 Found 178 hits with Last Name = 'nguyen' and Initial = 'jt'
Found 178 hits with Last Name = 'nguyen' and Initial = 'jt' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50480930
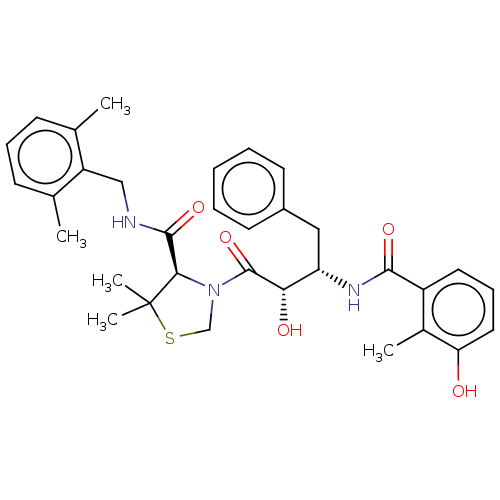
(CHEMBL584130 | KNI-814)Show SMILES Cc1cccc(C)c1CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(O)c1C |r| Show InChI InChI=1S/C33H39N3O5S/c1-20-11-9-12-21(2)25(20)18-34-31(40)29-33(4,5)42-19-36(29)32(41)28(38)26(17-23-13-7-6-8-14-23)35-30(39)24-15-10-16-27(37)22(24)3/h6-16,26,28-29,37-38H,17-19H2,1-5H3,(H,34,40)(H,35,39)/t26-,28-,29+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.00240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay |
J Med Chem 52: 7604-17 (2009)
Article DOI: 10.1021/jm9005115
BindingDB Entry DOI: 10.7270/Q2FR00F2 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM580

