,19-,20-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277815
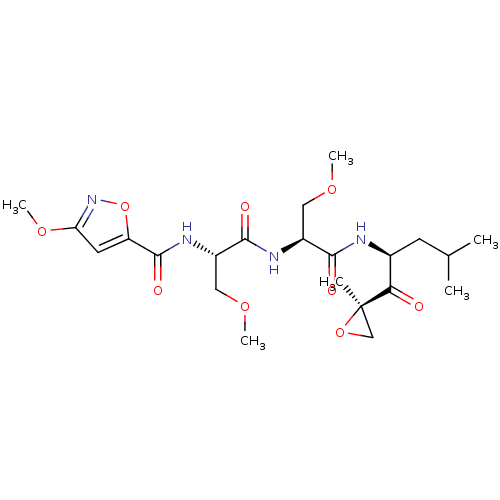
(3-methoxy-N-((S)-3-methoxy-1-((S)-3-methoxy-1-((S)...)Show SMILES COC[C@H](NC(=O)[C@H](COC)NC(=O)c1cc(OC)no1)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C22H34N4O9/c1-12(2)7-13(18(27)22(3)11-34-22)23-19(28)14(9-31-4)24-20(29)15(10-32-5)25-21(30)16-8-17(33-6)26-35-16/h8,12-15H,7,9-11H2,1-6H3,(H,23,28)(H,24,29)(H,25,30)/t13-,14-,15-,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50529639

(CHEMBL4454809)Show SMILES COc1ccc(C[C@H](NC(=O)[C@@H](C)NC(=O)[C@H]2CC[C@H](O)CC2)C(=O)N[C@@H](Cc2ccccc2)C(=O)[C@@]2(C)CO2)cc1 |r,wU:26.27,19.19,wD:16.15,11.11,7.6,36.38,(66.97,-27.92,;66.97,-26.38,;65.64,-25.61,;64.3,-26.38,;62.97,-25.61,;62.98,-24.07,;61.64,-23.3,;61.65,-21.76,;60.31,-20.99,;58.98,-21.76,;58.98,-23.3,;57.65,-20.98,;57.65,-19.44,;56.31,-21.75,;54.98,-20.98,;54.98,-19.44,;53.64,-21.75,;52.31,-20.97,;50.98,-21.73,;50.97,-23.27,;49.63,-24.04,;52.3,-24.05,;53.65,-23.29,;62.98,-20.99,;62.98,-19.45,;64.31,-21.76,;65.65,-21,;65.65,-19.46,;66.85,-18.48,;68.28,-19.04,;69.47,-18.07,;69.23,-16.55,;67.78,-16,;66.59,-16.97,;66.98,-21.77,;66.98,-23.31,;68.31,-20.99,;69.64,-21.76,;67.53,-19.65,;69.08,-19.65,;64.3,-23.3,;65.63,-24.06,)| Show InChI InChI=1S/C32H41N3O7/c1-20(33-30(39)23-11-13-24(36)14-12-23)29(38)35-27(18-22-9-15-25(41-3)16-10-22)31(40)34-26(28(37)32(2)19-42-32)17-21-7-5-4-6-8-21/h4-10,15-16,20,23-24,26-27,36H,11-14,17-19H2,1-3H3,(H,33,39)(H,34,40)(H,35,38)/t20-,23-,24-,26+,27+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50529639

(CHEMBL4454809)Show SMILES COc1ccc(C[C@H](NC(=O)[C@@H](C)NC(=O)[C@H]2CC[C@H](O)CC2)C(=O)N[C@@H](Cc2ccccc2)C(=O)[C@@]2(C)CO2)cc1 |r,wU:26.27,19.19,wD:16.15,11.11,7.6,36.38,(66.97,-27.92,;66.97,-26.38,;65.64,-25.61,;64.3,-26.38,;62.97,-25.61,;62.98,-24.07,;61.64,-23.3,;61.65,-21.76,;60.31,-20.99,;58.98,-21.76,;58.98,-23.3,;57.65,-20.98,;57.65,-19.44,;56.31,-21.75,;54.98,-20.98,;54.98,-19.44,;53.64,-21.75,;52.31,-20.97,;50.98,-21.73,;50.97,-23.27,;49.63,-24.04,;52.3,-24.05,;53.65,-23.29,;62.98,-20.99,;62.98,-19.45,;64.31,-21.76,;65.65,-21,;65.65,-19.46,;66.85,-18.48,;68.28,-19.04,;69.47,-18.07,;69.23,-16.55,;67.78,-16,;66.59,-16.97,;66.98,-21.77,;66.98,-23.31,;68.31,-20.99,;69.64,-21.76,;67.53,-19.65,;69.08,-19.65,;64.3,-23.3,;65.63,-24.06,)| Show InChI InChI=1S/C32H41N3O7/c1-20(33-30(39)23-11-13-24(36)14-12-23)29(38)35-27(18-22-9-15-25(41-3)16-10-22)31(40)34-26(28(37)32(2)19-42-32)17-21-7-5-4-6-8-21/h4-10,15-16,20,23-24,26-27,36H,11-14,17-19H2,1-3H3,(H,33,39)(H,34,40)(H,35,38)/t20-,23-,24-,26+,27+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50529641

