

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein kinase C alpha type (Bos taurus (bovine)) | BDBM50057512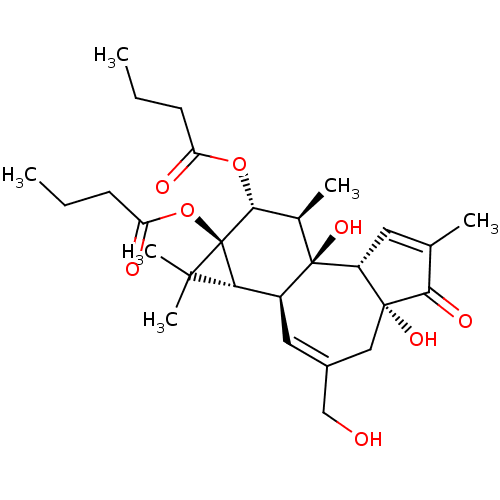 ((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute at Frederick Curated by ChEMBL | Assay Description Ability to displace bound [20-3H]-PDBU from a recombinant single isozyme (PKCalpha) in the presence of phosphatidylserine | J Med Chem 44: 1892-904 (2001) BindingDB Entry DOI: 10.7270/Q2H995WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protein kinase C alpha type (Homo sapiens (Human)) | BDBM50057512 ((1aR,1bS,4aR,7aS,7bS,8R,9R,9aS)-4a,7b-dihydroxy-3-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute Curated by ChEMBL | Assay Description Binding Affinity against protein kinase C alpha | J Med Chem 43: 921-44 (2000) BindingDB Entry DOI: 10.7270/Q2RN372X | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104371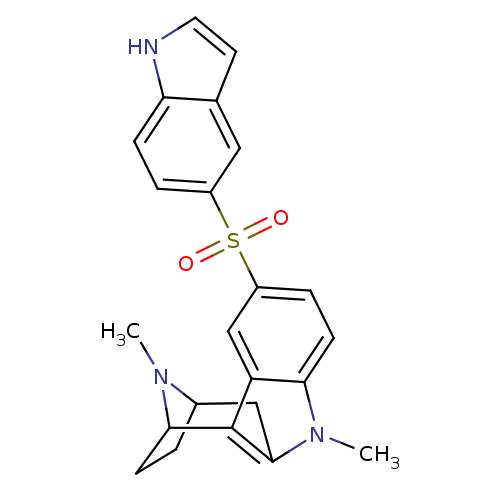 (US8575186, 134/183/184 | US8575186, 199 | US857518...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104408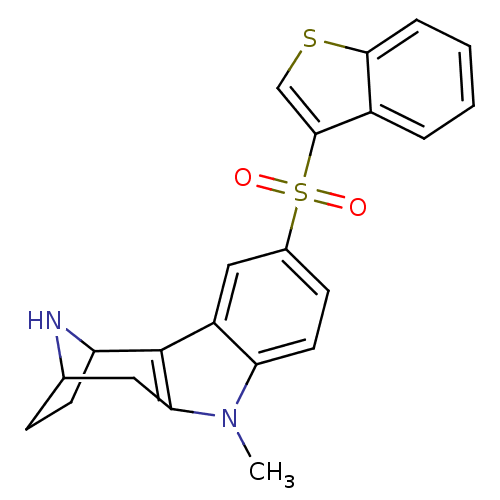 (US8575186, 171/172 | US8575186, 188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104331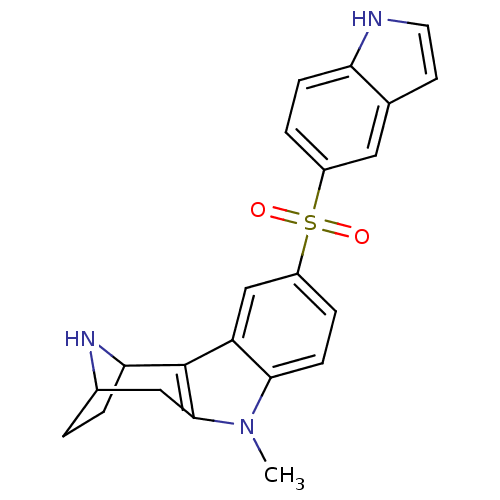 (US8575186, 177 | US8575186, 178 | US8575186, 94/16...