((4R)-3-[(2S,3S)-2-hydroxy-3-[(3-hydroxy-2-methylph...)Show SMILES Cc1ccccc1CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(O)c1C |r| Show InChI InChI=1S/C32H37N3O5S/c1-20-11-8-9-14-23(20)18-33-30(39)28-32(3,4)41-19-35(28)31(40)27(37)25(17-22-12-6-5-7-13-22)34-29(38)24-15-10-16-26(36)21(24)2/h5-16,25,27-28,36-37H,17-19H2,1-4H3,(H,33,39)(H,34,38)/t25-,27-,28+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay |
J Med Chem 52: 7604-17 (2009)
Article DOI: 10.1021/jm9005115
BindingDB Entry DOI: 10.7270/Q2FR00F2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50480931
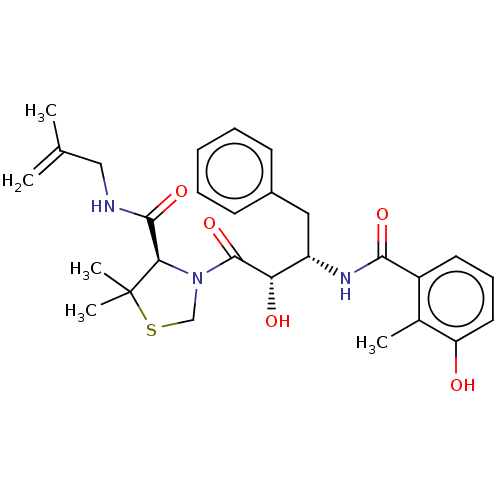
(CHEMBL575512 | KNI-1614)Show SMILES CC(=C)CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)c1cccc(O)c1C |r| Show InChI InChI=1S/C28H35N3O5S/c1-17(2)15-29-26(35)24-28(4,5)37-16-31(24)27(36)23(33)21(14-19-10-7-6-8-11-19)30-25(34)20-12-9-13-22(32)18(20)3/h6-13,21,23-24,32-33H,1,14-16H2,2-5H3,(H,29,35)(H,30,34)/t21-,23-,24+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay |
J Med Chem 52: 7604-17 (2009)
Article DOI: 10.1021/jm9005115
BindingDB Entry DOI: 10.7270/Q2FR00F2 |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50480929
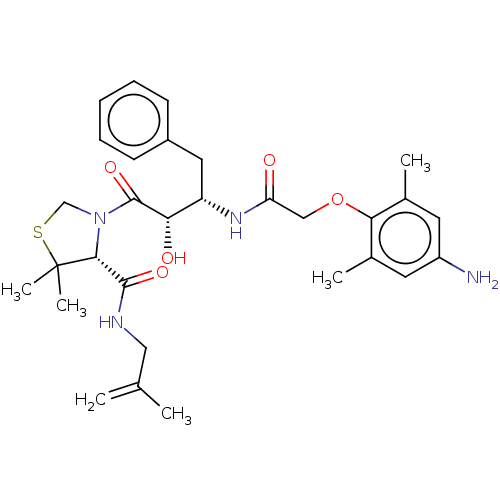
(CHEMBL573975 | KNI-1689)Show SMILES CC(=C)CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)COc1c(C)cc(N)cc1C |r| Show InChI InChI=1S/C30H40N4O5S/c1-18(2)15-32-28(37)27-30(5,6)40-17-34(27)29(38)25(36)23(14-21-10-8-7-9-11-21)33-24(35)16-39-26-19(3)12-22(31)13-20(26)4/h7-13,23,25,27,36H,1,14-17,31H2,2-6H3,(H,32,37)(H,33,35)/t23-,25-,27+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 recombinant protease after 15 mins by fluorescence assay |
J Med Chem 52: 7604-17 (2009)
Article DOI: 10.1021/jm9005115
BindingDB Entry DOI: 10.7270/Q2FR00F2 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50478722
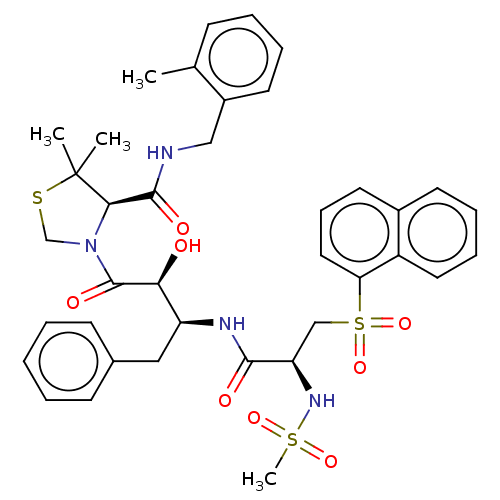
(CHEMBL452953 | KNI-1933)Show SMILES Cc1ccccc1CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CS(=O)(=O)c1cccc2ccccc12)NS(C)(=O)=O |r| Show InChI InChI=1S/C38H44N4O8S3/c1-25-13-8-9-17-28(25)22-39-36(45)34-38(2,3)51-24-42(34)37(46)33(43)30(21-26-14-6-5-7-15-26)40-35(44)31(41-52(4,47)48)23-53(49,50)32-20-12-18-27-16-10-11-19-29(27)32/h5-20,30-31,33-34,41,43H,21-24H2,1-4H3,(H,39,45)(H,40,44)/t30-,31+,33-,34+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 51: 2992-3004 (2008)
Article DOI: 10.1021/jm701555p
BindingDB Entry DOI: 10.7270/Q2QC069F |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50478719
(CHEMBL509400 | KNI-1965)Show SMILES CC(=O)N[C@H](CS(=O)(=O)c1ccc2ccccc2c1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)NCc1ccccc1C |r| Show InChI InChI=1S/C39H44N4O7S2/c1-25-12-8-9-17-30(25)22-40-37(47)35-39(3,4)51-24-43(35)38(48)34(45)32(20-27-13-6-5-7-14-27)42-36(46)33(41-26(2)44)23-52(49,50)31-19-18-28-15-10-11-16-29(28)21-31/h5-19,21,32-35,45H,20,22-24H2,1-4H3,(H,40,47)(H,41,44)(H,42,46)/t32-,33+,34-,35+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0860 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 51: 2992-3004 (2008)
Article DOI: 10.1021/jm701555p
BindingDB Entry DOI: 10.7270/Q2QC069F |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50478712
(CHEMBL485911 | KNI-1969)Show SMILES Cc1ccccc1CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CS(=O)(=O)c1ccc2ccccc2c1)NS(C)(=O)=O |r| Show InChI InChI=1S/C38H44N4O8S3/c1-25-12-8-9-17-29(25)22-39-36(45)34-38(2,3)51-24-42(34)37(46)33(43)31(20-26-13-6-5-7-14-26)40-35(44)32(41-52(4,47)48)23-53(49,50)30-19-18-27-15-10-11-16-28(27)21-30/h5-19,21,31-34,41,43H,20,22-24H2,1-4H3,(H,39,45)(H,40,44)/t31-,32+,33-,34+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 51: 2992-3004 (2008)
Article DOI: 10.1021/jm701555p
BindingDB Entry DOI: 10.7270/Q2QC069F |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50478713
(CHEMBL510136 | KNI-1961)Show SMILES CC(=O)N[C@H](CS(=O)(=O)c1cccc2ccccc12)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)NCc1ccccc1C |r| Show InChI InChI=1S/C39H44N4O7S2/c1-25-13-8-9-17-29(25)22-40-37(47)35-39(3,4)51-24-43(35)38(48)34(45)31(21-27-14-6-5-7-15-27)42-36(46)32(41-26(2)44)23-52(49,50)33-20-12-18-28-16-10-11-19-30(28)33/h5-20,31-32,34-35,45H,21-24H2,1-4H3,(H,40,47)(H,41,44)(H,42,46)/t31-,32+,34-,35+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 51: 2992-3004 (2008)
Article DOI: 10.1021/jm701555p
BindingDB Entry DOI: 10.7270/Q2QC069F |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50478715
(CHEMBL452873 | KNI-1910)Show SMILES Cc1ccccc1CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](CS(=O)(=O)c1ccc(Cl)cc1)NS(C)(=O)=O |r| Show InChI InChI=1S/C34H41ClN4O8S3/c1-22-10-8-9-13-24(22)19-36-32(42)30-34(2,3)48-21-39(30)33(43)29(40)27(18-23-11-6-5-7-12-23)37-31(41)28(38-49(4,44)45)20-50(46,47)26-16-14-25(35)15-17-26/h5-17,27-30,38,40H,18-21H2,1-4H3,(H,36,42)(H,37,41)/t27-,28+,29-,30+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 51: 2992-3004 (2008)
Article DOI: 10.1021/jm701555p
BindingDB Entry DOI: 10.7270/Q2QC069F |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50478721
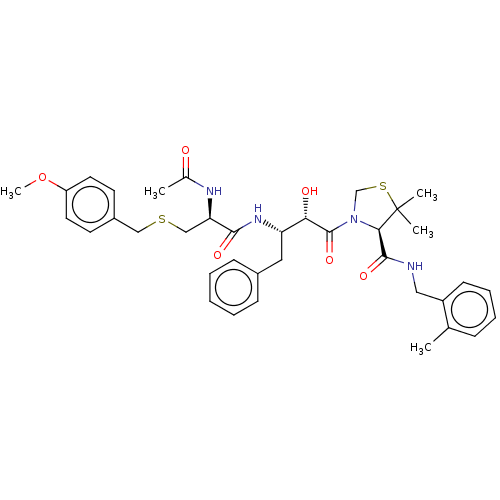
(CHEMBL230484 | KNI-1931)Show SMILES COc1ccc(CSC[C@@H](NC(C)=O)C(=O)N[C@@H](Cc2ccccc2)[C@H](O)C(=O)N2CSC(C)(C)[C@H]2C(=O)NCc2ccccc2C)cc1 |r| Show InChI InChI=1S/C37H46N4O6S2/c1-24-11-9-10-14-28(24)20-38-35(45)33-37(3,4)49-23-41(33)36(46)32(43)30(19-26-12-7-6-8-13-26)40-34(44)31(39-25(2)42)22-48-21-27-15-17-29(47-5)18-16-27/h6-18,30-33,43H,19-23H2,1-5H3,(H,38,45)(H,39,42)(H,40,44)/t30-,31+,32-,33+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 51: 2992-3004 (2008)
Article DOI: 10.1021/jm701555p
BindingDB Entry DOI: 10.7270/Q2QC069F |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50478714
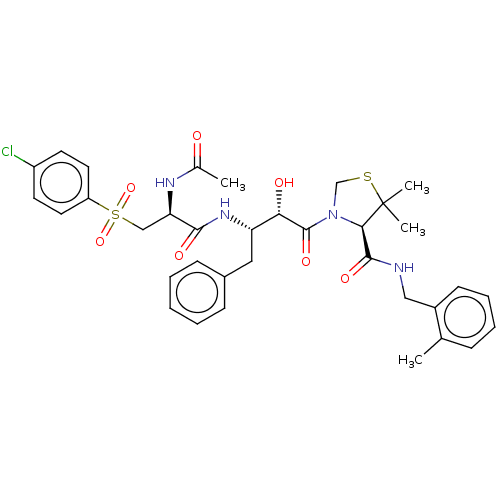
(CHEMBL462726 | KNI-1909)Show SMILES CC(=O)N[C@H](CS(=O)(=O)c1ccc(Cl)cc1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)NCc1ccccc1C |r| Show InChI InChI=1S/C35H41ClN4O7S2/c1-22-10-8-9-13-25(22)19-37-33(44)31-35(3,4)48-21-40(31)34(45)30(42)28(18-24-11-6-5-7-12-24)39-32(43)29(38-23(2)41)20-49(46,47)27-16-14-26(36)15-17-27/h5-17,28-31,42H,18-21H2,1-4H3,(H,37,44)(H,38,41)(H,39,43)/t28-,29+,30-,31+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 51: 2992-3004 (2008)
Article DOI: 10.1021/jm701555p
BindingDB Entry DOI: 10.7270/Q2QC069F |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50478718
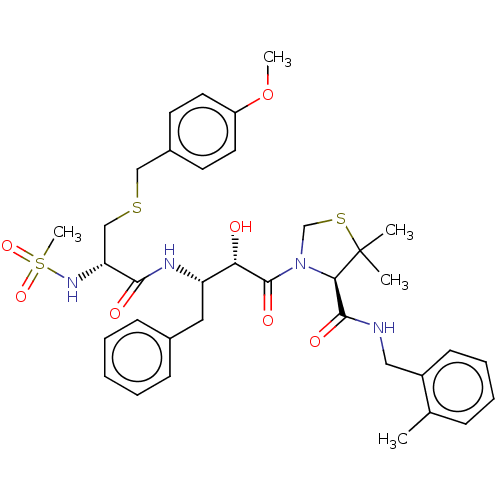
(CHEMBL455816 | KNI-1932)Show SMILES COc1ccc(CSC[C@@H](NS(C)(=O)=O)C(=O)N[C@@H](Cc2ccccc2)[C@H](O)C(=O)N2CSC(C)(C)[C@H]2C(=O)NCc2ccccc2C)cc1 |r| Show InChI InChI=1S/C36H46N4O7S3/c1-24-11-9-10-14-27(24)20-37-34(43)32-36(2,3)49-23-40(32)35(44)31(41)29(19-25-12-7-6-8-13-25)38-33(42)30(39-50(5,45)46)22-48-21-26-15-17-28(47-4)18-16-26/h6-18,29-32,39,41H,19-23H2,1-5H3,(H,37,43)(H,38,42)/t29-,30+,31-,32+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 51: 2992-3004 (2008)
Article DOI: 10.1021/jm701555p
BindingDB Entry DOI: 10.7270/Q2QC069F |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50478720
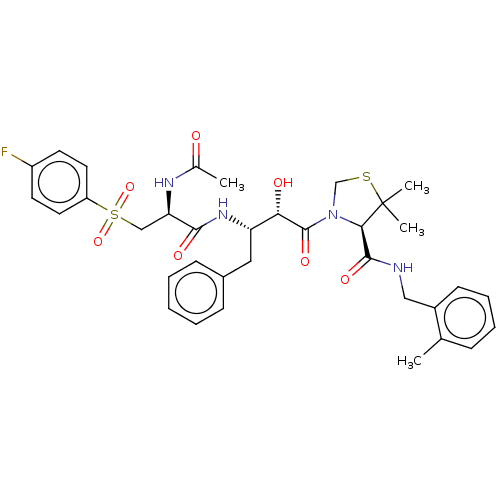
(CHEMBL508278 | KNI-1960)Show SMILES CC(=O)N[C@H](CS(=O)(=O)c1ccc(F)cc1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)NCc1ccccc1C |r| Show InChI InChI=1S/C35H41FN4O7S2/c1-22-10-8-9-13-25(22)19-37-33(44)31-35(3,4)48-21-40(31)34(45)30(42)28(18-24-11-6-5-7-12-24)39-32(43)29(38-23(2)41)20-49(46,47)27-16-14-26(36)15-17-27/h5-17,28-31,42H,18-21H2,1-4H3,(H,37,44)(H,38,41)(H,39,43)/t28-,29+,30-,31+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 51: 2992-3004 (2008)
Article DOI: 10.1021/jm701555p
BindingDB Entry DOI: 10.7270/Q2QC069F |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50478716
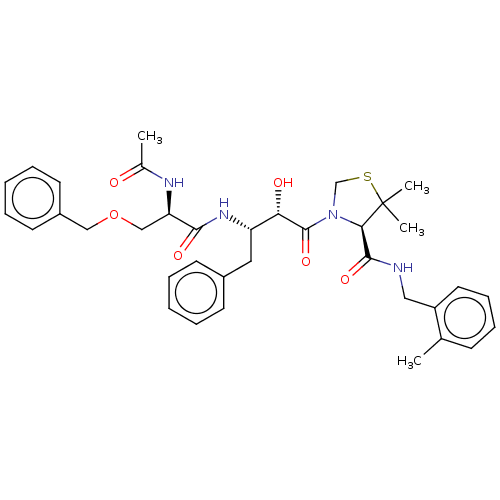
(CHEMBL449836 | KNI-1878)Show SMILES CC(=O)N[C@H](COCc1ccccc1)C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)NCc1ccccc1C |r| Show InChI InChI=1S/C36H44N4O6S/c1-24-13-11-12-18-28(24)20-37-34(44)32-36(3,4)47-23-40(32)35(45)31(42)29(19-26-14-7-5-8-15-26)39-33(43)30(38-25(2)41)22-46-21-27-16-9-6-10-17-27/h5-18,29-32,42H,19-23H2,1-4H3,(H,37,44)(H,38,41)(H,39,43)/t29-,30+,31-,32+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 51: 2992-3004 (2008)
Article DOI: 10.1021/jm701555p
BindingDB Entry DOI: 10.7270/Q2QC069F |
More data for this
Ligand-Target Pair | |
Protease
(Human immunodeficiency virus 1 (HIV-1)) | BDBM50478717
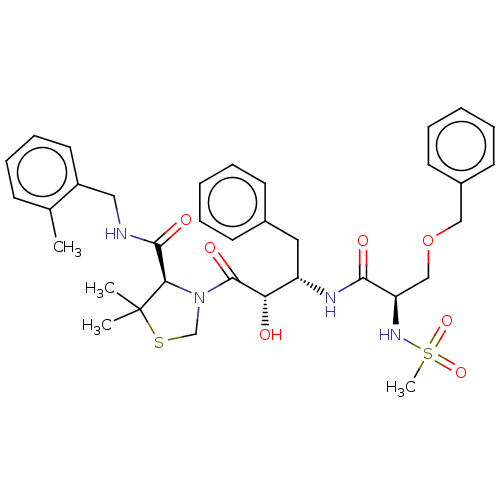
(CHEMBL503829 | KNI-1876)Show SMILES Cc1ccccc1CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](COCc1ccccc1)NS(C)(=O)=O |r| Show InChI InChI=1S/C35H44N4O7S2/c1-24-13-11-12-18-27(24)20-36-33(42)31-35(2,3)47-23-39(31)34(43)30(40)28(19-25-14-7-5-8-15-25)37-32(41)29(38-48(4,44)45)22-46-21-26-16-9-6-10-17-26/h5-18,28-31,38,40H,19-23H2,1-4H3,(H,36,42)(H,37,41)/t28-,29+,30-,31+/m0/s1 | PDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.560 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HIV1 protease |
J Med Chem 51: 2992-3004 (2008)
Article DOI: 10.1021/jm701555p
BindingDB Entry DOI: 10.7270/Q2QC069F |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50182869
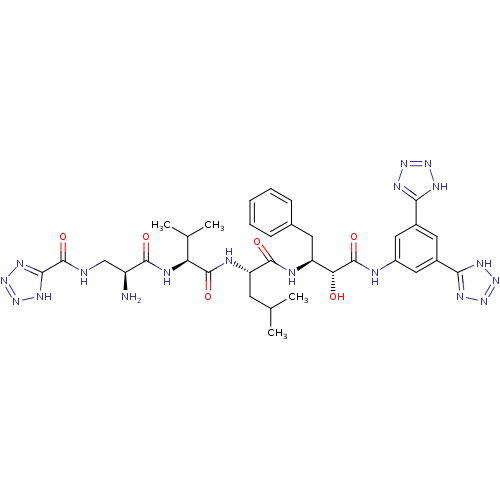
(CHEMBL439521 | N-((S)-2-amino-3-((S)-1-((S)-1-((2S...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cc(cc(c1)-c1nnn[nH]1)-c1nnn[nH]1 Show InChI InChI=1S/C34H44N18O6/c1-16(2)10-24(39-32(56)25(17(3)4)40-30(54)22(35)15-36-34(58)29-45-51-52-46-29)31(55)38-23(11-18-8-6-5-7-9-18)26(53)33(57)37-21-13-19(27-41-47-48-42-27)12-20(14-21)28-43-49-50-44-28/h5-9,12-14,16-17,22-26,53H,10-11,15,35H2,1-4H3,(H,36,58)(H,37,57)(H,38,55)(H,39,56)(H,40,54)(H,41,42,47,48)(H,43,44,49,50)(H,45,46,51,52)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1649-53 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.058
BindingDB Entry DOI: 10.7270/Q2PV6M6V |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50182869