(CHEMBL4464640)Show SMILES COc1ccc(C[C@H](NC(=O)[C@@H](C)NC(=O)C2CCCCC2)C(=O)N[C@@H](Cc2ccccc2)C(=O)[C@@]2(C)CO2)cc1 |r| Show InChI InChI=1S/C32H41N3O6/c1-21(33-30(38)24-12-8-5-9-13-24)29(37)35-27(19-23-14-16-25(40-3)17-15-23)31(39)34-26(28(36)32(2)20-41-32)18-22-10-6-4-7-11-22/h4,6-7,10-11,14-17,21,24,26-27H,5,8-9,12-13,18-20H2,1-3H3,(H,33,38)(H,34,39)(H,35,37)/t21-,26+,27+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50529641

(CHEMBL4464640)Show SMILES COc1ccc(C[C@H](NC(=O)[C@@H](C)NC(=O)C2CCCCC2)C(=O)N[C@@H](Cc2ccccc2)C(=O)[C@@]2(C)CO2)cc1 |r| Show InChI InChI=1S/C32H41N3O6/c1-21(33-30(38)24-12-8-5-9-13-24)29(37)35-27(19-23-14-16-25(40-3)17-15-23)31(39)34-26(28(36)32(2)20-41-32)18-22-10-6-4-7-11-22/h4,6-7,10-11,14-17,21,24,26-27H,5,8-9,12-13,18-20H2,1-3H3,(H,33,38)(H,34,39)(H,35,37)/t21-,26+,27+,32-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50526812

(CHEMBL4441524)Show SMILES COc1ccc(C[C@H](NC(=O)[C@@H](C)NC(=O)[C@H]2CC[C@H](O)CC2)C(=O)N[C@@H](CC2=CCCC2)C(=O)[C@@]2(C)CO2)cc1 |r,wU:19.19,26.27,wD:16.15,35.37,7.6,11.11,t:29,(25.81,-33.19,;24.47,-33.96,;23.14,-33.19,;23.14,-31.65,;21.81,-30.89,;20.48,-31.65,;19.15,-30.88,;19.15,-29.34,;17.81,-28.57,;16.48,-29.34,;16.48,-30.88,;15.15,-28.57,;15.15,-27.03,;13.81,-29.34,;12.48,-28.57,;12.48,-27.03,;11.14,-29.34,;9.81,-28.57,;8.47,-29.34,;8.47,-30.89,;7.14,-31.65,;9.81,-31.65,;11.14,-30.89,;20.48,-28.57,;20.48,-27.03,;21.81,-29.34,;23.14,-28.57,;23.14,-27.03,;24.23,-25.95,;25.75,-26.18,;26.46,-24.81,;25.37,-23.72,;24,-24.42,;24.48,-29.34,;24.48,-30.88,;25.81,-28.57,;27.14,-29.34,;25.05,-27.23,;26.58,-27.23,;20.48,-33.2,;21.82,-33.96,)| Show InChI InChI=1S/C31H43N3O7/c1-19(32-29(38)22-10-12-23(35)13-11-22)28(37)34-26(17-21-8-14-24(40-3)15-9-21)30(39)33-25(16-20-6-4-5-7-20)27(36)31(2)18-41-31/h6,8-9,14-15,19,22-23,25-26,35H,4-5,7,10-13,16-18H2,1-3H3,(H,32,38)(H,33,39)(H,34,37)/t19-,22-,23-,25+,26+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50526812