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104378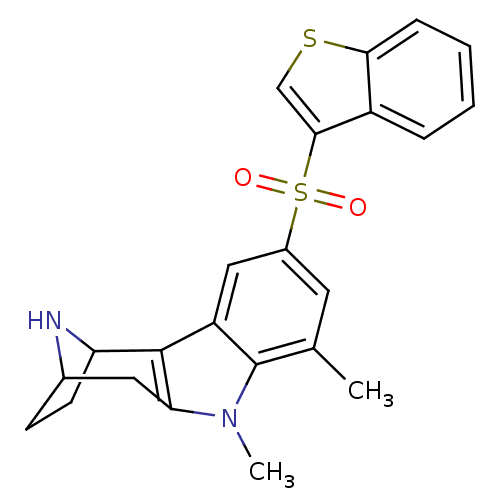 (US8575186, 141) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104467 (US8575186, 230/231 | US8575186, 247) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104382 (US8575186, 145) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50364980 (CHEMBL1950775) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50364980 (CHEMBL1950775) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104331 (US8575186, 177 | US8575186, 178 | US8575186, 94/16...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50364981 (CHEMBL1950776) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104361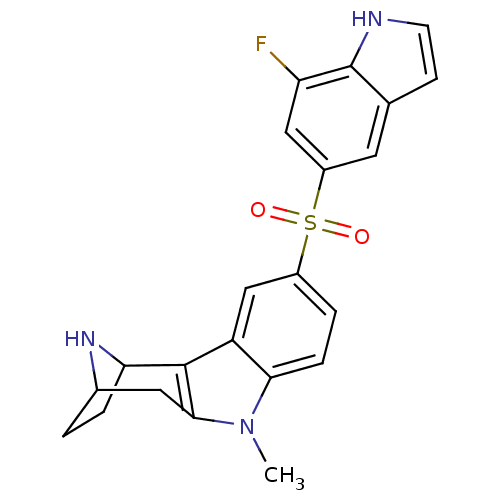 (US8575186, 124) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104371 (US8575186, 134/183/184 | US8575186, 199 | US857518...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104477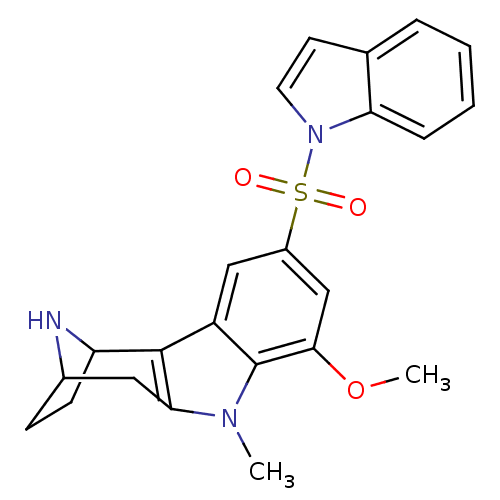 (US8575186, 240/241 | US8575186, 257) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50364981 (CHEMBL1950776) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104408 (US8575186, 171/172 | US8575186, 188) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50364981 (CHEMBL1950776) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50364980 (CHEMBL1950775) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104331 (US8575186, 177 | US8575186, 178 | US8575186, 94/16...