(CHEMBL439521 | N-((S)-2-amino-3-((S)-1-((S)-1-((2S...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cc(cc(c1)-c1nnn[nH]1)-c1nnn[nH]1 Show InChI InChI=1S/C34H44N18O6/c1-16(2)10-24(39-32(56)25(17(3)4)40-30(54)22(35)15-36-34(58)29-45-51-52-46-29)31(55)38-23(11-18-8-6-5-7-9-18)26(53)33(57)37-21-13-19(27-41-47-48-42-27)12-20(14-21)28-43-49-50-44-28/h5-9,12-14,16-17,22-26,53H,10-11,15,35H2,1-4H3,(H,36,58)(H,37,57)(H,38,55)(H,39,56)(H,40,54)(H,41,42,47,48)(H,43,44,49,50)(H,45,46,51,52)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 16: 2380-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.108
BindingDB Entry DOI: 10.7270/Q2TD9WXG |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50188338

(CHEMBL411711 | N-((2S,5S)-5-((S)-1-((1R,2S)-1-(3-(...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C36H48N14O6/c1-20(2)28(42-32(52)25(37)19-38-36(56)31-45-49-50-46-31)34(54)41-27(17-22-12-7-4-8-13-22)33(53)40-26(16-21-10-5-3-6-11-21)29(51)35(55)39-24-15-9-14-23(18-24)30-43-47-48-44-30/h3,5-6,9-11,14-15,18,20,22,25-29,51H,4,7-8,12-13,16-17,19,37H2,1-2H3,(H,38,56)(H,39,55)(H,40,53)(H,41,54)(H,42,52)(H,43,44,47,48)(H,45,46,49,50)/t25-,26-,27-,28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET assay |
Bioorg Med Chem 18: 3175-86 (2010)
Article DOI: 10.1016/j.bmc.2010.03.032
BindingDB Entry DOI: 10.7270/Q26M36ZV |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50188338

