

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50491400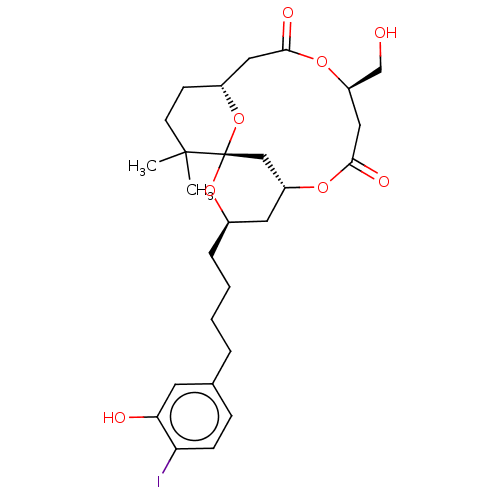 (CHEMBL2381165) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1B domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50491401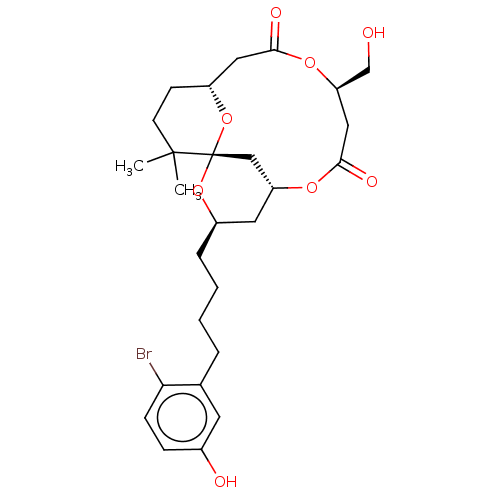 (CHEMBL2381162) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1B domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50491402 (CHEMBL2381163) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1B domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50491404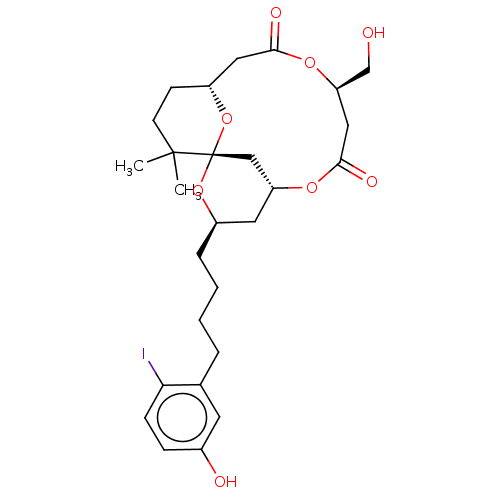 (CHEMBL2381166) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1B domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50491406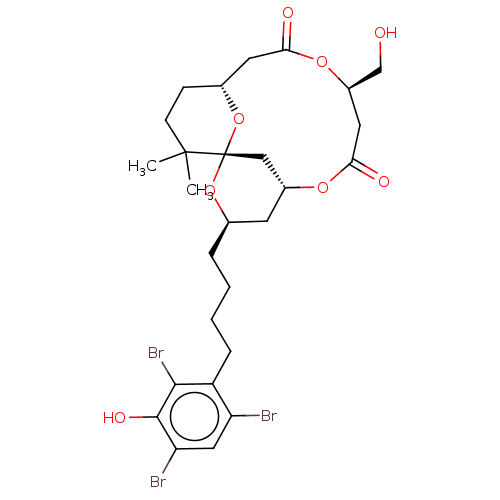 (CHEMBL2381164) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1B domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50327946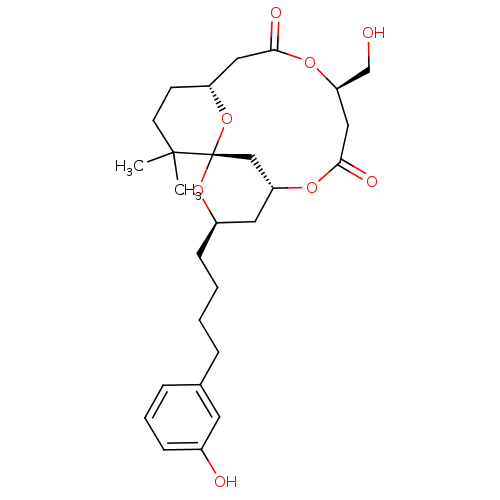 (1S,3R,5R,9R,13R)-9-Hydroxymethyl-3-[4-(3-hydroxy-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1B domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50391386 (CHEMBL2148106) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1A domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50491403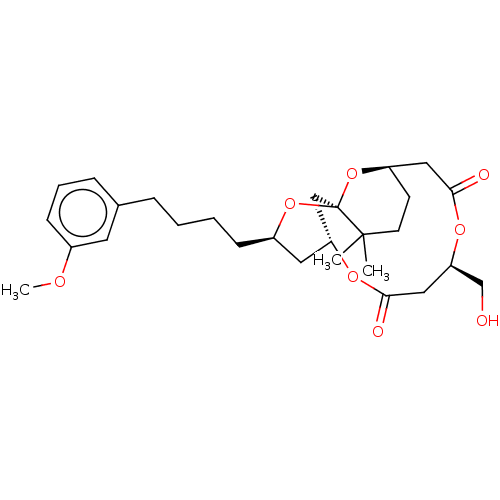 (CHEMBL2381167) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1B domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50391388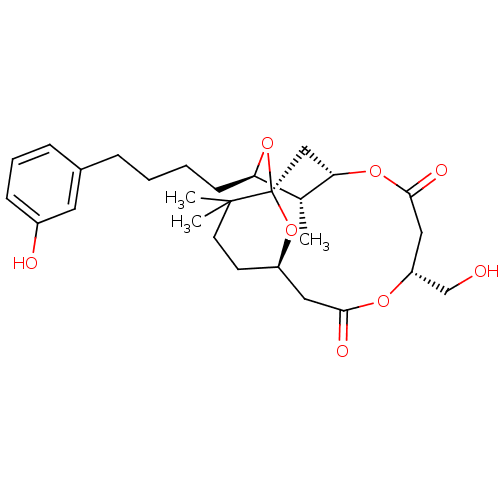 (CHEMBL2148108) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1A domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50491407 (CHEMBL2381161) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1B domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50491401 (CHEMBL2381162) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1A domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50491406 (CHEMBL2381164) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 79 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1A domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50491400 (CHEMBL2381165) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 82 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1A domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50491402 (CHEMBL2381163) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1A domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50491404 (CHEMBL2381166) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1A domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50327946 (1S,3R,5R,9R,13R)-9-Hydroxymethyl-3-[4-(3-hydroxy-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1A domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50491407 (CHEMBL2381161) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1A domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50491403 (CHEMBL2381167) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1A domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50491405 (CHEMBL2381160) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1B domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332461 ((R)-3-carba cyclic-phosphatidic acid | CHEMBL16300...) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Inhibition of recombinant ATX mediated hydrolysis of FS-3 | Bioorg Med Chem Lett 20: 7525-8 (2010) Article DOI: 10.1016/j.bmcl.2010.09.115 BindingDB Entry DOI: 10.7270/Q2XD11X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Ectonucleotide pyrophosphatase/phosphodiesterase family member 2 (Homo sapiens (Human)) | BDBM50332460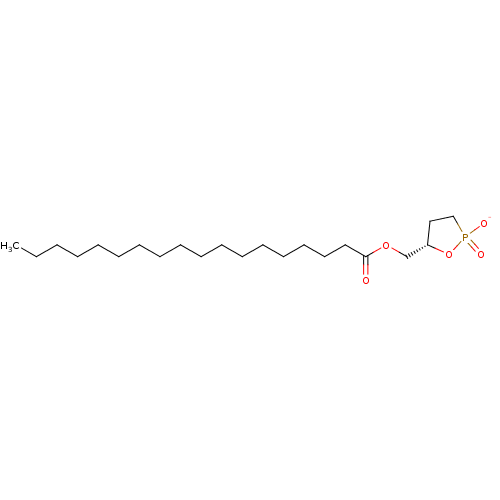 ((S)-carba cyclic-phosphatidic acid | CHEMBL1630084) | PDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tennessee Health Science Center Curated by ChEMBL | Assay Description Inhibition of recombinant ATX mediated hydrolysis of FS-3 | Bioorg Med Chem Lett 20: 7525-8 (2010) Article DOI: 10.1016/j.bmcl.2010.09.115 BindingDB Entry DOI: 10.7270/Q2XD11X1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C delta type (Homo sapiens (Human)) | BDBM50491405 (CHEMBL2381160) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto University Curated by ChEMBL | Assay Description Inhibition of [3H]PDBu binding to PKCdelta-C1A domain peptide (unknown origin) | Bioorg Med Chem 21: 2695-702 (2013) Article DOI: 10.1016/j.bmc.2013.03.013 BindingDB Entry DOI: 10.7270/Q26M39RD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50137956 (Biphenyl-2-carboxylic acid [2-[4-(4-methyl-piperaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V2 receptor using [3H]-AVP as radioligand in rat adrenal medulla | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50137945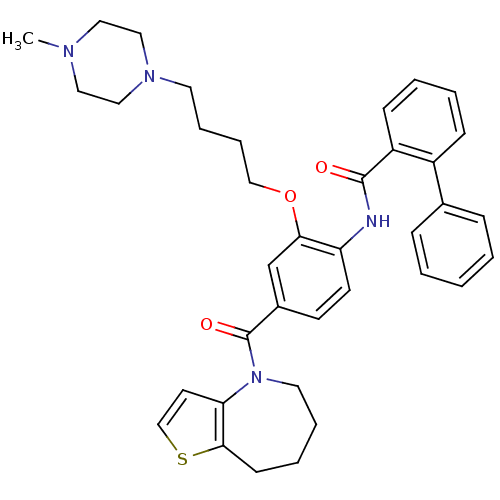 (Biphenyl-2-carboxylic acid [2-[4-(4-methyl-piperaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]-AVP as radioligand in CHO cells | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50137948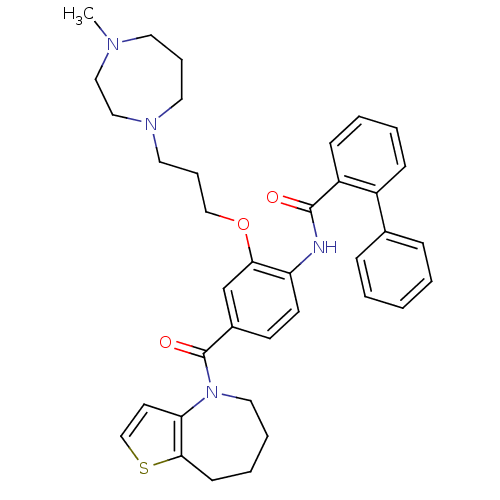 (Biphenyl-2-carboxylic acid [2-[3-(4-methyl-[1,4]di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V2 receptor using [3H]AVP as radioligand in rat adrenal medulla | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (Homo sapiens (Human)) | BDBM50137948 (Biphenyl-2-carboxylic acid [2-[3-(4-methyl-[1,4]di...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in human platelet | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50137948 (Biphenyl-2-carboxylic acid [2-[3-(4-methyl-[1,4]di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]-AVP as radioligand in CHO cells | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50137948 (Biphenyl-2-carboxylic acid [2-[3-(4-methyl-[1,4]di...