(CHEMBL4441524)Show SMILES COc1ccc(C[C@H](NC(=O)[C@@H](C)NC(=O)[C@H]2CC[C@H](O)CC2)C(=O)N[C@@H](CC2=CCCC2)C(=O)[C@@]2(C)CO2)cc1 |r,wU:19.19,26.27,wD:16.15,35.37,7.6,11.11,t:29,(25.81,-33.19,;24.47,-33.96,;23.14,-33.19,;23.14,-31.65,;21.81,-30.89,;20.48,-31.65,;19.15,-30.88,;19.15,-29.34,;17.81,-28.57,;16.48,-29.34,;16.48,-30.88,;15.15,-28.57,;15.15,-27.03,;13.81,-29.34,;12.48,-28.57,;12.48,-27.03,;11.14,-29.34,;9.81,-28.57,;8.47,-29.34,;8.47,-30.89,;7.14,-31.65,;9.81,-31.65,;11.14,-30.89,;20.48,-28.57,;20.48,-27.03,;21.81,-29.34,;23.14,-28.57,;23.14,-27.03,;24.23,-25.95,;25.75,-26.18,;26.46,-24.81,;25.37,-23.72,;24,-24.42,;24.48,-29.34,;24.48,-30.88,;25.81,-28.57,;27.14,-29.34,;25.05,-27.23,;26.58,-27.23,;20.48,-33.2,;21.82,-33.96,)| Show InChI InChI=1S/C31H43N3O7/c1-19(32-29(38)22-10-12-23(35)13-11-22)28(37)34-26(17-21-8-14-24(40-3)15-9-21)30(39)33-25(16-20-6-4-5-7-20)27(36)31(2)18-41-31/h6,8-9,14-15,19,22-23,25-26,35H,4-5,7,10-13,16-18H2,1-3H3,(H,32,38)(H,33,39)(H,34,37)/t19-,22-,23-,25+,26+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277816
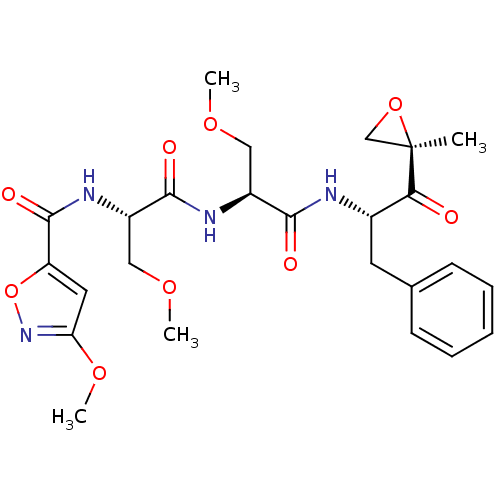
(3-methoxy-N-((S)-3-methoxy-1-((S)-3-methoxy-1-((S)...)Show SMILES COC[C@H](NC(=O)[C@H](COC)NC(=O)c1cc(OC)no1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C25H32N4O9/c1-25(14-37-25)21(30)16(10-15-8-6-5-7-9-15)26-22(31)17(12-34-2)27-23(32)18(13-35-3)28-24(33)19-11-20(36-4)29-38-19/h5-9,11,16-18H,10,12-14H2,1-4H3,(H,26,31)(H,27,32)(H,28,33)/t16-,17-,18-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50529638
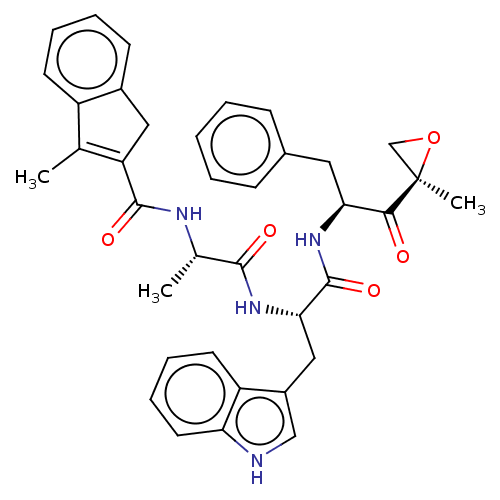
(CHEMBL4566534)Show SMILES C[C@H](NC(=O)C1=C(C)c2ccccc2C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r,c:5| Show InChI InChI=1S/C37H38N4O5/c1-22-27-14-8-7-13-25(27)18-29(22)35(44)39-23(2)34(43)41-32(19-26-20-38-30-16-10-9-15-28(26)30)36(45)40-31(33(42)37(3)21-46-37)17-24-11-5-4-6-12-24/h4-16,20,23,31-32,38H,17-19,21H2,1-3H3,(H,39,44)(H,40,45)(H,41,43)/t23-,31-,32-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Mus musculus) | BDBM50526813

(CHEMBL4465456)Show SMILES CC(C)C[C@H](NC(=O)CN1CCCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C35H48N4O5/c1-25(2)20-29(36-31(40)23-39-18-12-4-5-13-19-39)33(42)38-30(22-27-16-10-7-11-17-27)34(43)37-28(32(41)35(3)24-44-35)21-26-14-8-6-9-15-26/h6-11,14-17,25,28-30H,4-5,12-13,18-24H2,1-3H3,(H,36,40)(H,37,43)(H,38,42)/t28-,29-,30-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of 20S proteosome beta 5 in mouse A20 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Mus musculus) | BDBM50526813

(CHEMBL4465456)Show SMILES CC(C)C[C@H](NC(=O)CN1CCCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C35H48N4O5/c1-25(2)20-29(36-31(40)23-39-18-12-4-5-13-19-39)33(42)38-30(22-27-16-10-7-11-17-27)34(43)37-28(32(41)35(3)24-44-35)21-26-14-8-6-9-15-26/h6-11,14-17,25,28-30H,4-5,12-13,18-24H2,1-3H3,(H,36,40)(H,37,43)(H,38,42)/t28-,29-,30-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of 20S proteosome beta 5 in mouse A20 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50529638