(CHEMBL411711 | N-((2S,5S)-5-((S)-1-((1R,2S)-1-(3-(...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C36H48N14O6/c1-20(2)28(42-32(52)25(37)19-38-36(56)31-45-49-50-46-31)34(54)41-27(17-22-12-7-4-8-13-22)33(53)40-26(16-21-10-5-3-6-11-21)29(51)35(55)39-24-15-9-14-23(18-24)30-43-47-48-44-30/h3,5-6,9-11,14-15,18,20,22,25-29,51H,4,7-8,12-13,16-17,19,37H2,1-2H3,(H,38,56)(H,39,55)(H,40,53)(H,41,54)(H,42,52)(H,43,44,47,48)(H,45,46,49,50)/t25-,26-,27-,28-,29+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 |
Bioorg Med Chem Lett 16: 4354-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.046
BindingDB Entry DOI: 10.7270/Q26M36F4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50157441
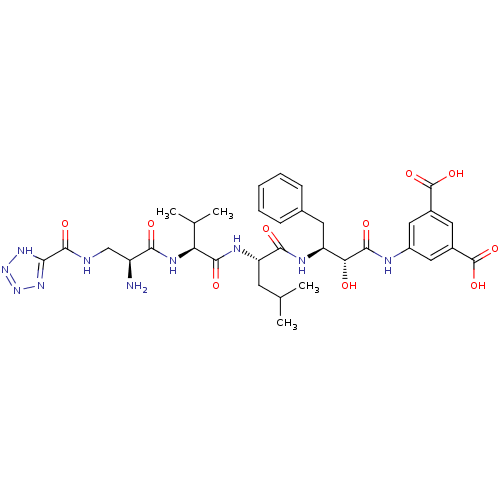
(5-((2R,3S)-3-((S)-2-((S)-2-((S)-2-amino-3-(2H-tetr...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cc(cc(c1)C(O)=O)C(O)=O Show InChI InChI=1S/C34H44N10O10/c1-16(2)10-24(39-30(48)25(17(3)4)40-28(46)22(35)15-36-32(50)27-41-43-44-42-27)29(47)38-23(11-18-8-6-5-7-9-18)26(45)31(49)37-21-13-19(33(51)52)12-20(14-21)34(53)54/h5-9,12-14,16-17,22-26,45H,10-11,15,35H2,1-4H3,(H,36,50)(H,37,49)(H,38,47)(H,39,48)(H,40,46)(H,51,52)(H,53,54)(H,41,42,43,44)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1649-53 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.058
BindingDB Entry DOI: 10.7270/Q2PV6M6V |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50157441

(5-((2R,3S)-3-((S)-2-((S)-2-((S)-2-amino-3-(2H-tetr...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cc(cc(c1)C(O)=O)C(O)=O Show InChI InChI=1S/C34H44N10O10/c1-16(2)10-24(39-30(48)25(17(3)4)40-28(46)22(35)15-36-32(50)27-41-43-44-42-27)29(47)38-23(11-18-8-6-5-7-9-18)26(45)31(49)37-21-13-19(33(51)52)12-20(14-21)34(53)54/h5-9,12-14,16-17,22-26,45H,10-11,15,35H2,1-4H3,(H,36,50)(H,37,49)(H,38,47)(H,39,48)(H,40,46)(H,51,52)(H,53,54)(H,41,42,43,44)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 16: 2380-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.108
BindingDB Entry DOI: 10.7270/Q2TD9WXG |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50157441

(5-((2R,3S)-3-((S)-2-((S)-2-((S)-2-amino-3-(2H-tetr...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cc(cc(c1)C(O)=O)C(O)=O Show InChI InChI=1S/C34H44N10O10/c1-16(2)10-24(39-30(48)25(17(3)4)40-28(46)22(35)15-36-32(50)27-41-43-44-42-27)29(47)38-23(11-18-8-6-5-7-9-18)26(45)31(49)37-21-13-19(33(51)52)12-20(14-21)34(53)54/h5-9,12-14,16-17,22-26,45H,10-11,15,35H2,1-4H3,(H,36,50)(H,37,49)(H,38,47)(H,39,48)(H,40,46)(H,51,52)(H,53,54)(H,41,42,43,44)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem 19: 5238-46 (2011)
Article DOI: 10.1016/j.bmc.2011.07.002
BindingDB Entry DOI: 10.7270/Q20Z73NP |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50182871

(CHEMBL381826 | N-((2S,5S)-5-((S)-1-((1R,2S)-1-(3-(...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C33H44N14O6/c1-17(2)13-24(38-31(51)25(18(3)4)39-29(49)22(34)16-35-33(53)28-42-46-47-43-28)30(50)37-23(14-19-9-6-5-7-10-19)26(48)32(52)36-21-12-8-11-20(15-21)27-40-44-45-41-27/h5-12,15,17-18,22-26,48H,13-14,16,34H2,1-4H3,(H,35,53)(H,36,52)(H,37,50)(H,38,51)(H,39,49)(H,40,41,44,45)(H,42,43,46,47)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET assay |
Bioorg Med Chem 18: 3175-86 (2010)
Article DOI: 10.1016/j.bmc.2010.03.032
BindingDB Entry DOI: 10.7270/Q26M36ZV |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50182871

(CHEMBL381826 | N-((2S,5S)-5-((S)-1-((1R,2S)-1-(3-(...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C33H44N14O6/c1-17(2)13-24(38-31(51)25(18(3)4)39-29(49)22(34)16-35-33(53)28-42-46-47-43-28)30(50)37-23(14-19-9-6-5-7-10-19)26(48)32(52)36-21-12-8-11-20(15-21)27-40-44-45-41-27/h5-12,15,17-18,22-26,48H,13-14,16,34H2,1-4H3,(H,35,53)(H,36,52)(H,37,50)(H,38,51)(H,39,49)(H,40,41,44,45)(H,42,43,46,47)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 |
Bioorg Med Chem Lett 16: 4354-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.046
BindingDB Entry DOI: 10.7270/Q26M36F4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50182871

(CHEMBL381826 | N-((2S,5S)-5-((S)-1-((1R,2S)-1-(3-(...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C33H44N14O6/c1-17(2)13-24(38-31(51)25(18(3)4)39-29(49)22(34)16-35-33(53)28-42-46-47-43-28)30(50)37-23(14-19-9-6-5-7-10-19)26(48)32(52)36-21-12-8-11-20(15-21)27-40-44-45-41-27/h5-12,15,17-18,22-26,48H,13-14,16,34H2,1-4H3,(H,35,53)(H,36,52)(H,37,50)(H,38,51)(H,39,49)(H,40,41,44,45)(H,42,43,46,47)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 16: 2380-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.108
BindingDB Entry DOI: 10.7270/Q2TD9WXG |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50234178
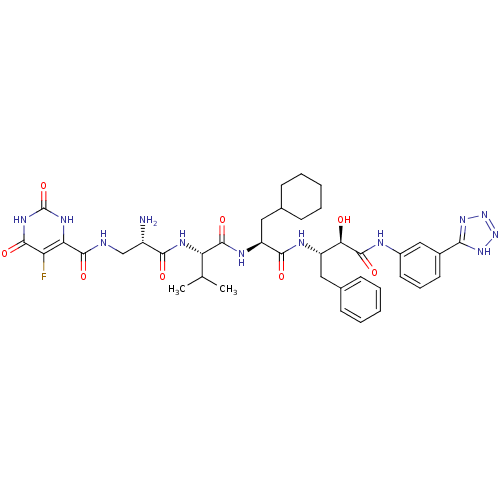
(CHEMBL255838 | N-((S)-3-((S)-1-((S)-1-((2S,3R)-4-(...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](N)CNC(=O)c1[nH]c(=O)[nH]c(=O)c1F)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C39H49FN12O8/c1-20(2)29(46-33(54)25(41)19-42-36(57)30-28(40)35(56)48-39(60)47-30)37(58)45-27(17-22-12-7-4-8-13-22)34(55)44-26(16-21-10-5-3-6-11-21)31(53)38(59)43-24-15-9-14-23(18-24)32-49-51-52-50-32/h3,5-6,9-11,14-15,18,20,22,25-27,29,31,53H,4,7-8,12-13,16-17,19,41H2,1-2H3,(H,42,57)(H,43,59)(H,44,55)(H,45,58)(H,46,54)(H2,47,48,56,60)(H,49,50,51,52)/t25-,26-,27-,29-,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1649-53 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.058
BindingDB Entry DOI: 10.7270/Q2PV6M6V |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50234178