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in rat liver | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50137949 (Biphenyl-2-carboxylic acid [2-[4-(4-methyl-[1,4]di...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V2 receptor using [3H]-AVP as radioligand in rat adrenal medulla | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50137945 (Biphenyl-2-carboxylic acid [2-[4-(4-methyl-piperaz...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in rat liver | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50137940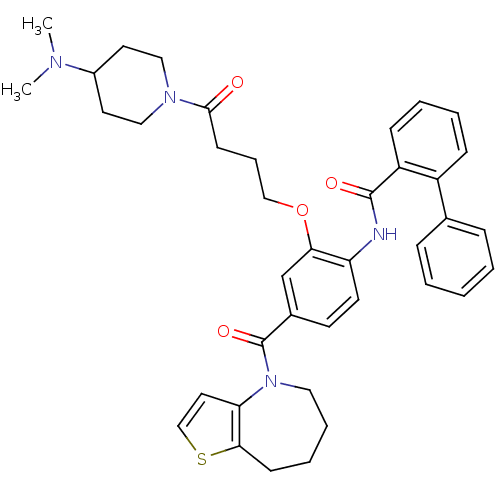 (Biphenyl-2-carboxylic acid [2-[4-(4-dimethylamino-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V2 receptor using [3H]-AVP as radioligand in rat adrenal medulla | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (Homo sapiens (Human)) | BDBM50137940 (Biphenyl-2-carboxylic acid [2-[4-(4-dimethylamino-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in human platelet | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50137953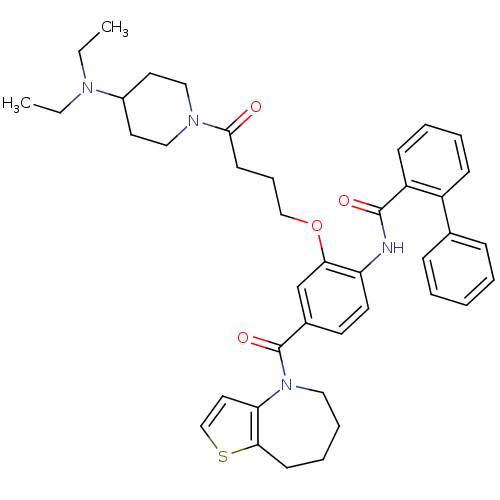 (Biphenyl-2-carboxylic acid [2-[4-(4-diethylamino-p...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in rat liver | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (Homo sapiens (Human)) | BDBM50137953 (Biphenyl-2-carboxylic acid [2-[4-(4-diethylamino-p...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in human platelet | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50137946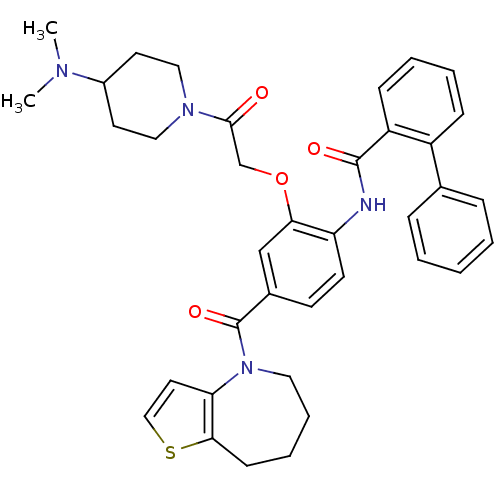 (Biphenyl-2-carboxylic acid [2-[2-(4-dimethylamino-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V2 receptor using [3H]-AVP as radioligand in rat adrenal medulla | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (Homo sapiens (Human)) | BDBM50137951 (Biphenyl-2-carboxylic acid [2-{4-[(2-dimethylamino...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in human platelet | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (Homo sapiens (Human)) | BDBM50137945 (Biphenyl-2-carboxylic acid [2-[4-(4-methyl-piperaz...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in human platelet | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (RAT) | BDBM50137940 (Biphenyl-2-carboxylic acid [2-[4-(4-dimethylamino-...) | MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in rat liver | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50137953 (Biphenyl-2-carboxylic acid [2-[4-(4-diethylamino-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V2 receptor using [3H]-AVP as radioligand in rat adrenal medulla | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50137956 (Biphenyl-2-carboxylic acid [2-[4-(4-methyl-piperaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]-AVP as radioligand in CHO cells | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50137945 (Biphenyl-2-carboxylic acid [2-[4-(4-methyl-piperaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V2 receptor using [3H]-AVP as radioligand in rat adrenal medulla | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50137946 (Biphenyl-2-carboxylic acid [2-[2-(4-dimethylamino-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]-AVP as radioligand in CHO cells | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50137951 (Biphenyl-2-carboxylic acid [2-{4-[(2-dimethylamino...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V2 receptor using [3H]-AVP as radioligand in rat adrenal medulla | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50137953 (Biphenyl-2-carboxylic acid [2-[4-(4-diethylamino-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]-AVP as radioligand in CHO cells | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50137955 (Biphenyl-2-carboxylic acid [4-(8-dimethylamino-5,6...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V2 receptor using [3H]-AVP as radioligand in rat adrenal medulla | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glycogen synthase kinase-3 beta (Homo sapiens (Human)) | BDBM50362881 (CHEMBL1940907) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyorin Pharmaceutical Co., Ltd Curated by ChEMBL | Assay Description Inhibition of human recombinant GSK3-beta after 1 hr by KinaseGlo luciferase assay | Bioorg Med Chem 20: 1188-200 (2012) Article DOI: 10.1016/j.bmc.2011.12.046 BindingDB Entry DOI: 10.7270/Q29W0FXV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50137943 (Biphenyl-2-carboxylic acid (4-{8-[2-(4-methyl-pipe...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V2 receptor using [3H]-AVP as radioligand in rat adrenal medulla | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a/V1b receptor (Homo sapiens (Human)) | BDBM50137942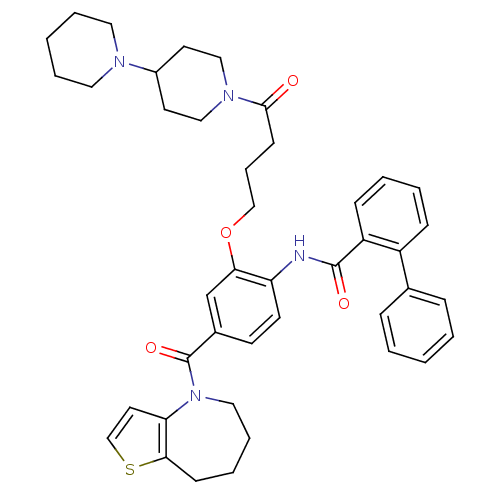 (Biphenyl-2-carboxylic acid [2-(4-[1,4']bipiperidin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against arginine vasopressin V1 receptor using [3H]AVP as radioligand in human platelet | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50137949 (Biphenyl-2-carboxylic acid [2-[4-(4-methyl-[1,4]di...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]-AVP as radioligand in CHO cells | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50204026 (CHEMBL369150) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | <10 | n/a | n/a | n/a | n/a | n/a | n/a |
Central Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human recombinant arginine vasopressin V2 receptor using [3H]AVP as radioligand in CHO cells | J Med Chem 47: 101-9 (2003) Article DOI: 10.1021/jm030287l BindingDB Entry DOI: 10.7270/Q2TX3DT7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 491 total ) | Next | Last >> |