

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inositol phosphorylceramide synthase (Candida albicans) | BDBM50408926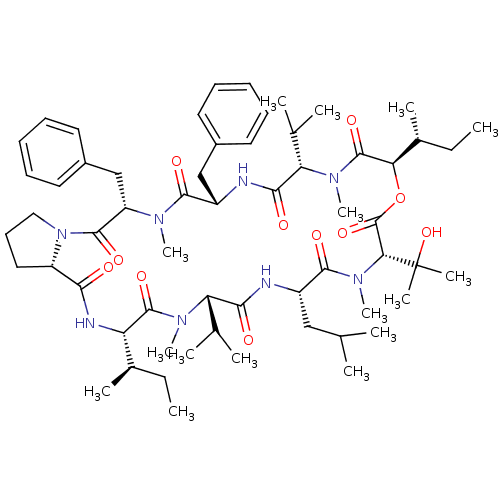 (Aureobasidin A | CHEMBL1793802) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp. Curated by ChEMBL | Assay Description Inhibition of Candida albicans ATCC 38247 inositol phosphorylceramide synthase preincubated for 30 mins | Antimicrob Agents Chemother 53: 496-504 (2009) Article DOI: 10.1128/AAC.00633-08 BindingDB Entry DOI: 10.7270/Q27H1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol phosphorylceramide synthase catalytic subunit AUR1 (Saccharomyces cerevisiae S288c) | BDBM50408926 (Aureobasidin A | CHEMBL1793802) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp. Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae SJ21R inositol phosphorylceramide synthase preincubated for 30 mins | Antimicrob Agents Chemother 53: 496-504 (2009) Article DOI: 10.1128/AAC.00633-08 BindingDB Entry DOI: 10.7270/Q27H1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol phosphorylceramide synthase (Candida albicans) | BDBM50333121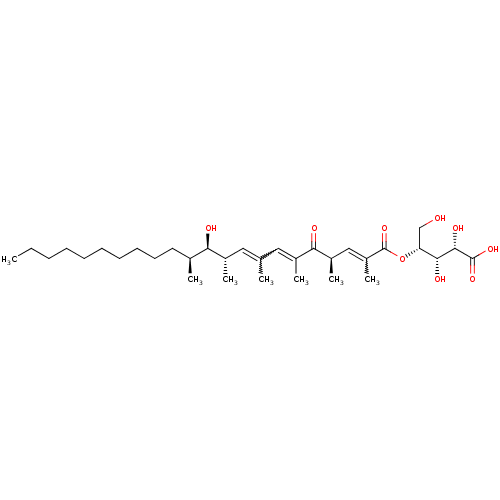 (CHEMBL1631582 | Khafrefungin) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp. Curated by ChEMBL | Assay Description Inhibition of Candida albicans ATCC 38247 inositol phosphorylceramide synthase preincubated for 30 mins | Antimicrob Agents Chemother 53: 496-504 (2009) Article DOI: 10.1128/AAC.00633-08 BindingDB Entry DOI: 10.7270/Q27H1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol phosphorylceramide synthase (Candida albicans) | BDBM50408926 (Aureobasidin A | CHEMBL1793802) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp. Curated by ChEMBL | Assay Description Inhibition of Candida albicans inositol phosphorylceramide synthase | Antimicrob Agents Chemother 53: 496-504 (2009) Article DOI: 10.1128/AAC.00633-08 BindingDB Entry DOI: 10.7270/Q27H1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol phosphorylceramide synthase (Candida albicans) | BDBM50333120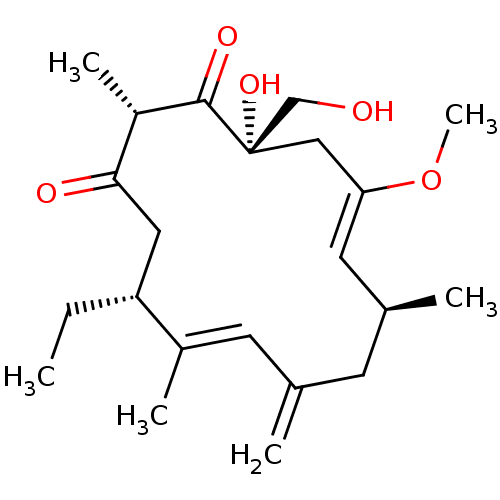 (CHEMBL1631581 | Rustmicin) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp. Curated by ChEMBL | Assay Description Inhibition of Candida albicans ATCC 38247 inositol phosphorylceramide synthase preincubated for 30 mins | Antimicrob Agents Chemother 53: 496-504 (2009) Article DOI: 10.1128/AAC.00633-08 BindingDB Entry DOI: 10.7270/Q27H1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol phosphorylceramide synthase catalytic subunit AUR1 (Saccharomyces cerevisiae S288c) | BDBM50408926 (Aureobasidin A | CHEMBL1793802) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp. Curated by ChEMBL | Assay Description Inhibition of aureobasidin A-resistant Saccharomyces cerevisiae inositol phosphorylceramide synthase preincubated for 30 mins | Antimicrob Agents Chemother 53: 496-504 (2009) Article DOI: 10.1128/AAC.00633-08 BindingDB Entry DOI: 10.7270/Q27H1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol phosphorylceramide synthase (Candida albicans) | BDBM50333124 ((R)-2-((3,4-dimethoxyphenethyl)(methyl)amino)-2-ox...