

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenylate cyclase type 4 (Homo sapiens (Human)) | BDBM50226415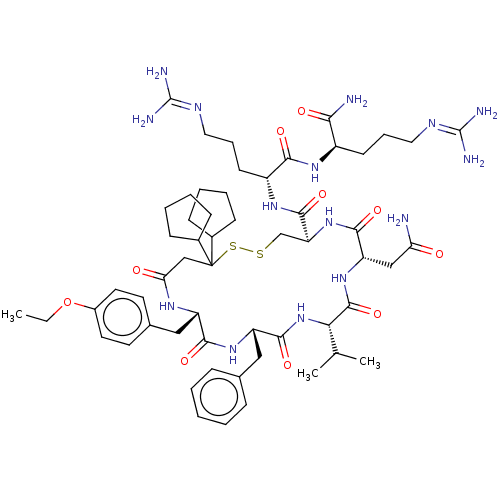 (CHEMBL3142312) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Division Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone | J Med Chem 30: 2291-4 (1987) BindingDB Entry DOI: 10.7270/Q2MG7RRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020671 (13-(2-Amino-ethyl)-19-benzyl-22-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of LVP stimulated adenylate cyclase activity in pig kidney medullary membrane | J Med Chem 29: 984-8 (1986) BindingDB Entry DOI: 10.7270/Q27M06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 4 (Homo sapiens (Human)) | BDBM50226410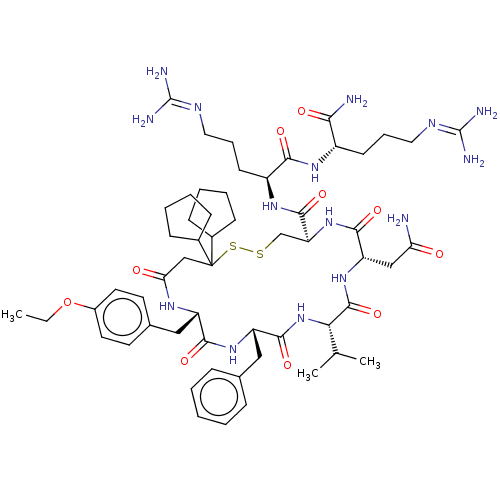 (CHEMBL3142318) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Division Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone | J Med Chem 30: 2291-4 (1987) BindingDB Entry DOI: 10.7270/Q2MG7RRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 4 (Homo sapiens (Human)) | BDBM50226412 (CHEMBL3142332) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Division Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone | J Med Chem 30: 2291-4 (1987) BindingDB Entry DOI: 10.7270/Q2MG7RRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 4 (Homo sapiens (Human)) | BDBM50226411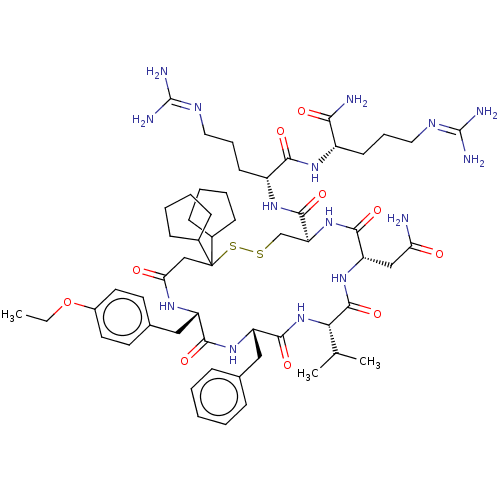 (CHEMBL3142329) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Division Curated by ChEMBL | Assay Description Binding affinity against sigma receptor | J Med Chem 30: 2291-4 (1987) BindingDB Entry DOI: 10.7270/Q2MG7RRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020669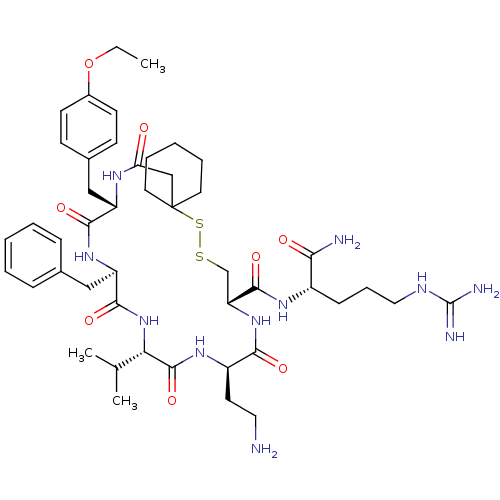 (13-(2-Amino-ethyl)-19-benzyl-22-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of LVP stimulated adenylate cyclase activity in pig kidney medullary membrane | J Med Chem 29: 984-8 (1986) BindingDB Entry DOI: 10.7270/Q27M06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 4 (Homo sapiens (Human)) | BDBM50226417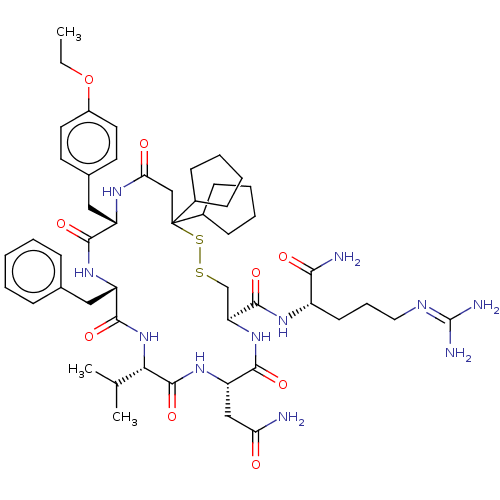 (CHEMBL3142331) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Division Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone | J Med Chem 30: 2291-4 (1987) BindingDB Entry DOI: 10.7270/Q2MG7RRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 