(CHEMBL4566534)Show SMILES C[C@H](NC(=O)C1=C(C)c2ccccc2C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r,c:5| Show InChI InChI=1S/C37H38N4O5/c1-22-27-14-8-7-13-25(27)18-29(22)35(44)39-23(2)34(43)41-32(19-26-20-38-30-16-10-9-15-28(26)30)36(45)40-31(33(42)37(3)21-46-37)17-24-11-5-4-6-12-24/h4-16,20,23,31-32,38H,17-19,21H2,1-3H3,(H,39,44)(H,40,45)(H,41,43)/t23-,31-,32-,37+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50526811
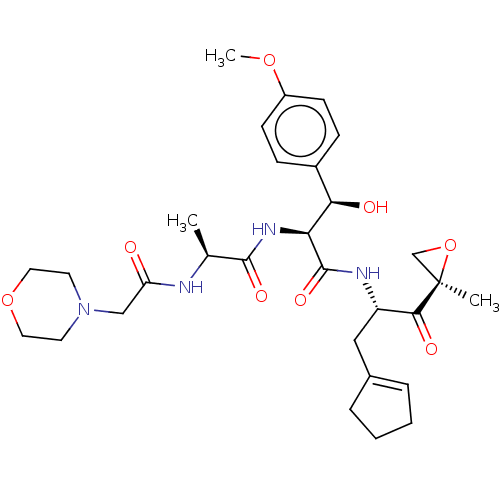
(Kzr-616)Show SMILES COc1ccc(cc1)[C@@H](O)[C@H](NC(=O)[C@H](C)NC(=O)CN1CCOCC1)C(=O)N[C@@H](CC1=CCCC1)C(=O)[C@@]1(C)CO1 |t:33| Show InChI InChI=1S/C30H42N4O8/c1-19(31-24(35)17-34-12-14-41-15-13-34)28(38)33-25(26(36)21-8-10-22(40-3)11-9-21)29(39)32-23(16-20-6-4-5-7-20)27(37)30(2)18-42-30/h6,8-11,19,23,25-26,36H,4-5,7,12-18H2,1-3H3,(H,31,35)(H,32,39)(H,33,38)/t19-,23-,25-,26+,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50526811

(Kzr-616)Show SMILES COc1ccc(cc1)[C@@H](O)[C@H](NC(=O)[C@H](C)NC(=O)CN1CCOCC1)C(=O)N[C@@H](CC1=CCCC1)C(=O)[C@@]1(C)CO1 |t:33| Show InChI InChI=1S/C30H42N4O8/c1-19(31-24(35)17-34-12-14-41-15-13-34)28(38)33-25(26(36)21-8-10-22(40-3)11-9-21)29(39)32-23(16-20-6-4-5-7-20)27(37)30(2)18-42-30/h6,8-11,19,23,25-26,36H,4-5,7,12-18H2,1-3H3,(H,31,35)(H,32,39)(H,33,38)/t19-,23-,25-,26+,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50099663
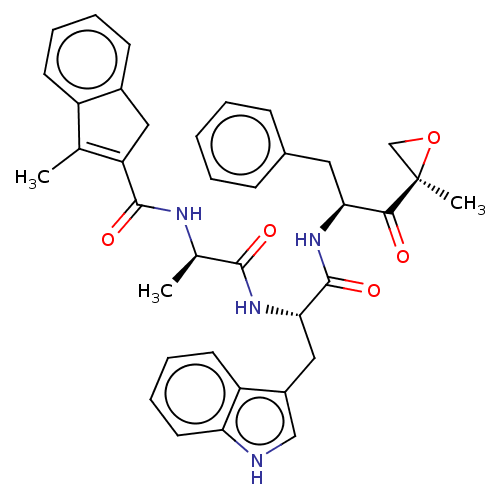
(CHEMBL3319482)Show SMILES C[C@@H](NC(=O)C1=C(C)c2ccccc2C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r,c:5| Show InChI InChI=1S/C22H16ClF2N3O/c23-17-6-2-4-8-20(17)28-21(13-26-12-14-5-1-3-7-18(14)25)27-19-10-9-15(24)11-16(19)22(28)29/h1-11,26H,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50007203
(CHEMBL3237875)Show SMILES COc1ccc(C[C@H](NC(=O)[C@H](C)NC(=O)CN2CCOCC2)C(=O)N[C@@H](Cc2ccccc2)C(=O)[C@@]2(C)CO2)cc1 |r| Show InChI InChI=1S/C31H40N4O7/c1-21(32-27(36)19-35-13-15-41-16-14-35)29(38)34-26(18-23-9-11-24(40-3)12-10-23)30(39)33-25(28(37)31(2)20-42-31)17-22-7-5-4-6-8-22/h4-12,21,25-26H,13-20H2,1-3H3,(H,32,36)(H,33,39)(H,34,38)/t21-,25-,26-,31+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50526813

(CHEMBL4465456)Show SMILES CC(C)C[C@H](NC(=O)CN1CCCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C35H48N4O5/c1-25(2)20-29(36-31(40)23-39-18-12-4-5-13-19-39)33(42)38-30(22-27-16-10-7-11-17-27)34(43)37-28(32(41)35(3)24-44-35)21-26-14-8-6-9-15-26/h6-11,14-17,25,28-30H,4-5,12-13,18-24H2,1-3H3,(H,36,40)(H,37,43)(H,38,42)/t28-,29-,30-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of 20S proteosome beta 5 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50099663