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp. Curated by ChEMBL | Assay Description Inhibition of Candida albicans ATCC 38247 inositol phosphorylceramide synthase preincubated for 30 mins | Antimicrob Agents Chemother 53: 496-504 (2009) Article DOI: 10.1128/AAC.00633-08 BindingDB Entry DOI: 10.7270/Q27H1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol phosphorylceramide synthase (Candida albicans) | BDBM50333123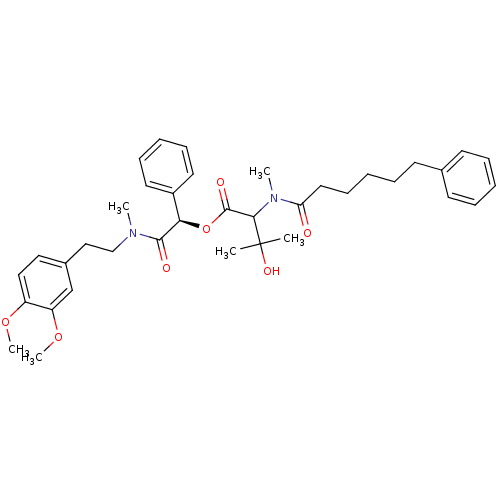 ((R)-2-((3,4-dimethoxyphenethyl)(methyl)amino)-2-ox...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.05E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp. Curated by ChEMBL | Assay Description Inhibition of Candida albicans ATCC 38247 inositol phosphorylceramide synthase preincubated for 30 mins | Antimicrob Agents Chemother 53: 496-504 (2009) Article DOI: 10.1128/AAC.00633-08 BindingDB Entry DOI: 10.7270/Q27H1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol phosphorylceramide synthase (Candida albicans) | BDBM50333122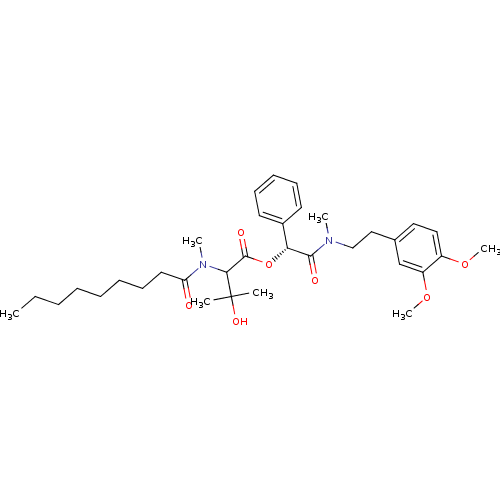 ((R)-2-((3,4-dimethoxyphenethyl)(methyl)amino)-2-ox...) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp. Curated by ChEMBL | Assay Description Inhibition of Candida albicans ATCC 38247 inositol phosphorylceramide synthase preincubated for 30 mins | Antimicrob Agents Chemother 53: 496-504 (2009) Article DOI: 10.1128/AAC.00633-08 BindingDB Entry DOI: 10.7270/Q27H1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237417 (CHEMBL4077280) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of SPHK1 in human MDA1483 cells using C17-sphingosine as substrate assessed as reduction in C17-S1P formation preincubated for 30 mins wit... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237435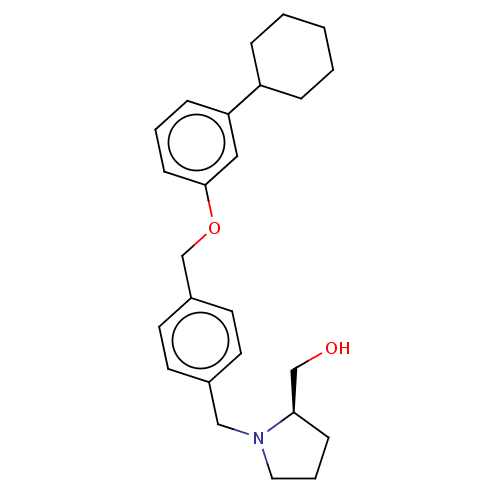 (CHEMBL4066270) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human progesterone receptor | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237418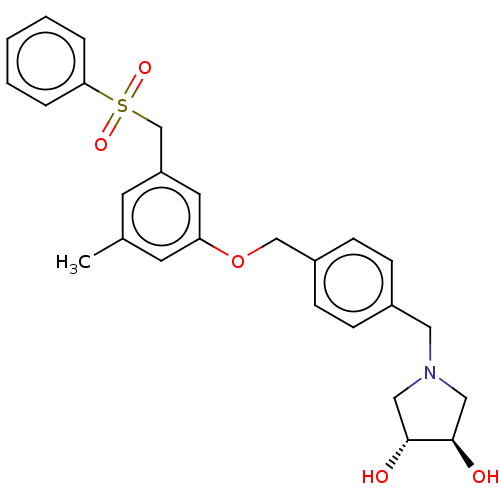 (CHEMBL4061789) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of SPHK1 in human MDA1483 cells using C17-sphingosine as substrate assessed as reduction in C17-S1P formation preincubated for 30 mins wit... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol phosphorylceramide synthase (Candida albicans) | BDBM50408926 (Aureobasidin A | CHEMBL1793802) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp. Curated by ChEMBL | Assay Description Inhibition of Candida albicans inositol phosphorylceramide synthase | Antimicrob Agents Chemother 53: 496-504 (2009) Article DOI: 10.1128/AAC.00633-08 BindingDB Entry DOI: 10.7270/Q27H1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol phosphorylceramide synthase catalytic subunit AUR1 (Saccharomyces cerevisiae S288c) | BDBM50408926 (Aureobasidin A | CHEMBL1793802) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp. Curated by ChEMBL | Assay Description Inhibition of Saccharomyces cerevisiae inositol phosphorylceramide synthase | Antimicrob Agents Chemother 53: 496-504 (2009) Article DOI: 10.1128/AAC.00633-08 BindingDB Entry DOI: 10.7270/Q27H1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aureobasidin-resistance protein (Neosartorya fumigata) | BDBM50408926 (Aureobasidin A | CHEMBL1793802) | UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp. Curated by ChEMBL | Assay Description Inhibition of Aspergillus fumigatus inositol phosphorylceramide synthase | Antimicrob Agents Chemother 53: 496-504 (2009) Article DOI: 10.1128/AAC.00633-08 BindingDB Entry DOI: 10.7270/Q27H1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237420 (CHEMBL4090361) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of SPHK1 in human MDA1483 cells using C17-sphingosine as substrate assessed as reduction in C17-S1P formation preincubated for 30 mins wit... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237422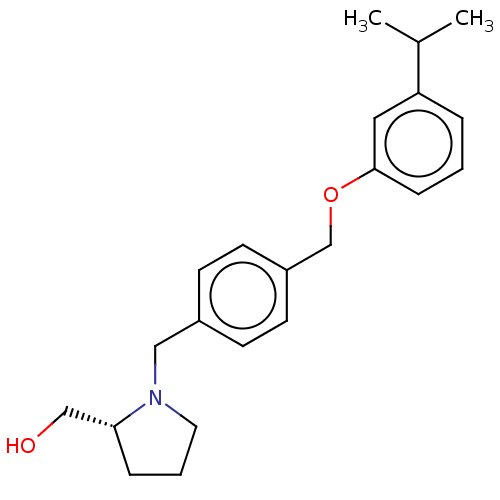 (CHEMBL4086094) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of SPHK1 in human MDA1483 cells using C17-sphingosine as substrate assessed as reduction in C17-S1P formation preincubated for 30 mins wit... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol phosphorylceramide synthase (Candida albicans) | BDBM50333121 (CHEMBL1631582 | Khafrefungin) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp. Curated by ChEMBL | Assay Description Inhibition of Candida albicans inositol phosphorylceramide synthase | Antimicrob Agents Chemother 53: 496-504 (2009) Article DOI: 10.1128/AAC.00633-08 BindingDB Entry DOI: 10.7270/Q27H1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237435 (CHEMBL4066270) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity at human progesterone receptor. | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237422 (CHEMBL4086094) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged SPHK1 expressed in fall armyworm sf9 cells assessed as reduction in ADP formation using sphingosine as sub... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50041978 (CHEMBL3134157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of SPHK1 in human MDA1483 cells using C17-sphingosine as substrate assessed as reduction in C17-S1P formation preincubated for 30 mins wit... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237431 (CHEMBL4063424) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of SPHK1 in human MDA1483 cells using C17-sphingosine as substrate assessed as reduction in C17-S1P formation preincubated for 30 mins wit... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237435 (CHEMBL4066270) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of SPHK1 in human whole-blood using C17-sphingosine as substrate assessed as reduction in C17-S1P production preincubated for 30 mins foll... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237421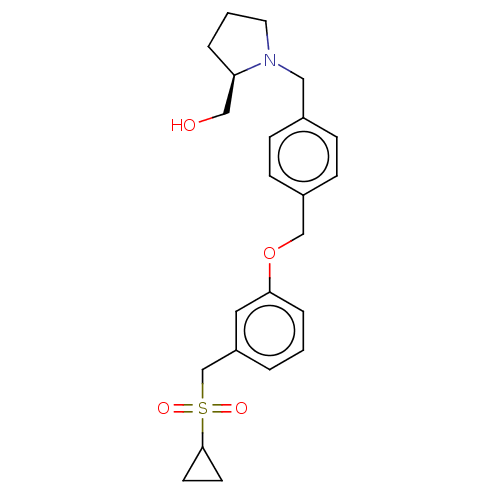 (CHEMBL4069327) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of SPHK1 in human MDA1483 cells using C17-sphingosine as substrate assessed as reduction in C17-S1P formation preincubated for 30 mins wit... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237423 (CHEMBL4104017) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of SPHK1 in human MDA1483 cells using C17-sphingosine as substrate assessed as reduction in C17-S1P formation preincubated for 30 mins wit... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237435 (CHEMBL4066270) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged SPHK1 expressed in fall armyworm sf9 cells assessed as reduction in S1P formation using sphingosine as sub... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50237435 (CHEMBL4066270) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of SPHK2 (unknown origin) by FITC-based caliper assay | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50237422 (CHEMBL4086094) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | <1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of SPHK2 (unknown origin) by FITC-based caliper assay | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237418 (CHEMBL4061789) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged SPHK1 expressed in fall armyworm sf9 cells assessed as reduction in S1P formation using sphingosine as sub... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237418 (CHEMBL4061789) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged SPHK1 expressed in fall armyworm sf9 cells assessed as reduction in ADP formation using sphingosine as sub... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50041978 (CHEMBL3134157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged SPHK1 expressed in fall armyworm sf9 cells assessed as reduction in ADP formation using sphingosine as sub... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237420 (CHEMBL4090361) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged SPHK1 expressed in fall armyworm sf9 cells assessed as reduction in ADP formation using sphingosine as sub... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 2 (Homo sapiens (Human)) | BDBM50237423 (CHEMBL4104017) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of SPHK2 (unknown origin) by FITC-based caliper assay | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237420 (CHEMBL4090361) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged SPHK1 expressed in fall armyworm sf9 cells assessed as reduction in S1P formation using sphingosine as sub... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237417 (CHEMBL4077280) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged SPHK1 expressed in fall armyworm sf9 cells assessed as reduction in S1P formation using sphingosine as sub... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50041978 (CHEMBL3134157) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Effective concentration for human progesterone receptor in T47D human breast cancer cell | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237417 (CHEMBL4077280) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged SPHK1 expressed in fall armyworm sf9 cells assessed as reduction in ADP formation using sphingosine as sub... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237419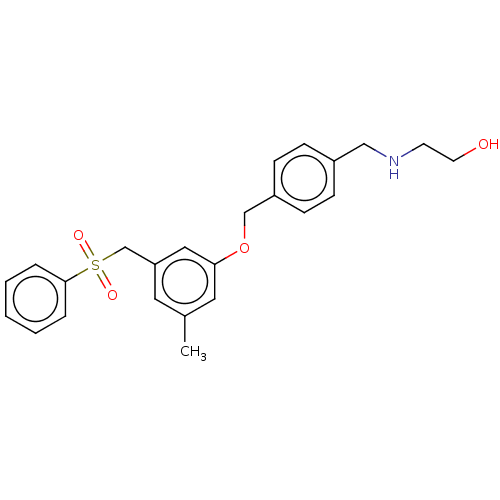 (CHEMBL4103414) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of SPHK1 in human MDA1483 cells using C17-sphingosine as substrate assessed as reduction in C17-S1P formation preincubated for 30 mins wit... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol phosphorylceramide synthase (Candida albicans) | BDBM50333120 (CHEMBL1631581 | Rustmicin) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp. Curated by ChEMBL | Assay Description Reversible inhibition of Candida albicans inositol phosphorylceramide synthase | Antimicrob Agents Chemother 53: 496-504 (2009) Article DOI: 10.1128/AAC.00633-08 BindingDB Entry DOI: 10.7270/Q27H1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Inositol phosphorylceramide synthase catalytic subunit AUR1 (Saccharomyces cerevisiae S288c) | BDBM50333120 (CHEMBL1631581 | Rustmicin) | KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pharmacia Corp. Curated by ChEMBL | Assay Description Reversible inhibition of Saccharomyces cerevisiae inositol phosphorylceramide synthase | Antimicrob Agents Chemother 53: 496-504 (2009) Article DOI: 10.1128/AAC.00633-08 BindingDB Entry DOI: 10.7270/Q27H1JV5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237431 (CHEMBL4063424) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged SPHK1 expressed in fall armyworm sf9 cells assessed as reduction in ADP formation using sphingosine as sub... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237431 (CHEMBL4063424) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Antagonistic activity at human progesterone receptor in CV-1 cells. | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237436 (CHEMBL4086161) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of SPHK1 in human MDA1483 cells using C17-sphingosine as substrate assessed as reduction in C17-S1P formation preincubated for 30 mins wit... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237426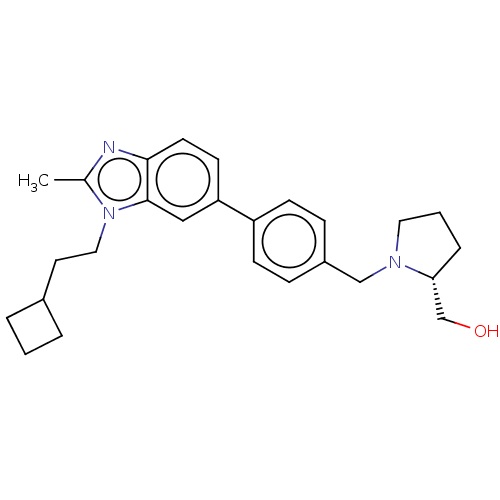 (CHEMBL4091588) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Effective concentration for agonist activity towards human progesterone receptor (hPR) using the cotransfection assay in CV-1 cells | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237423 (CHEMBL4104017) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged SPHK1 expressed in fall armyworm sf9 cells assessed as reduction in ADP formation using sphingosine as sub... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237422 (CHEMBL4086094) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged SPHK1 expressed in fall armyworm sf9 cells assessed as reduction in S1P formation using sphingosine as sub... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237417 (CHEMBL4077280) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Binding affinity to human progesterone receptor | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237419 (CHEMBL4103414) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged SPHK1 expressed in fall armyworm sf9 cells assessed as reduction in ADP formation using sphingosine as sub... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237418 (CHEMBL4061789) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of SPHK1 in human whole-blood using C17-sphingosine as substrate assessed as reduction in C17-S1P production preincubated for 30 mins foll... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine kinase 1 (Homo sapiens (Human)) | BDBM50237436 (CHEMBL4086161) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human C-terminal His6-tagged SPHK1 expressed in fall armyworm sf9 cells assessed as reduction in ADP formation using sphingosine as sub... | J Med Chem 60: 2562-2572 (2017) Article DOI: 10.1021/acs.jmedchem.7b00070 BindingDB Entry DOI: 10.7270/Q27P91PG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 158 total ) | Next | Last >> |