4 (Homo sapiens (Human)) | BDBM50226413 (CHEMBL2369777) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Division Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone | J Med Chem 30: 2291-4 (1987) BindingDB Entry DOI: 10.7270/Q2MG7RRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020665 (13-(2-Amino-ethyl)-19-benzyl-22-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of LVP stimulated adenylate cyclase activity in pig kidney medullary membrane | J Med Chem 29: 984-8 (1986) BindingDB Entry DOI: 10.7270/Q27M06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 4 (Homo sapiens (Human)) | BDBM50226416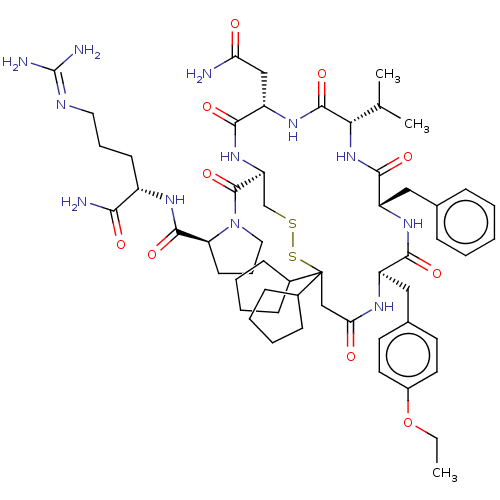 (CHEMBL2369525) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Division Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone | J Med Chem 30: 2291-4 (1987) BindingDB Entry DOI: 10.7270/Q2MG7RRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020654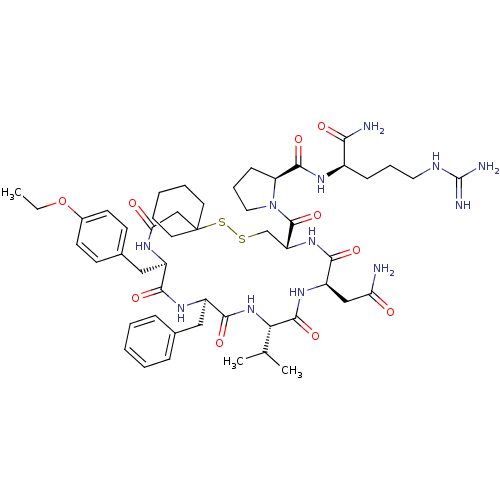 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition against V2 vasopressin receptor in pig renal medullary membrane preparations. | J Med Chem 29: 2425-6 (1987) BindingDB Entry DOI: 10.7270/Q23T9G6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020667 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of LVP stimulated adenylate cyclase activity in pig kidney medullary membrane | J Med Chem 29: 984-8 (1986) BindingDB Entry DOI: 10.7270/Q27M06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 4 (Homo sapiens (Human)) | BDBM50226414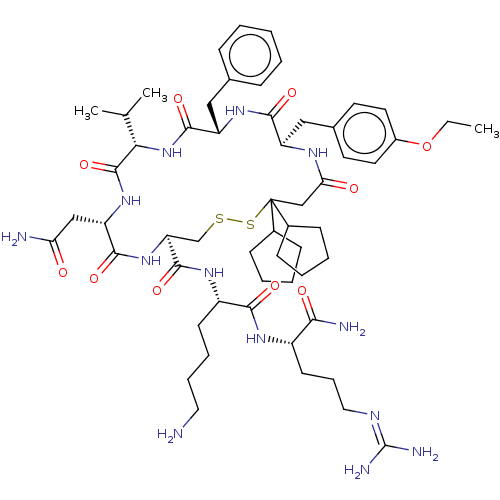 (CHEMBL2369778) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Division Curated by ChEMBL | Assay Description Binding affinity against sigma receptor | J Med Chem 30: 2291-4 (1987) BindingDB Entry DOI: 10.7270/Q2MG7RRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020675 (1-[13-Benzyl-7-carbamoylmethyl-16-(4-ethoxy-benzyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition against V2 vasopressin receptor in pig renal medullary membrane preparations. | J Med Chem 29: 2425-6 (1987) BindingDB Entry DOI: 10.7270/Q23T9G6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020666 (13-(2-Amino-ethyl)-19-benzyl-22-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of LVP stimulated adenylate cyclase activity in pig kidney