(CHEMBL255838 | N-((S)-3-((S)-1-((S)-1-((2S,3R)-4-(...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](N)CNC(=O)c1[nH]c(=O)[nH]c(=O)c1F)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C39H49FN12O8/c1-20(2)29(46-33(54)25(41)19-42-36(57)30-28(40)35(56)48-39(60)47-30)37(58)45-27(17-22-12-7-4-8-13-22)34(55)44-26(16-21-10-5-3-6-11-21)31(53)38(59)43-24-15-9-14-23(18-24)32-49-51-52-50-32/h3,5-6,9-11,14-15,18,20,22,25-27,29,31,53H,4,7-8,12-13,16-17,19,41H2,1-2H3,(H,42,57)(H,43,59)(H,44,55)(H,45,58)(H,46,54)(H2,47,48,56,60)(H,49,50,51,52)/t25-,26-,27-,29-,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 |
Bioorg Med Chem Lett 16: 4354-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.046
BindingDB Entry DOI: 10.7270/Q26M36F4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50234178

(CHEMBL255838 | N-((S)-3-((S)-1-((S)-1-((2S,3R)-4-(...)Show SMILES CC(C)[C@H](NC(=O)[C@@H](N)CNC(=O)c1[nH]c(=O)[nH]c(=O)c1F)C(=O)N[C@@H](CC1CCCCC1)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C39H49FN12O8/c1-20(2)29(46-33(54)25(41)19-42-36(57)30-28(40)35(56)48-39(60)47-30)37(58)45-27(17-22-12-7-4-8-13-22)34(55)44-26(16-21-10-5-3-6-11-21)31(53)38(59)43-24-15-9-14-23(18-24)32-49-51-52-50-32/h3,5-6,9-11,14-15,18,20,22,25-27,29,31,53H,4,7-8,12-13,16-17,19,41H2,1-2H3,(H,42,57)(H,43,59)(H,44,55)(H,45,58)(H,46,54)(H2,47,48,56,60)(H,49,50,51,52)/t25-,26-,27-,29-,31+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET assay |
Bioorg Med Chem 18: 3175-86 (2010)
Article DOI: 10.1016/j.bmc.2010.03.032
BindingDB Entry DOI: 10.7270/Q26M36ZV |
More data for this
Ligand-Target Pair | |
Protease
(Human T-cell leukemia virus type I) | BDBM50483605
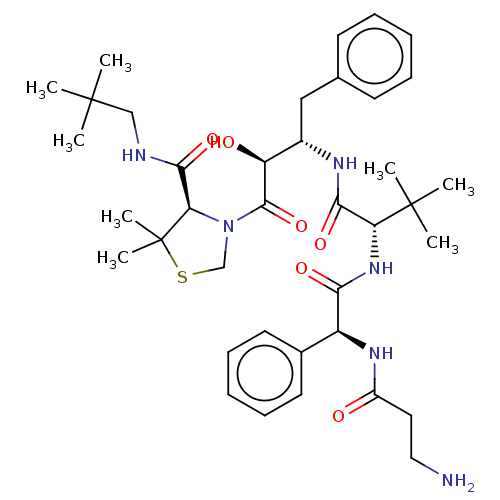
(CHEMBL1761462 | KNI-10838)Show SMILES CC(C)(C)CNC(=O)[C@H]1N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CCN)c1ccccc1)C(C)(C)C |r| Show InChI InChI=1S/C38H56N6O6S/c1-36(2,3)22-40-34(49)31-38(7,8)51-23-44(31)35(50)29(46)26(21-24-15-11-9-12-16-24)41-33(48)30(37(4,5)6)43-32(47)28(42-27(45)19-20-39)25-17-13-10-14-18-25/h9-18,26,28-31,46H,19-23,39H2,1-8H3,(H,40,49)(H,41,48)(H,42,45)(H,43,47)/t26-,28-,29-,30+,31+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HTLV-1 recombinant protease L40I mutant |
Bioorg Med Chem Lett 21: 2425-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.066
BindingDB Entry DOI: 10.7270/Q27W6G18 |
More data for this
Ligand-Target Pair | |
Protease
(Human T-cell leukemia virus type I) | BDBM50483483
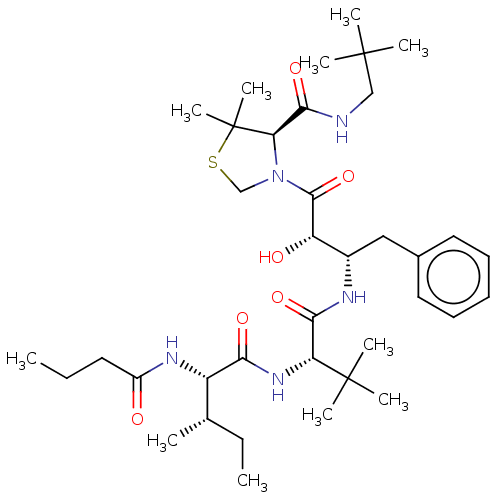
(CHEMBL1682244 | KNI-10842)Show SMILES [H][C@@]1(N(CSC1(C)C)C(=O)[C@@H](O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CCC)[C@@H](C)CC)C(C)(C)C)C(=O)NCC(C)(C)C |r| Show InChI InChI=1S/C37H61N5O6S/c1-12-17-26(43)40-27(23(3)13-2)31(45)41-29(36(7,8)9)32(46)39-25(20-24-18-15-14-16-19-24)28(44)34(48)42-22-49-37(10,11)30(42)33(47)38-21-35(4,5)6/h14-16,18-19,23,25,27-30,44H,12-13,17,20-22H2,1-11H3,(H,38,47)(H,39,46)(H,40,43)(H,41,45)/t23-,25-,27-,28-,29+,30+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HTLV-1 protease L40I mutant by fluorimetric assay |
Bioorg Med Chem Lett 21: 1832-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.048
BindingDB Entry DOI: 10.7270/Q2JD50N4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50182870
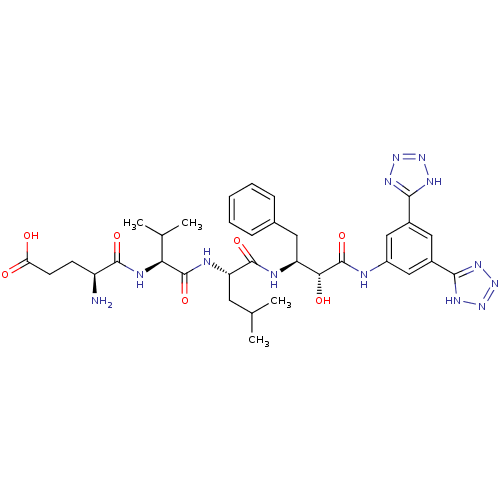
((S)-4-amino-5-((S)-1-((S)-1-((2S,3R)-4-(3,5-di(2H-...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CCC(O)=O)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cc(cc(c1)-c1nnn[nH]1)-c1nnn[nH]1 Show InChI InChI=1S/C34H45N13O7/c1-17(2)12-25(38-33(53)27(18(3)4)39-31(51)23(35)10-11-26(48)49)32(52)37-24(13-19-8-6-5-7-9-19)28(50)34(54)36-22-15-20(29-40-44-45-41-29)14-21(16-22)30-42-46-47-43-30/h5-9,14-18,23-25,27-28,50H,10-13,35H2,1-4H3,(H,36,54)(H,37,52)(H,38,53)(H,39,51)(H,48,49)(H,40,41,44,45)(H,42,43,46,47)/t23-,24-,25-,27-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 16: 2380-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.108
BindingDB Entry DOI: 10.7270/Q2TD9WXG |
More data for this
Ligand-Target Pair | |
Protease
(Human T-cell leukemia virus type I) | BDBM50478933
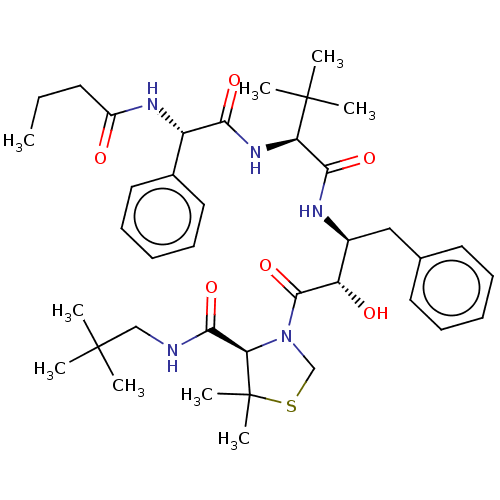
(CHEMBL444457 | KNI-10635)Show SMILES CCCC(=O)N[C@H](C(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)NCC(C)(C)C)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C39H57N5O6S/c1-10-17-28(45)42-29(26-20-15-12-16-21-26)33(47)43-31(38(5,6)7)34(48)41-27(22-25-18-13-11-14-19-25)30(46)36(50)44-24-51-39(8,9)32(44)35(49)40-23-37(2,3)4/h11-16,18-21,27,29-32,46H,10,17,22-24H2,1-9H3,(H,40,49)(H,41,48)(H,42,45)(H,43,47)/t27-,29-,30-,31+,32+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HTLV-1 recombinant protease L40I mutant |
Bioorg Med Chem Lett 21: 2425-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.066
BindingDB Entry DOI: 10.7270/Q27W6G18 |
More data for this
Ligand-Target Pair | |
Protease
(Human T-cell leukemia virus type I) | BDBM50478933