(CHEMBL3319482)Show SMILES C[C@@H](NC(=O)C1=C(C)c2ccccc2C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r,c:5| Show InChI InChI=1S/C22H16ClF2N3O/c23-17-6-2-4-8-20(17)28-21(13-26-12-14-5-1-3-7-18(14)25)27-19-10-9-15(24)11-16(19)22(28)29/h1-11,26H,12-13H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50007203

(CHEMBL3237875)Show SMILES COc1ccc(C[C@H](NC(=O)[C@H](C)NC(=O)CN2CCOCC2)C(=O)N[C@@H](Cc2ccccc2)C(=O)[C@@]2(C)CO2)cc1 |r| Show InChI InChI=1S/C31H40N4O7/c1-21(32-27(36)19-35-13-15-41-16-14-35)29(38)34-26(18-23-9-11-24(40-3)12-10-23)30(39)33-25(28(37)31(2)20-42-31)17-22-7-5-4-6-8-22/h4-12,21,25-26H,13-20H2,1-3H3,(H,32,36)(H,33,39)(H,34,38)/t21-,25-,26-,31+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50007203

(CHEMBL3237875)Show SMILES COc1ccc(C[C@H](NC(=O)[C@H](C)NC(=O)CN2CCOCC2)C(=O)N[C@@H](Cc2ccccc2)C(=O)[C@@]2(C)CO2)cc1 |r| Show InChI InChI=1S/C31H40N4O7/c1-21(32-27(36)19-35-13-15-41-16-14-35)29(38)34-26(18-23-9-11-24(40-3)12-10-23)30(39)33-25(28(37)31(2)20-42-31)17-22-7-5-4-6-8-22/h4-12,21,25-26H,13-20H2,1-3H3,(H,32,36)(H,33,39)(H,34,38)/t21-,25-,26-,31+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cell lysate after 1 hr by ELISA |
ACS Med Chem Lett 8: 413-417 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00496
BindingDB Entry DOI: 10.7270/Q28W3GKM |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50526813

(CHEMBL4465456)Show SMILES CC(C)C[C@H](NC(=O)CN1CCCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C35H48N4O5/c1-25(2)20-29(36-31(40)23-39-18-12-4-5-13-19-39)33(42)38-30(22-27-16-10-7-11-17-27)34(43)37-28(32(41)35(3)24-44-35)21-26-14-8-6-9-15-26/h6-11,14-17,25,28-30H,4-5,12-13,18-24H2,1-3H3,(H,36,40)(H,37,43)(H,38,42)/t28-,29-,30-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of 20S proteosome beta 5 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277780

(5-ethoxy-N-((S)-3-methoxy-1-((S)-3-methoxy-1-((S)-...)Show SMILES CCOc1cc(no1)C(=O)N[C@@H](COC)C(=O)N[C@@H](COC)C(=O)N[C@@H](CC(C)C)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C23H36N4O9/c1-7-34-18-9-15(27-36-18)20(29)25-17(11-33-6)22(31)26-16(10-32-5)21(30)24-14(8-13(2)3)19(28)23(4)12-35-23/h9,13-14,16-17H,7-8,10-12H2,1-6H3,(H,24,30)(H,25,29)(H,26,31)/t14-,16-,17-,23+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Mus musculus) | BDBM50529638

(CHEMBL4566534)Show SMILES C[C@H](NC(=O)C1=C(C)c2ccccc2C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r,c:5| Show InChI InChI=1S/C37H38N4O5/c1-22-27-14-8-7-13-25(27)18-29(22)35(44)39-23(2)34(43)41-32(19-26-20-38-30-16-10-9-15-28(26)30)36(45)40-31(33(42)37(3)21-46-37)17-24-11-5-4-6-12-24/h4-16,20,23,31-32,38H,17-19,21H2,1-3H3,(H,39,44)(H,40,45)(H,41,43)/t23-,31-,32-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of 20S proteosome beta 5 in mouse A20 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Mus musculus) | BDBM50529638

(CHEMBL4566534)Show SMILES C[C@H](NC(=O)C1=C(C)c2ccccc2C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r,c:5| Show InChI InChI=1S/C37H38N4O5/c1-22-27-14-8-7-13-25(27)18-29(22)35(44)39-23(2)34(43)41-32(19-26-20-38-30-16-10-9-15-28(26)30)36(45)40-31(33(42)37(3)21-46-37)17-24-11-5-4-6-12-24/h4-16,20,23,31-32,38H,17-19,21H2,1-3H3,(H,39,44)(H,40,45)(H,41,43)/t23-,31-,32-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of 20S proteosome beta 5 in mouse A20 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50526813