medullary membrane | J Med Chem 29: 984-8 (1986) BindingDB Entry DOI: 10.7270/Q27M06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020673 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of LVP stimulated adenylate cyclase activity in pig kidney medullary membrane | J Med Chem 29: 984-8 (1986) BindingDB Entry DOI: 10.7270/Q27M06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020653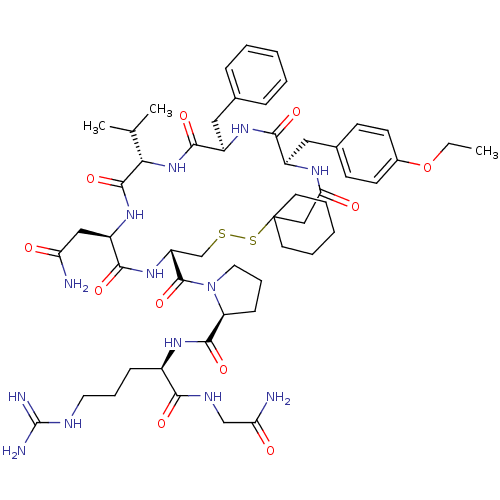 (1-[19-Benzyl-13-carbamoylmethyl-22-(4-ethoxy-benzy...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition against V2 vasopressin receptor in pig renal medullary membrane preparations. | J Med Chem 29: 2425-6 (1987) BindingDB Entry DOI: 10.7270/Q23T9G6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenylate cyclase type 4 (Homo sapiens (Human)) | BDBM50226418 (CHEMBL3142313) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Research and Development Division Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone | J Med Chem 30: 2291-4 (1987) BindingDB Entry DOI: 10.7270/Q2MG7RRN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020674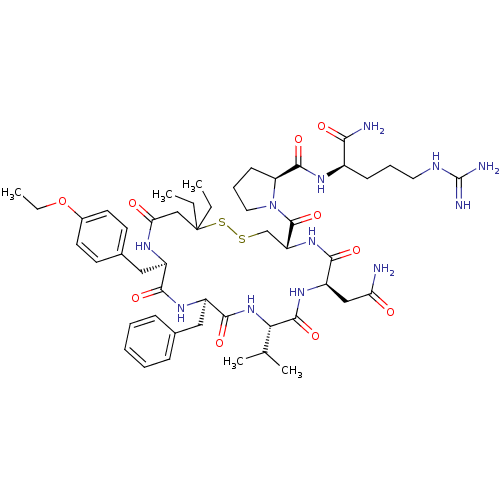 (1-[13-Benzyl-7-carbamoylmethyl-16-(4-ethoxy-benzyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition against V2 vasopressin receptor in pig renal medullary membrane preparations. | J Med Chem 29: 2425-6 (1987) BindingDB Entry DOI: 10.7270/Q23T9G6Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020672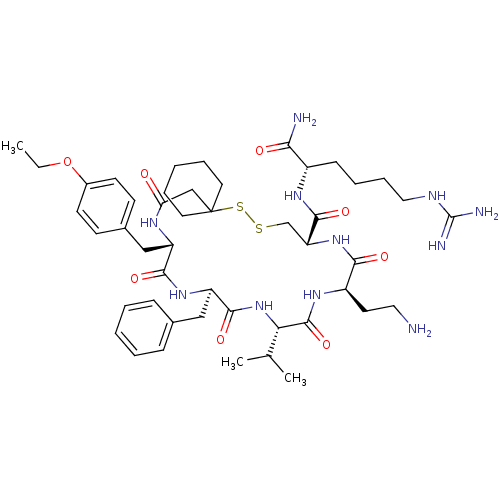 (13-(2-Amino-ethyl)-19-benzyl-22-(4-ethoxy-benzyl)-...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of LVP stimulated adenylate cyclase activity in pig kidney medullary membrane | J Med Chem 29: 984-8 (1986) BindingDB Entry DOI: 10.7270/Q27M06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020668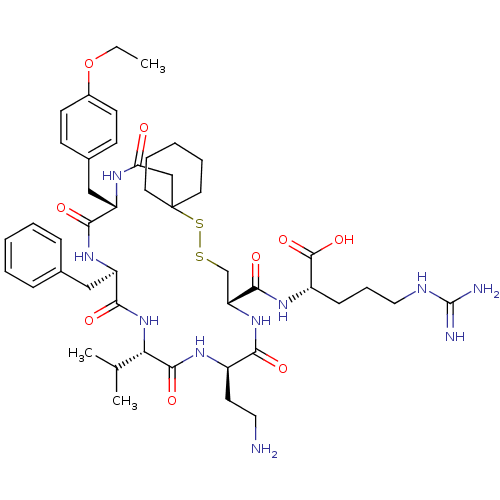 (2-{[13-(2-Amino-ethyl)-19-benzyl-22-(4-ethoxy-benz...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of LVP stimulated adenylate cyclase activity in pig kidney medullary membrane | J Med Chem 29: 984-8 (1986) BindingDB Entry DOI: 10.7270/Q27M06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of human AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition by Lineweaver-B... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Vasopressin V2 receptor (Sus scrofa) | BDBM50020670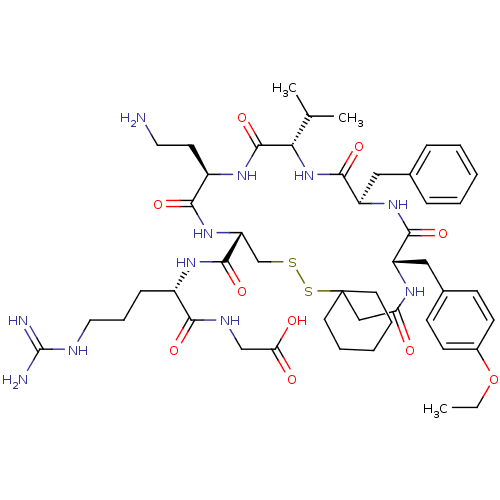 ((2-{[13-(2-Amino-ethyl)-19-benzyl-22-(4-ethoxy-ben...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of LVP stimulated adenylate cyclase activity in pig kidney medullary membrane | J Med Chem 29: 984-8 (1986) BindingDB Entry DOI: 10.7270/Q27M06W4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50462868 (CHEMBL4251246) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Mixed-type inhibition of Helicobacter pylori ATCC 43504 urease assessed as enzyme-inhibitor complex using urea as substrate preincubated for 1.5 hrs | Bioorg Med Chem 26: 4145-4152 (2018) Article DOI: 10.1016/j.bmc.2018.07.003 BindingDB Entry DOI: 10.7270/Q2N87DFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urease subunit beta (Helicobacter pylori (strain ATCC 700392 / 26695) (...) | BDBM50462868 (CHEMBL4251246) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Jishou University Curated by ChEMBL | Assay Description Mixed-type inhibition of Helicobacter pylori ATCC 43504 urease assessed as enzyme-substrate-inhibitor complex using urea as substrate preincubated fo... | Bioorg Med Chem 26: 4145-4152 (2018) Article DOI: 10.1016/j.bmc.2018.07.003 BindingDB Entry DOI: 10.7270/Q2N87DFQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 175 | -38.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | Biochemistry 41: 10810-8 (2002) Article DOI: 10.1021/bi020151+ BindingDB Entry DOI: 10.7270/Q2HQ3X52 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Induced myeloid leukemia cell differentiation protein Mcl-1 (Homo sapiens (Human)) | BDBM50196032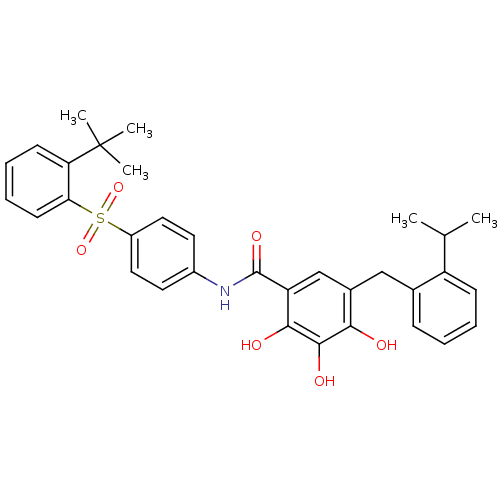 ((N-[(2-tert-butylbenzenesulfonyl)phenyl]-2,3,4-tri...) | PDB MMDB NCI pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FAM-Bid BH3 peptide binding to recombinant human MCL1 by fluorescence polarization assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112201 BindingDB Entry DOI: 10.7270/Q26D5XMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Apoptosis regulator Bcl-2 (Homo sapiens (Human)) | BDBM50196032 ((N-[(2-tert-butylbenzenesulfonyl)phenyl]-2,3,4-tri...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FAM-Bid BH3 peptide binding to recombinant human BCL2 by fluorescence polarization assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112201 BindingDB Entry DOI: 10.7270/Q26D5XMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10632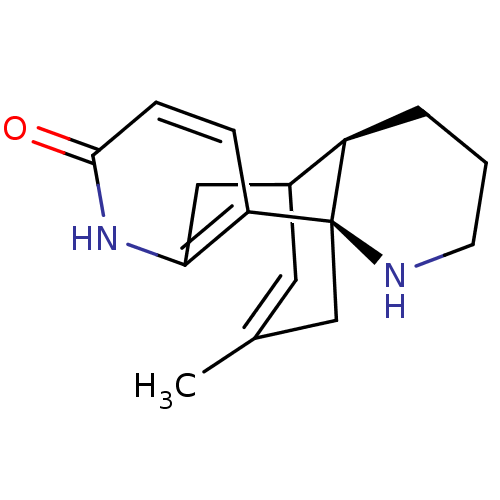 ((-)-Huperzine B | (1R,10R)-16-methyl-6,14-diazatet...