(CHEMBL444457 | KNI-10635)Show SMILES CCCC(=O)N[C@H](C(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)NCC(C)(C)C)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C39H57N5O6S/c1-10-17-28(45)42-29(26-20-15-12-16-21-26)33(47)43-31(38(5,6)7)34(48)41-27(22-25-18-13-11-14-19-25)30(46)36(50)44-24-51-39(8,9)32(44)35(49)40-23-37(2,3)4/h11-16,18-21,27,29-32,46H,10,17,22-24H2,1-9H3,(H,40,49)(H,41,48)(H,42,45)(H,43,47)/t27-,29-,30-,31+,32+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HTLV-1 protease L40I mutant by fluorimetric assay |
Bioorg Med Chem Lett 21: 1832-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.048
BindingDB Entry DOI: 10.7270/Q2JD50N4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50182872

(CHEMBL208060 | N-((S)-2-amino-3-((S)-1-((S)-1-((2S...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nc(=S)o[nH]1 Show InChI InChI=1S/C34H44N12O7S/c1-17(2)13-24(39-31(50)25(18(3)4)40-29(48)22(35)16-36-33(52)28-42-45-46-43-28)30(49)38-23(14-19-9-6-5-7-10-19)26(47)32(51)37-21-12-8-11-20(15-21)27-41-34(54)53-44-27/h5-12,15,17-18,22-26,47H,13-14,16,35H2,1-4H3,(H,36,52)(H,37,51)(H,38,49)(H,39,50)(H,40,48)(H,41,44,54)(H,42,43,45,46)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 16: 2380-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.108
BindingDB Entry DOI: 10.7270/Q2TD9WXG |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50188339

(CHEMBL380381 | N-((2S,5S)-5-((S)-1-((1R,2S)-1-(3-(...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1[nH]c(=O)[nH]c(=O)c1F)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C36H45FN12O8/c1-17(2)13-24(42-34(55)26(18(3)4)43-30(51)22(38)16-39-33(54)27-25(37)32(53)45-36(57)44-27)31(52)41-23(14-19-9-6-5-7-10-19)28(50)35(56)40-21-12-8-11-20(15-21)29-46-48-49-47-29/h5-12,15,17-18,22-24,26,28,50H,13-14,16,38H2,1-4H3,(H,39,54)(H,40,56)(H,41,52)(H,42,55)(H,43,51)(H2,44,45,53,57)(H,46,47,48,49)/t22-,23-,24-,26-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 |
Bioorg Med Chem Lett 16: 4354-9 (2006)
Article DOI: 10.1016/j.bmcl.2006.05.046
BindingDB Entry DOI: 10.7270/Q26M36F4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50188339

(CHEMBL380381 | N-((2S,5S)-5-((S)-1-((1R,2S)-1-(3-(...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1[nH]c(=O)[nH]c(=O)c1F)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C36H45FN12O8/c1-17(2)13-24(42-34(55)26(18(3)4)43-30(51)22(38)16-39-33(54)27-25(37)32(53)45-36(57)44-27)31(52)41-23(14-19-9-6-5-7-10-19)28(50)35(56)40-21-12-8-11-20(15-21)29-46-48-49-47-29/h5-12,15,17-18,22-24,26,28,50H,13-14,16,38H2,1-4H3,(H,39,54)(H,40,56)(H,41,52)(H,42,55)(H,43,51)(H2,44,45,53,57)(H,46,47,48,49)/t22-,23-,24-,26-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET assay |
Bioorg Med Chem 18: 3175-86 (2010)
Article DOI: 10.1016/j.bmc.2010.03.032
BindingDB Entry DOI: 10.7270/Q26M36ZV |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50182873

(CHEMBL205333 | N-((S)-2-amino-3-((S)-1-((S)-1-((2S...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nc(=O)o[nH]1 Show InChI InChI=1S/C34H44N12O8/c1-17(2)13-24(39-31(50)25(18(3)4)40-29(48)22(35)16-36-33(52)28-42-45-46-43-28)30(49)38-23(14-19-9-6-5-7-10-19)26(47)32(51)37-21-12-8-11-20(15-21)27-41-34(53)54-44-27/h5-12,15,17-18,22-26,47H,13-14,16,35H2,1-4H3,(H,36,52)(H,37,51)(H,38,49)(H,39,50)(H,40,48)(H,41,44,53)(H,42,43,45,46)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 16: 2380-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.108
BindingDB Entry DOI: 10.7270/Q2TD9WXG |
More data for this
Ligand-Target Pair | |
Protease
(Human T-cell leukemia virus type I) | BDBM50483598
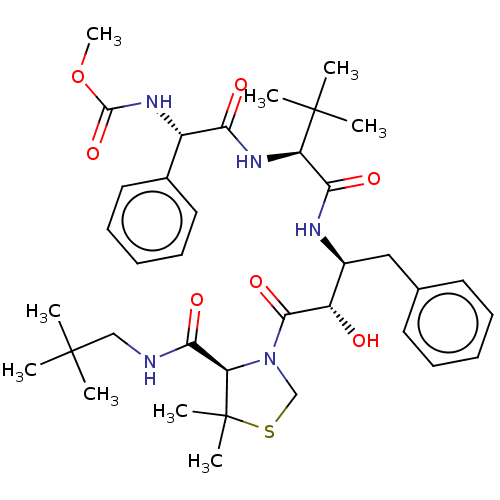
(CHEMBL1232433 | KNI-10562)Show SMILES COC(=O)N[C@H](C(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)NCC(C)(C)C)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C37H53N5O7S/c1-35(2,3)21-38-32(46)29-37(7,8)50-22-42(29)33(47)27(43)25(20-23-16-12-10-13-17-23)39-31(45)28(36(4,5)6)41-30(44)26(40-34(48)49-9)24-18-14-11-15-19-24/h10-19,25-29,43H,20-22H2,1-9H3,(H,38,46)(H,39,45)(H,40,48)(H,41,44)/t25-,26-,27-,28+,29+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 7.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HTLV-1 recombinant protease L40I mutant |
Bioorg Med Chem Lett 21: 2425-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.066
BindingDB Entry DOI: 10.7270/Q27W6G18 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317669