(CHEMBL4465456)Show SMILES CC(C)C[C@H](NC(=O)CN1CCCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C35H48N4O5/c1-25(2)20-29(36-31(40)23-39-18-12-4-5-13-19-39)33(42)38-30(22-27-16-10-7-11-17-27)34(43)37-28(32(41)35(3)24-44-35)21-26-14-8-6-9-15-26/h6-11,14-17,25,28-30H,4-5,12-13,18-24H2,1-3H3,(H,36,40)(H,37,43)(H,38,42)/t28-,29-,30-,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50526813

(CHEMBL4465456)Show SMILES CC(C)C[C@H](NC(=O)CN1CCCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C35H48N4O5/c1-25(2)20-29(36-31(40)23-39-18-12-4-5-13-19-39)33(42)38-30(22-27-16-10-7-11-17-27)34(43)37-28(32(41)35(3)24-44-35)21-26-14-8-6-9-15-26/h6-11,14-17,25,28-30H,4-5,12-13,18-24H2,1-3H3,(H,36,40)(H,37,43)(H,38,42)/t28-,29-,30-,35+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Mus musculus) | BDBM50529638

(CHEMBL4566534)Show SMILES C[C@H](NC(=O)C1=C(C)c2ccccc2C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r,c:5| Show InChI InChI=1S/C37H38N4O5/c1-22-27-14-8-7-13-25(27)18-29(22)35(44)39-23(2)34(43)41-32(19-26-20-38-30-16-10-9-15-28(26)30)36(45)40-31(33(42)37(3)21-46-37)17-24-11-5-4-6-12-24/h4-16,20,23,31-32,38H,17-19,21H2,1-3H3,(H,39,44)(H,40,45)(H,41,43)/t23-,31-,32-,37+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in mouse A20 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Mus musculus) | BDBM50529638

(CHEMBL4566534)Show SMILES C[C@H](NC(=O)C1=C(C)c2ccccc2C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r,c:5| Show InChI InChI=1S/C37H38N4O5/c1-22-27-14-8-7-13-25(27)18-29(22)35(44)39-23(2)34(43)41-32(19-26-20-38-30-16-10-9-15-28(26)30)36(45)40-31(33(42)37(3)21-46-37)17-24-11-5-4-6-12-24/h4-16,20,23,31-32,38H,17-19,21H2,1-3H3,(H,39,44)(H,40,45)(H,41,43)/t23-,31-,32-,37+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in mouse A20 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-9
(Homo sapiens (Human)) | BDBM50232675
(CHEMBL4100295)Show SMILES C[C@@]1(CO1)C(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](CO)NC(=O)c1cccc(=O)[nH]1 |r| Show InChI InChI=1S/C21H23N3O6/c1-21(12-30-21)18(27)15(10-13-6-3-2-4-7-13)23-20(29)16(11-25)24-19(28)14-8-5-9-17(26)22-14/h2-9,15-16,25H,10-12H2,1H3,(H,22,26)(H,23,29)(H,24,28)/t15-,16-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP2 in human MOLT4 cell lysate after 1 hr by ELISA |
ACS Med Chem Lett 8: 413-417 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00496
BindingDB Entry DOI: 10.7270/Q28W3GKM |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50529638

(CHEMBL4566534)Show SMILES C[C@H](NC(=O)C1=C(C)c2ccccc2C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r,c:5| Show InChI InChI=1S/C37H38N4O5/c1-22-27-14-8-7-13-25(27)18-29(22)35(44)39-23(2)34(43)41-32(19-26-20-38-30-16-10-9-15-28(26)30)36(45)40-31(33(42)37(3)21-46-37)17-24-11-5-4-6-12-24/h4-16,20,23,31-32,38H,17-19,21H2,1-3H3,(H,39,44)(H,40,45)(H,41,43)/t23-,31-,32-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of 20S proteosome beta 5 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-5
(Homo sapiens (Human)) | BDBM50529638

(CHEMBL4566534)Show SMILES C[C@H](NC(=O)C1=C(C)c2ccccc2C1)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r,c:5| Show InChI InChI=1S/C37H38N4O5/c1-22-27-14-8-7-13-25(27)18-29(22)35(44)39-23(2)34(43)41-32(19-26-20-38-30-16-10-9-15-28(26)30)36(45)40-31(33(42)37(3)21-46-37)17-24-11-5-4-6-12-24/h4-16,20,23,31-32,38H,17-19,21H2,1-3H3,(H,39,44)(H,40,45)(H,41,43)/t23-,31-,32-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of 20S proteosome beta 5 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Mus musculus) | BDBM50526813

(CHEMBL4465456)Show SMILES CC(C)C[C@H](NC(=O)CN1CCCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C35H48N4O5/c1-25(2)20-29(36-31(40)23-39-18-12-4-5-13-19-39)33(42)38-30(22-27-16-10-7-11-17-27)34(43)37-28(32(41)35(3)24-44-35)21-26-14-8-6-9-15-26/h6-11,14-17,25,28-30H,4-5,12-13,18-24H2,1-3H3,(H,36,40)(H,37,43)(H,38,42)/t28-,29-,30-,35+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in mouse A20 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Mus musculus) | BDBM50526813