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 334 | -37.0 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | Biochemistry 41: 10810-8 (2002) Article DOI: 10.1021/bi020151+ BindingDB Entry DOI: 10.7270/Q2HQ3X52 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Bcl-2-like protein 1 (Homo sapiens (Human)) | BDBM50196032 ((N-[(2-tert-butylbenzenesulfonyl)phenyl]-2,3,4-tri...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of FAM-Bid BH3 peptide binding to recombinant human BCL-XL by fluorescence polarization assay | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112201 BindingDB Entry DOI: 10.7270/Q26D5XMR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469009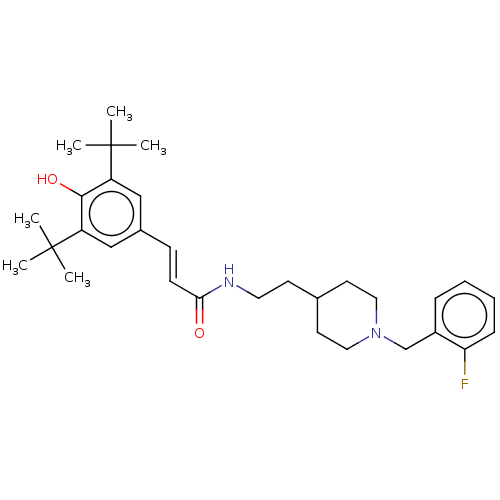 (CHEMBL4292766) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of human AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition by Lineweaver-B... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50072297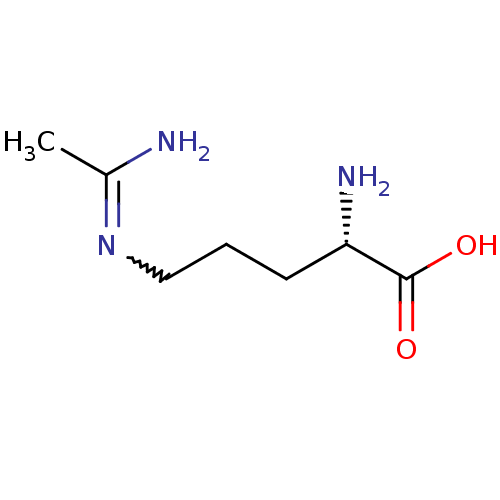 ((S)-5-Acetimidoylamino-2-amino-pent | (S)-5-Acetim...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 1.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University at Buffalo Curated by ChEMBL | Assay Description Compound was tested for competitive antagonist of Nitric oxide synthase | Bioorg Med Chem Lett 10: 1077-80 (2000) BindingDB Entry DOI: 10.7270/Q2959GSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cocaine esterase (Homo sapiens (Human)) | BDBM50130903 (CHEMBL3632950) | NCI pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Liaoning University of Traditional Chinese Medicine Curated by ChEMBL | Assay Description Fixed inhibition of human carboxylesterase 2 using 4-benzoyl-N-butyl-1,8-naphthalimide as substrate by Dixon and Lineweaver-Burk plot analysis | J Nat Prod 78: 2372-80 (2015) Article DOI: 10.1021/acs.jnatprod.5b00321 BindingDB Entry DOI: 10.7270/Q20K2BDR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50063300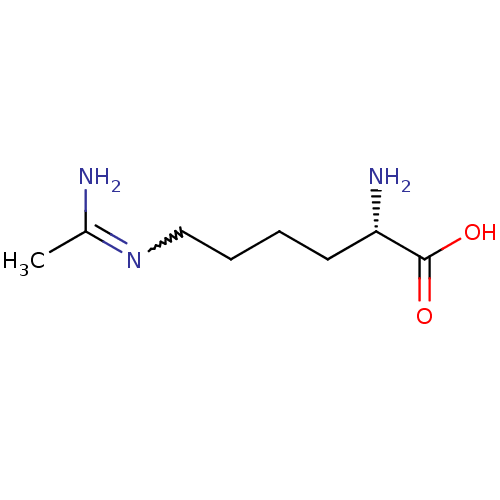 ((L-N6-1-iminoethyl)lysine | (S)-6-Acetimidoylamino...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University at Buffalo Curated by ChEMBL | Assay Description The compound was tested for competitive antagonist of Nitric oxide synthase | Bioorg Med Chem Lett 10: 1077-80 (2000) BindingDB Entry DOI: 10.7270/Q2959GSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50469008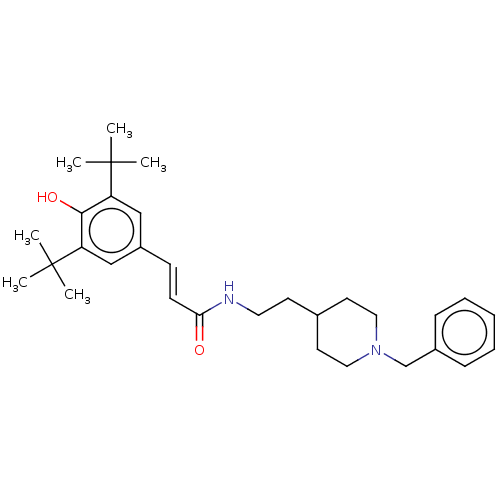 (CHEMBL4283390) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