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104402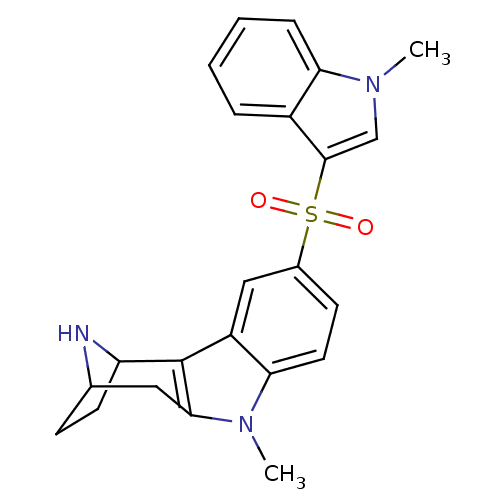 (US8575186, 165/166 | US8575186, 182) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104321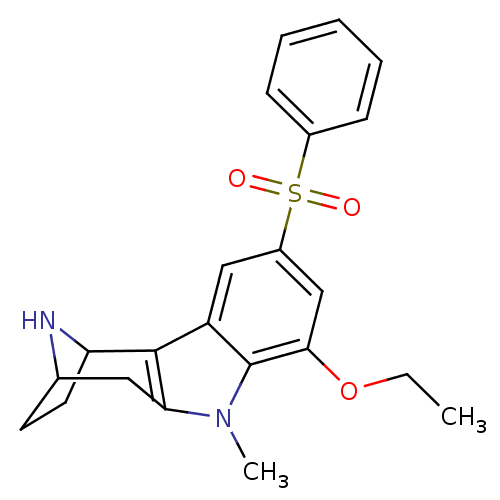 (US8575186, 250 | US8575186, 251 | US8575186, 84/23...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104371 (US8575186, 134/183/184 | US8575186, 199 | US857518...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104475 (US8575186, 238/239 | US8575186, 255) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104454 (US8575186, 217/218 | US8575186, 234) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104376 (US8575186, 139) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104360 (US8575186, 123) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104430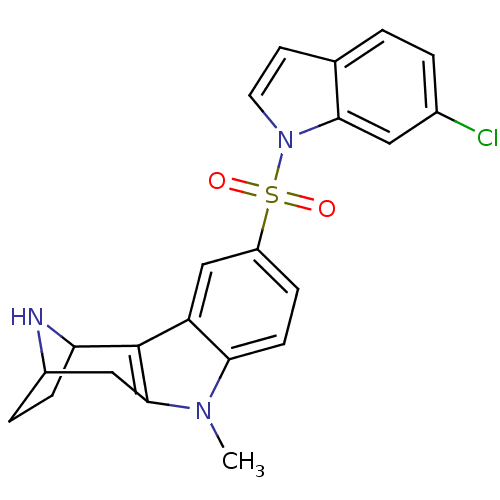 (US8575186, 193/194 | US8575186, 210) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104456 (US8575186, 219/220 | US8575186, 236) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104302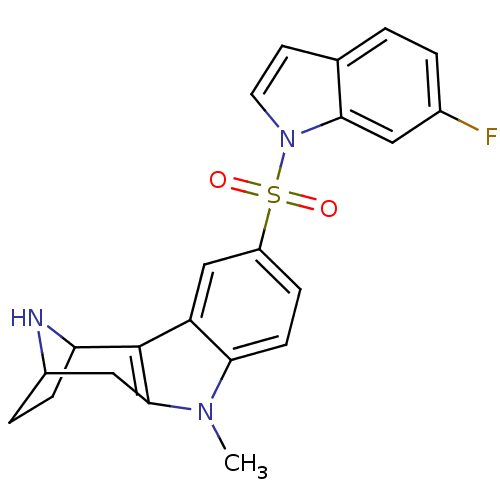 (US8575186, 203 | US8575186, 204 | US8575186, 65/18...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104462 (US8575186, 225/226 | US8575186, 242) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104446 (US8575186, 209/215/216 | US8575186, 231 | US857518...