(CHEMBL1094659 | N-((2S,5S)-5-((S)-1-((1R,2S)-1-(3-...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1[nH]c(=O)[nH]c(=O)c1F)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nc(=O)o[nH]1 |r| Show InChI InChI=1S/C37H45FN10O10/c1-17(2)13-24(43-34(54)26(18(3)4)44-30(50)22(39)16-40-33(53)27-25(38)32(52)47-36(56)45-27)31(51)42-23(14-19-9-6-5-7-10-19)28(49)35(55)41-21-12-8-11-20(15-21)29-46-37(57)58-48-29/h5-12,15,17-18,22-24,26,28,49H,13-14,16,39H2,1-4H3,(H,40,53)(H,41,55)(H,42,51)(H,43,54)(H,44,50)(H,46,48,57)(H2,45,47,52,56)/t22-,23-,24-,26-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET assay |
Bioorg Med Chem 18: 3175-86 (2010)
Article DOI: 10.1016/j.bmc.2010.03.032
BindingDB Entry DOI: 10.7270/Q26M36ZV |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50157439

(3-((2R,3S)-3-((S)-2-((S)-2-((S)-2-amino-3-(2H-tetr...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)C(O)=O Show InChI InChI=1S/C33H44N10O8/c1-17(2)13-24(38-30(47)25(18(3)4)39-28(45)22(34)16-35-32(49)27-40-42-43-41-27)29(46)37-23(14-19-9-6-5-7-10-19)26(44)31(48)36-21-12-8-11-20(15-21)33(50)51/h5-12,15,17-18,22-26,44H,13-14,16,34H2,1-4H3,(H,35,49)(H,36,48)(H,37,46)(H,38,47)(H,39,45)(H,50,51)(H,40,41,42,43)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 16: 2380-6 (2006)
Article DOI: 10.1016/j.bmcl.2006.01.108
BindingDB Entry DOI: 10.7270/Q2TD9WXG |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50157439

(3-((2R,3S)-3-((S)-2-((S)-2-((S)-2-amino-3-(2H-tetr...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)C(O)=O Show InChI InChI=1S/C33H44N10O8/c1-17(2)13-24(38-30(47)25(18(3)4)39-28(45)22(34)16-35-32(49)27-40-42-43-41-27)29(46)37-23(14-19-9-6-5-7-10-19)26(44)31(48)36-21-12-8-11-20(15-21)33(50)51/h5-12,15,17-18,22-26,44H,13-14,16,34H2,1-4H3,(H,35,49)(H,36,48)(H,37,46)(H,38,47)(H,39,45)(H,50,51)(H,40,41,42,43)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem Lett 18: 1649-53 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.058
BindingDB Entry DOI: 10.7270/Q2PV6M6V |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50157439