China Pharmaceutical University Curated by ChEMBL | Assay Description Mixed-type inhibition of human AChE using acetylthiocholine chloride as substrate incubated for 5 mins followed by substrate addition by Lineweaver-B... | Eur J Med Chem 157: 161-176 (2018) Article DOI: 10.1016/j.ejmech.2018.08.005 BindingDB Entry DOI: 10.7270/Q29P34C1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Tetronarce californica (Pacific electric ray) (Tor...) | BDBM10441 ((+)-Huperzine A | (+/-)Huperzine A | (-)-Huperzine...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 4.30E+3 | -30.6 | n/a | n/a | n/a | n/a | n/a | 7.0 | 25 |
Weizmann Institute of Science | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. Enzyme activity was determined by measuring the absorbance at ... | Biochemistry 41: 10810-8 (2002) Article DOI: 10.1021/bi020151+ BindingDB Entry DOI: 10.7270/Q2HQ3X52 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50088667 (2-(S)-Amino-5-[(N-hydroxy-acetimidoyl)-amino]-pent...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University at Buffalo Curated by ChEMBL | Assay Description The compound was tested for competitive antagonist of Nitric oxide synthase | Bioorg Med Chem Lett 10: 1077-80 (2000) BindingDB Entry DOI: 10.7270/Q2959GSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Nitric oxide synthase, inducible (Mus musculus (mouse)) | BDBM50088666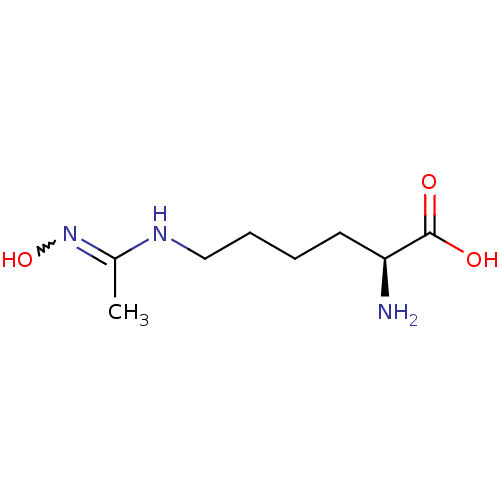 ((S)-2-Amino-6-[(N-hydroxy-acetimidoyl)-amino]-hexa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University at Buffalo Curated by ChEMBL | Assay Description The compound was tested for competitive antagonist of Nitric oxide synthase | Bioorg Med Chem Lett 10: 1077-80 (2000) BindingDB Entry DOI: 10.7270/Q2959GSN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50517420 (CHEMBL4513589) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of full length recombinant PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues preincubated for 