(CHEMBL4465456)Show SMILES CC(C)C[C@H](NC(=O)CN1CCCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C35H48N4O5/c1-25(2)20-29(36-31(40)23-39-18-12-4-5-13-19-39)33(42)38-30(22-27-16-10-7-11-17-27)34(43)37-28(32(41)35(3)24-44-35)21-26-14-8-6-9-15-26/h6-11,14-17,25,28-30H,4-5,12-13,18-24H2,1-3H3,(H,36,40)(H,37,43)(H,38,42)/t28-,29-,30-,35+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 56 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in mouse A20 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Mus musculus) | BDBM50526811

(Kzr-616)Show SMILES COc1ccc(cc1)[C@@H](O)[C@H](NC(=O)[C@H](C)NC(=O)CN1CCOCC1)C(=O)N[C@@H](CC1=CCCC1)C(=O)[C@@]1(C)CO1 |t:33| Show InChI InChI=1S/C30H42N4O8/c1-19(31-24(35)17-34-12-14-41-15-13-34)28(38)33-25(26(36)21-8-10-22(40-3)11-9-21)29(39)32-23(16-20-6-4-5-7-20)27(37)30(2)18-42-30/h6,8-11,19,23,25-26,36H,4-5,7,12-18H2,1-3H3,(H,31,35)(H,32,39)(H,33,38)/t19-,23-,25-,26+,30+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in mouse A20 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Mus musculus) | BDBM50526811

(Kzr-616)Show SMILES COc1ccc(cc1)[C@@H](O)[C@H](NC(=O)[C@H](C)NC(=O)CN1CCOCC1)C(=O)N[C@@H](CC1=CCCC1)C(=O)[C@@]1(C)CO1 |t:33| Show InChI InChI=1S/C30H42N4O8/c1-19(31-24(35)17-34-12-14-41-15-13-34)28(38)33-25(26(36)21-8-10-22(40-3)11-9-21)29(39)32-23(16-20-6-4-5-7-20)27(37)30(2)18-42-30/h6,8-11,19,23,25-26,36H,4-5,7,12-18H2,1-3H3,(H,31,35)(H,32,39)(H,33,38)/t19-,23-,25-,26+,30+/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in mouse A20 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-10
(Homo sapiens (Human)) | BDBM50529640

(CHEMBL4452892)Show SMILES CC(C)C[C@H](NC(=O)CN1CCCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCc1ccc(C)cc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C37H52N4O5/c1-26(2)22-31(38-33(42)24-41-20-10-5-6-11-21-41)35(44)40-32(23-29-12-8-7-9-13-29)36(45)39-30(34(43)37(4)25-46-37)19-18-28-16-14-27(3)15-17-28/h7-9,12-17,26,30-32H,5-6,10-11,18-25H2,1-4H3,(H,38,42)(H,39,45)(H,40,44)/t30-,31-,32-,37+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of MECL1 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-10
(Homo sapiens (Human)) | BDBM50529640

(CHEMBL4452892)Show SMILES CC(C)C[C@H](NC(=O)CN1CCCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCc1ccc(C)cc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C37H52N4O5/c1-26(2)22-31(38-33(42)24-41-20-10-5-6-11-21-41)35(44)40-32(23-29-12-8-7-9-13-29)36(45)39-30(34(43)37(4)25-46-37)19-18-28-16-14-27(3)15-17-28/h7-9,12-17,26,30-32H,5-6,10-11,18-25H2,1-4H3,(H,38,42)(H,39,45)(H,40,44)/t30-,31-,32-,37+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of MECL1 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50099829
(CHEMBL3319584)Show SMILES COc1ccc(C[C@H](NC(=O)[C@@H](C)NC(=O)C2=C(C)c3ccccc3C2)C(=O)N[C@@H](Cc2ccccc2)C(=O)[C@@]2(C)CO2)cc1 |r,c:16| Show InChI InChI=1S/C8H11NO3/c9-5-6(4-8(10)11)7-2-1-3-12-7/h1-3,6H,4-5,9H2,(H,10,11)/t6-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50099829

(CHEMBL3319584)Show SMILES COc1ccc(C[C@H](NC(=O)[C@@H](C)NC(=O)C2=C(C)c3ccccc3C2)C(=O)N[C@@H](Cc2ccccc2)C(=O)[C@@]2(C)CO2)cc1 |r,c:16| Show InChI InChI=1S/C8H11NO3/c9-5-6(4-8(10)11)7-2-1-3-12-7/h1-3,6H,4-5,9H2,(H,10,11)/t6-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50526813

(CHEMBL4465456)Show SMILES CC(C)C[C@H](NC(=O)CN1CCCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C35H48N4O5/c1-25(2)20-29(36-31(40)23-39-18-12-4-5-13-19-39)33(42)38-30(22-27-16-10-7-11-17-27)34(43)37-28(32(41)35(3)24-44-35)21-26-14-8-6-9-15-26/h6-11,14-17,25,28-30H,4-5,12-13,18-24H2,1-3H3,(H,36,40)(H,37,43)(H,38,42)/t28-,29-,30-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of 20S proteosome beta 2 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-2
(Homo sapiens (Human)) | BDBM50526813