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104377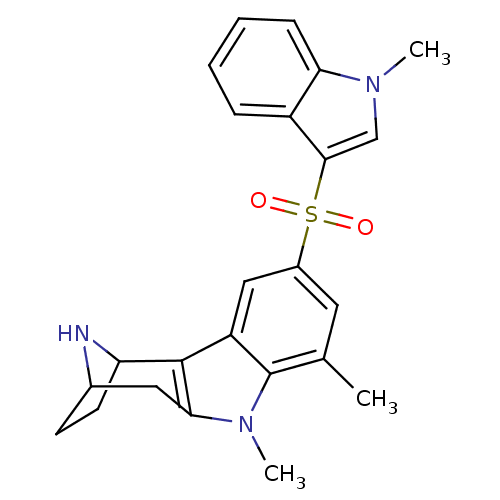 (US8575186, 140) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104458 (US8575186, 221/222 | US8575186, 238) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104320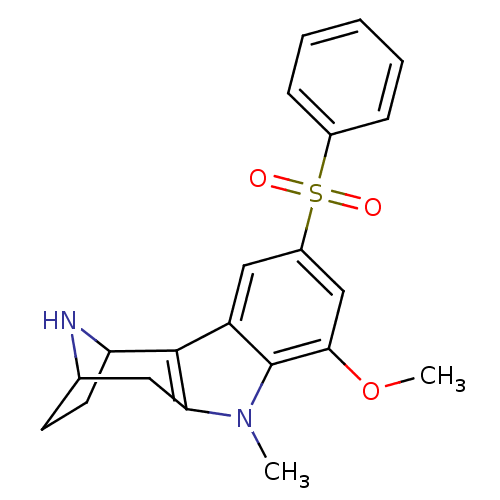 (US8575186, 228 | US8575186, 229 | US8575186, 83/21...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104479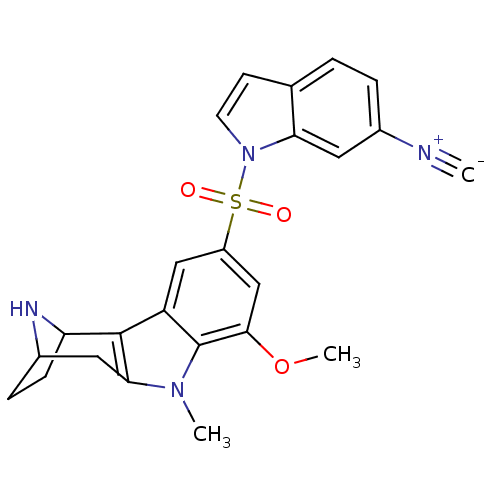 (US8575186, 242/243 | US8575186, 259) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104302 (US8575186, 203 | US8575186, 204 | US8575186, 65/18...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104464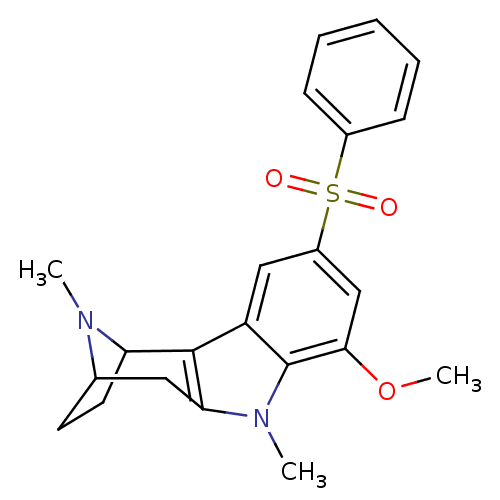 (US8575186, 227) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104299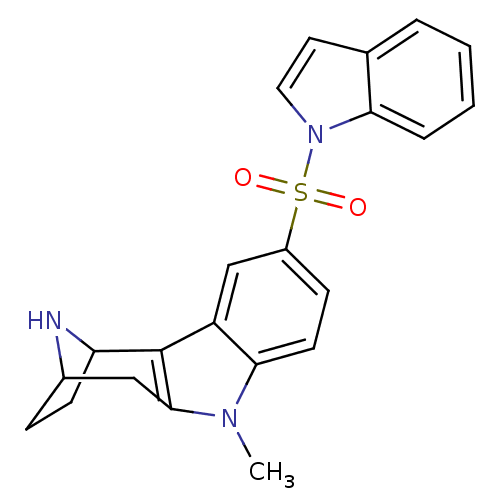 (US8575186, 205 | US8575186, 206 | US8575186, 62/18...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104444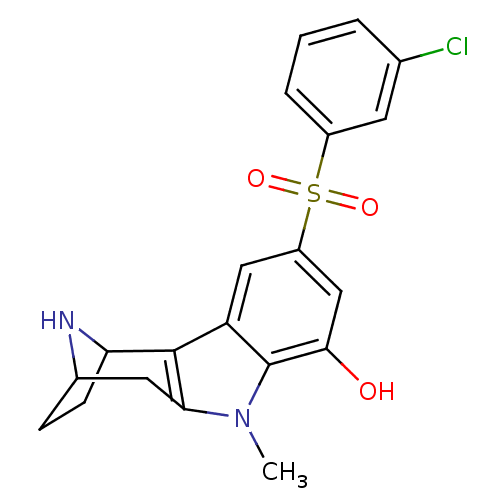 (US8575186, 207/208 | US8575186, 224) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104339 (US8575186, 102) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104346 (US8575186, 109) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104438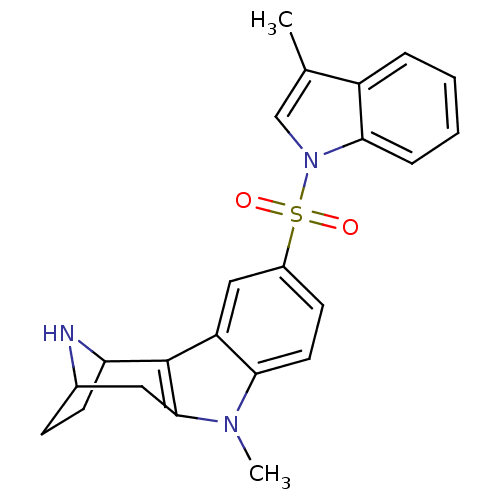 (US8575186, 201/202 | US8575186, 218) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104335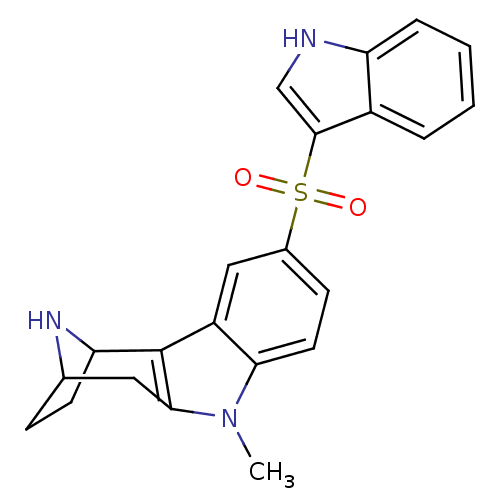 (US8575186, 179 | US8575186, 180 | US8575186, 98/16...