30 mins ... | J Med Chem 62: 1523-1540 (2019) Article DOI: 10.1021/acs.jmedchem.8b01733 BindingDB Entry DOI: 10.7270/Q25X2D97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM192642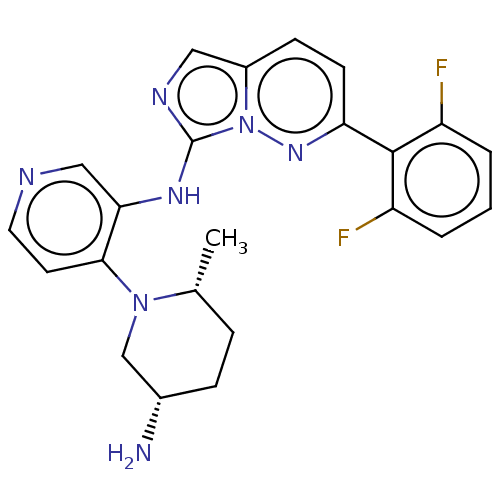 (US9187486, 87) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant Pim1 (unknown origin) by electrochemiluminescence assay | Bioorg Med Chem Lett 26: 5580-5590 (2016) Article DOI: 10.1016/j.bmcl.2016.09.067 BindingDB Entry DOI: 10.7270/Q261128M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM192574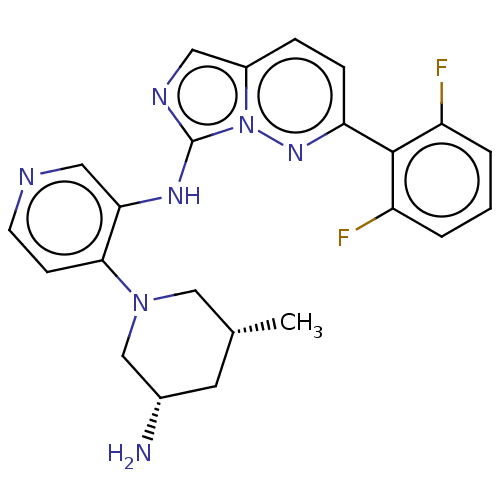 (US9187486, 37 | US9187486, 39 | US9187486, 60) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0240 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant Pim1 (unknown origin) by electrochemiluminescence assay | Bioorg Med Chem Lett 26: 5580-5590 (2016) Article DOI: 10.1016/j.bmcl.2016.09.067 BindingDB Entry DOI: 10.7270/Q261128M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-3 (Homo sapiens (Human)) | BDBM192574 (US9187486, 37 | US9187486, 39 | US9187486, 60) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0270 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of Pim3 (unknown origin) | Bioorg Med Chem Lett 26: 5580-5590 (2016) Article DOI: 10.1016/j.bmcl.2016.09.067 BindingDB Entry DOI: 10.7270/Q261128M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50517433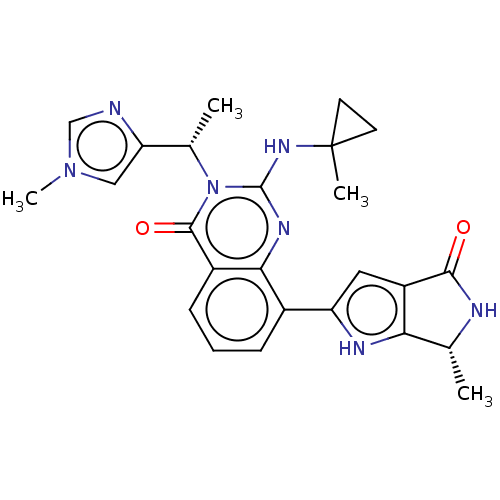 (CHEMBL4450494) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of full length recombinant PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues preincubated for 30 mins ... | J Med Chem 62: 1523-1540 (2019) Article DOI: 10.1021/acs.jmedchem.8b01733 BindingDB Entry DOI: 10.7270/Q25X2D97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50517431 (CHEMBL4471849) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of full length recombinant PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues preincubated for 30 mins ... | J Med Chem 62: 1523-1540 (2019) Article DOI: 10.1021/acs.jmedchem.8b01733 BindingDB Entry DOI: 10.7270/Q25X2D97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM192648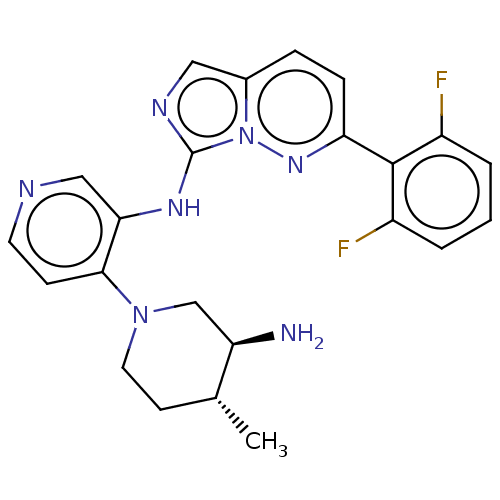 (US9187486, 100) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant Pim1 (unknown origin) by electrochemiluminescence assay | Bioorg Med Chem Lett 26: 