(CHEMBL4465456)Show SMILES CC(C)C[C@H](NC(=O)CN1CCCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C35H48N4O5/c1-25(2)20-29(36-31(40)23-39-18-12-4-5-13-19-39)33(42)38-30(22-27-16-10-7-11-17-27)34(43)37-28(32(41)35(3)24-44-35)21-26-14-8-6-9-15-26/h6-11,14-17,25,28-30H,4-5,12-13,18-24H2,1-3H3,(H,36,40)(H,37,43)(H,38,42)/t28-,29-,30-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of 20S proteosome beta 2 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277818
(2-Me-5-thiazole-Ser(OMe)-Ser(OMe)-Phe-ketoepoxide ...)Show SMILES COC[C@H](NC(=O)[C@H](COC)NC(=O)c1cnc(C)o1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C25H32N4O8/c1-15-26-11-20(37-15)24(33)29-19(13-35-4)23(32)28-18(12-34-3)22(31)27-17(21(30)25(2)14-36-25)10-16-8-6-5-7-9-16/h5-9,11,17-19H,10,12-14H2,1-4H3,(H,27,31)(H,28,32)(H,29,33)/t17-,18-,19-,25+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50526810

(CHEMBL4443505)Show SMILES COc1ccc(C[C@H](NC(=O)[C@H](C)NC(=O)CN2CCOCC2)C(=O)N[C@@H](CC2=CCCC2)C(=O)[C@@]2(C)CO2)cc1 |r,t:29| Show InChI InChI=1S/C30H42N4O7/c1-20(31-26(35)18-34-12-14-40-15-13-34)28(37)33-25(17-22-8-10-23(39-3)11-9-22)29(38)32-24(16-21-6-4-5-7-21)27(36)30(2)19-41-30/h6,8-11,20,24-25H,4-5,7,12-19H2,1-3H3,(H,31,35)(H,32,38)(H,33,37)/t20-,24-,25-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50526810

(CHEMBL4443505)Show SMILES COc1ccc(C[C@H](NC(=O)[C@H](C)NC(=O)CN2CCOCC2)C(=O)N[C@@H](CC2=CCCC2)C(=O)[C@@]2(C)CO2)cc1 |r,t:29| Show InChI InChI=1S/C30H42N4O7/c1-20(31-26(35)18-34-12-14-40-15-13-34)28(37)33-25(17-22-8-10-23(39-3)11-9-22)29(38)32-24(16-21-6-4-5-7-21)27(36)30(2)19-41-30/h6,8-11,20,24-25H,4-5,7,12-19H2,1-3H3,(H,31,35)(H,32,38)(H,33,37)/t20-,24-,25-,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of LMP7 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-8
(Homo sapiens (Human)) | BDBM50277778
(CHEMBL484002 | N-((S)-3-methoxy-1-((S)-3-methoxy-1...)Show SMILES COC[C@H](NC(=O)[C@H](COC)NC(=O)c1ccon1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C24H30N4O8/c1-24(14-35-24)20(29)17(11-15-7-5-4-6-8-15)25-22(31)18(12-33-2)27-23(32)19(13-34-3)26-21(30)16-9-10-36-28-16/h4-10,17-19H,11-14H2,1-3H3,(H,25,31)(H,26,30)(H,27,32)/t17-,18-,19-,24+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 93 | n/a | n/a | n/a | n/a | n/a | n/a |
Proteolix, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of chymotrypsin-like activity of LMP7 immunoproteasome by proteasome active site ELISA |
J Med Chem 52: 3028-38 (2009)
Article DOI: 10.1021/jm801329v
BindingDB Entry DOI: 10.7270/Q2ZW1KS0 |
More data for this
Ligand-Target Pair | |
Proteasome subunit beta type-10
(Homo sapiens (Human)) | BDBM50526813

(CHEMBL4465456)Show SMILES CC(C)C[C@H](NC(=O)CN1CCCCCC1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)[C@@]1(C)CO1 |r| Show InChI InChI=1S/C35H48N4O5/c1-25(2)20-29(36-31(40)23-39-18-12-4-5-13-19-39)33(42)38-30(22-27-16-10-7-11-17-27)34(43)37-28(32(41)35(3)24-44-35)21-26-14-8-6-9-15-26/h6-11,14-17,25,28-30H,4-5,12-13,18-24H2,1-3H3,(H,36,40)(H,37,43)(H,38,42)/t28-,29-,30-,35+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Kezar Life Sciences
Curated by ChEMBL
| Assay Description
Inhibition of MECL1 in human MOLT4 cells by ELISA |
J Med Chem 61: 11127-11143 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01201
BindingDB Entry DOI: 10.7270/Q2M61PQB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data