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104364 (US8575186, 127/199/200 | US8575186, 215 | US857518...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104422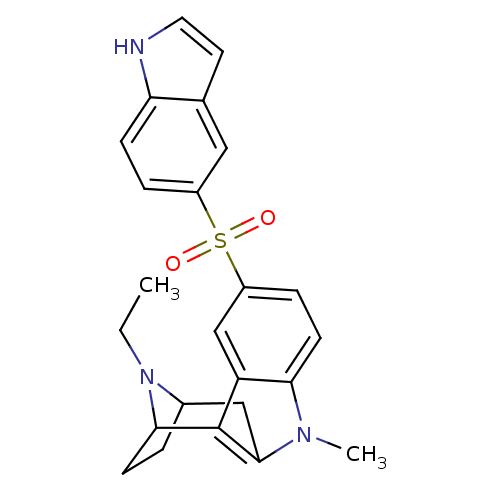 (US8575186, 185/186 | US8575186, 202) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104446 (US8575186, 209/215/216 | US8575186, 231 | US857518...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50365005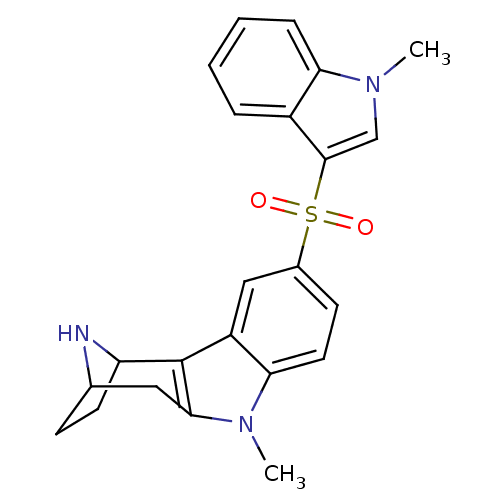 (CHEMBL1950777) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM104402 (US8575186, 165/166 | US8575186, 182) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Albany Molecular Research, Inc. US Patent | Assay Description The relative affinities of the various compounds for the 5-HT6 receptor were measured in a radioligand binding assay, using a sintillation proximity ... | US Patent US8575186 (2013) BindingDB Entry DOI: 10.7270/Q2BR8QTS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 6 (Homo sapiens (Human)) | BDBM50364978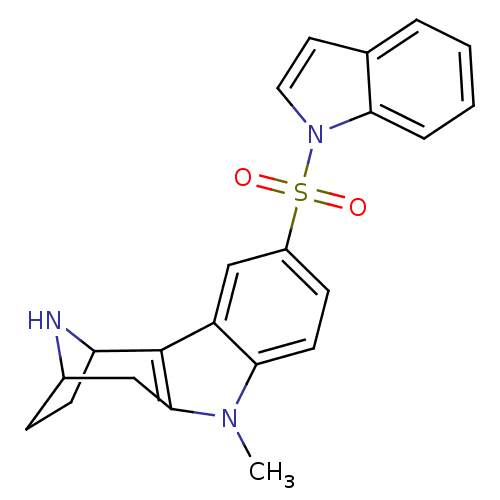 (CHEMBL1950773) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI Curated by ChEMBL | Assay Description Displacement of [3H]LSD from human 5-HT6 serotonin receptor by scintillation proximity assay | Bioorg Med Chem Lett 22: 1494-8 (2012) Article DOI: 10.1016/j.bmcl.2012.01.022 BindingDB Entry DOI: 10.7270/Q2RB753Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1386 total ) | Next | Last >> |