5580-5590 (2016) Article DOI: 10.1016/j.bmcl.2016.09.067 BindingDB Entry DOI: 10.7270/Q261128M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM192640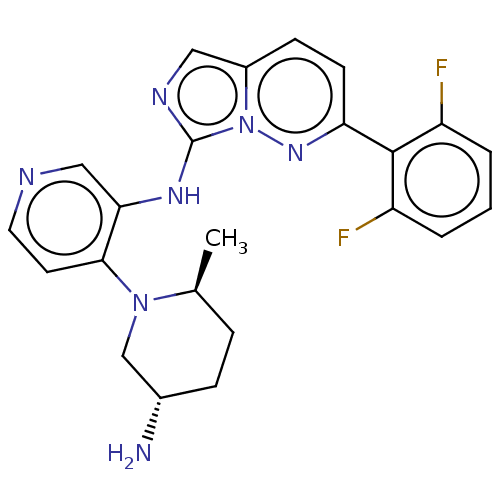 (US9187486, 85) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.0370 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant Pim1 (unknown origin) by electrochemiluminescence assay | Bioorg Med Chem Lett 26: 5580-5590 (2016) Article DOI: 10.1016/j.bmcl.2016.09.067 BindingDB Entry DOI: 10.7270/Q261128M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50200531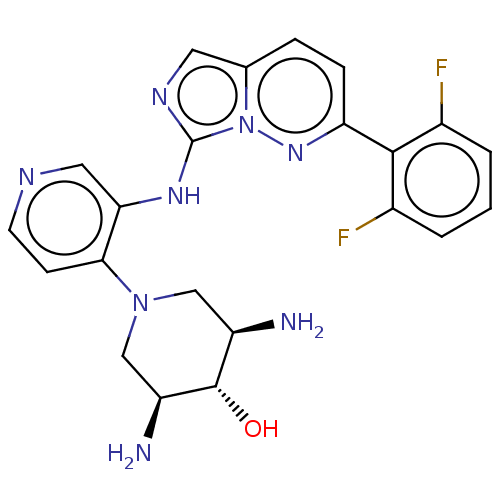 (CHEMBL3950251) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of recombinant Pim1 (unknown origin) by electrochemiluminescence assay | Bioorg Med Chem Lett 26: 5580-5590 (2016) Article DOI: 10.1016/j.bmcl.2016.09.067 BindingDB Entry DOI: 10.7270/Q261128M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-1 (Homo sapiens (Human)) | BDBM50517428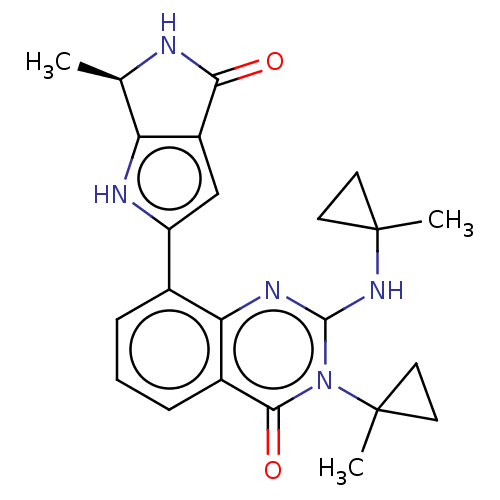 (CHEMBL4568404) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of full length recombinant PIM1 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues preincubated for 30 mins ... | J Med Chem 62: 1523-1540 (2019) Article DOI: 10.1021/acs.jmedchem.8b01733 BindingDB Entry DOI: 10.7270/Q25X2D97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50517428 (CHEMBL4568404) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of full length recombinant PIM2 (unknown origin) assessed as reduction in BAD phosphorylation at Ser112 residues preincubated for 30 mins ... | J Med Chem 62: 1523-1540 (2019) Article DOI: 10.1021/acs.jmedchem.8b01733 BindingDB Entry DOI: 10.7270/Q25X2D97 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase pim-2 (Homo sapiens (Human)) | BDBM50174153 (CHEMBL3808887) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem | Article PubMed | n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc. Curated by ChEMBL | Assay Description Inhibition of human full-length Pim2 expressed in Escherichia coli assessed as phosphorylation of biotinylated BAD peptide at Ser 112 preincubated fo... | ACS Med Chem Lett 7: 408-12 (2016) Article DOI: 10.1021/acsmedchemlett.5b00403 BindingDB Entry DOI: 10.7270/Q2MG7RD5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 2182 total ) | Next | Last >> |