(3-((2R,3S)-3-((S)-2-((S)-2-((S)-2-amino-3-(2H-tetr...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)C(O)=O Show InChI InChI=1S/C33H44N10O8/c1-17(2)13-24(38-30(47)25(18(3)4)39-28(45)22(34)16-35-32(49)27-40-42-43-41-27)29(46)37-23(14-19-9-6-5-7-10-19)26(44)31(48)36-21-12-8-11-20(15-21)33(50)51/h5-12,15,17-18,22-26,44H,13-14,16,34H2,1-4H3,(H,35,49)(H,36,48)(H,37,46)(H,38,47)(H,39,45)(H,50,51)(H,40,41,42,43)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of BACE1 |
Bioorg Med Chem 19: 5238-46 (2011)
Article DOI: 10.1016/j.bmc.2011.07.002
BindingDB Entry DOI: 10.7270/Q20Z73NP |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317671
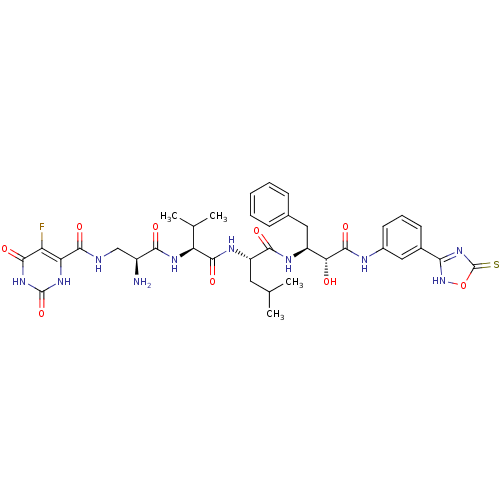
(CHEMBL1094663 | N-((2S,5S)-5-((S)-1-((1R,2S)-1-(3-...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1[nH]c(=O)[nH]c(=O)c1F)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nc(=S)o[nH]1 |r| Show InChI InChI=1S/C37H45FN10O9S/c1-17(2)13-24(43-34(54)26(18(3)4)44-30(50)22(39)16-40-33(53)27-25(38)32(52)47-36(56)45-27)31(51)42-23(14-19-9-6-5-7-10-19)28(49)35(55)41-21-12-8-11-20(15-21)29-46-37(58)57-48-29/h5-12,15,17-18,22-24,26,28,49H,13-14,16,39H2,1-4H3,(H,40,53)(H,41,55)(H,42,51)(H,43,54)(H,44,50)(H,46,48,58)(H2,45,47,52,56)/t22-,23-,24-,26-,28+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET assay |
Bioorg Med Chem 18: 3175-86 (2010)
Article DOI: 10.1016/j.bmc.2010.03.032
BindingDB Entry DOI: 10.7270/Q26M36ZV |
More data for this
Ligand-Target Pair | |
Protease
(Human T-cell leukemia virus type I) | BDBM50483599
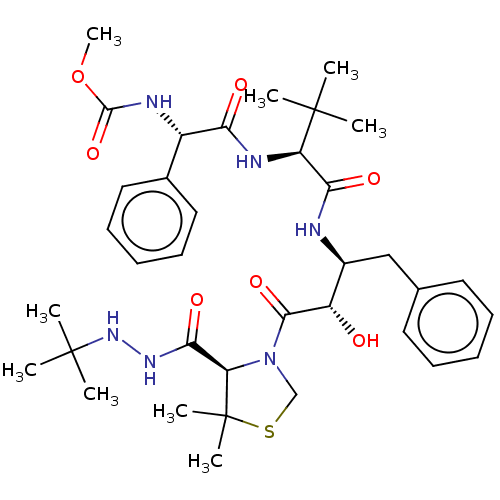
(CHEMBL1761460 | KNI-10605)Show SMILES COC(=O)N[C@H](C(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)NNC(C)(C)C)C(C)(C)C)c1ccccc1 |r| Show InChI InChI=1S/C36H52N6O7S/c1-34(2,3)27(39-29(44)25(38-33(48)49-9)23-18-14-11-15-19-23)30(45)37-24(20-22-16-12-10-13-17-22)26(43)32(47)42-21-50-36(7,8)28(42)31(46)40-41-35(4,5)6/h10-19,24-28,41,43H,20-21H2,1-9H3,(H,37,45)(H,38,48)(H,39,44)(H,40,46)/t24-,25-,26-,27+,28+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HTLV-1 recombinant protease L40I mutant |
Bioorg Med Chem Lett 21: 2425-9 (2011)
Article DOI: 10.1016/j.bmcl.2011.02.066
BindingDB Entry DOI: 10.7270/Q27W6G18 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317670
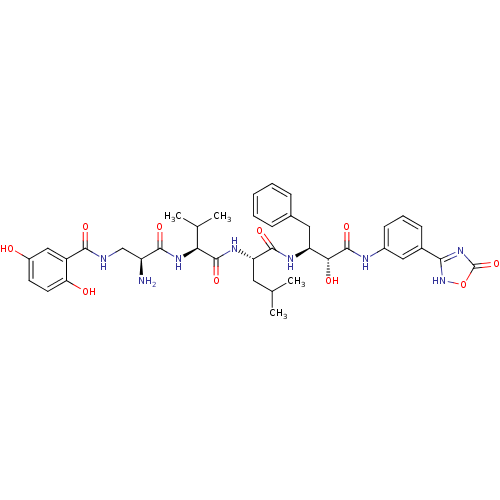
(CHEMBL1094660 | N-((2S,5S)-5-((S)-1-((1R,2S)-1-(3-...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1cc(O)ccc1O)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nc(=O)o[nH]1 |r| Show InChI InChI=1S/C39H48N8O10/c1-20(2)15-29(44-37(54)31(21(3)4)45-35(52)27(40)19-41-34(51)26-18-25(48)13-14-30(26)49)36(53)43-28(16-22-9-6-5-7-10-22)32(50)38(55)42-24-12-8-11-23(17-24)33-46-39(56)57-47-33/h5-14,17-18,20-21,27-29,31-32,48-50H,15-16,19,40H2,1-4H3,(H,41,51)(H,42,55)(H,43,53)(H,44,54)(H,45,52)(H,46,47,56)/t27-,28-,29-,31-,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET assay |
Bioorg Med Chem 18: 3175-86 (2010)
Article DOI: 10.1016/j.bmc.2010.03.032
BindingDB Entry DOI: 10.7270/Q26M36ZV |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50317672
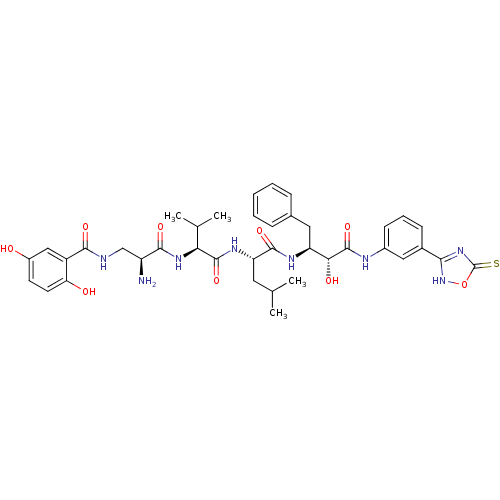
(CHEMBL1094664 | N-((2S,5S)-5-((S)-1-((1R,2S)-1-(3-...)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1cc(O)ccc1O)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cccc(c1)-c1nc(=S)o[nH]1 |r| Show InChI InChI=1S/C39H48N8O9S/c1-20(2)15-29(44-37(54)31(21(3)4)45-35(52)27(40)19-41-34(51)26-18-25(48)13-14-30(26)49)36(53)43-28(16-22-9-6-5-7-10-22)32(50)38(55)42-24-12-8-11-23(17-24)33-46-39(57)56-47-33/h5-14,17-18,20-21,27-29,31-32,48-50H,15-16,19,40H2,1-4H3,(H,41,51)(H,42,55)(H,43,53)(H,44,54)(H,45,52)(H,46,47,57)/t27-,28-,29-,31-,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human BACE1 by FRET assay |
Bioorg Med Chem 18: 3175-86 (2010)
Article DOI: 10.1016/j.bmc.2010.03.032
BindingDB Entry DOI: 10.7270/Q26M36ZV |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50351103
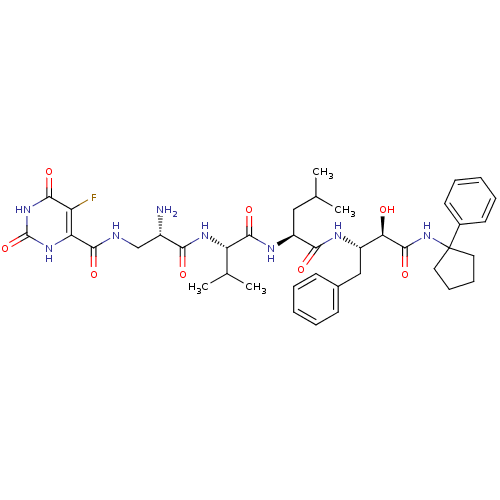
(CHEMBL1817745)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1[nH]c(=O)[nH]c(=O)c1F)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)NC1(CCCC1)c1ccccc1 |r| Show InChI InChI=1S/C40H53FN8O8/c1-22(2)19-28(45-37(55)30(23(3)4)46-33(51)26(42)21-43-36(54)31-29(41)35(53)48-39(57)47-31)34(52)44-27(20-24-13-7-5-8-14-24)32(50)38(56)49-40(17-11-12-18-40)25-15-9-6-10-16-25/h5-10,13-16,22-23,26-28,30,32,50H,11-12,17-21,42H2,1-4H3,(H,43,54)(H,44,52)(H,45,55)(H,46,51)(H,49,56)(H2,47,48,53,57)/t26-,27-,28-,30-,32+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 by FRET assay |
Bioorg Med Chem 19: 5238-46 (2011)
Article DOI: 10.1016/j.bmc.2011.07.002
BindingDB Entry DOI: 10.7270/Q20Z73NP |
More data for this
Ligand-Target Pair | |
Protease
(Human T-cell leukemia virus type I) | BDBM50483482
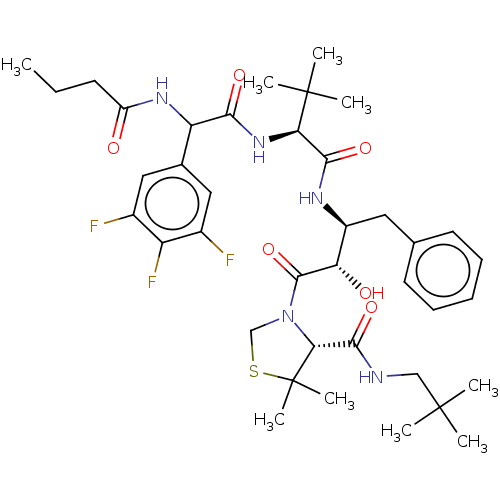
(CHEMBL3349018)Show SMILES CCCC(=O)NC(C(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)NCC(C)(C)C)C(C)(C)C)c1cc(F)c(F)c(F)c1 |r| Show InChI InChI=1S/C39H54F3N5O6S/c1-10-14-27(48)45-29(23-18-24(40)28(42)25(41)19-23)33(50)46-31(38(5,6)7)34(51)44-26(17-22-15-12-11-13-16-22)30(49)36(53)47-21-54-39(8,9)32(47)35(52)43-20-37(2,3)4/h11-13,15-16,18-19,26,29-32,49H,10,14,17,20-21H2,1-9H3,(H,43,52)(H,44,51)(H,45,48)(H,46,50)/t26-,29?,30-,31+,32+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HTLV-1 protease L40I mutant by fluorimetric assay |
Bioorg Med Chem Lett 21: 1832-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.048
BindingDB Entry DOI: 10.7270/Q2JD50N4 |
More data for this
Ligand-Target Pair | |
Protease
(Human T-cell leukemia virus type I) | BDBM50483482

(CHEMBL3349018)Show SMILES CCCC(=O)NC(C(=O)N[C@H](C(=O)N[C@@H](Cc1ccccc1)[C@H](O)C(=O)N1CSC(C)(C)[C@H]1C(=O)NCC(C)(C)C)C(C)(C)C)c1cc(F)c(F)c(F)c1 |r| Show InChI InChI=1S/C39H54F3N5O6S/c1-10-14-27(48)45-29(23-18-24(40)28(42)25(41)19-23)33(50)46-31(38(5,6)7)34(51)44-26(17-22-15-12-11-13-16-22)30(49)36(53)47-21-54-39(8,9)32(47)35(52)43-20-37(2,3)4/h11-13,15-16,18-19,26,29-32,49H,10,14,17,20-21H2,1-9H3,(H,43,52)(H,44,51)(H,45,48)(H,46,50)/t26-,29?,30-,31+,32+/m0/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of HTLV-1 protease L40I mutant by fluorimetric assay |
Bioorg Med Chem Lett 21: 1832-7 (2011)
Article DOI: 10.1016/j.bmcl.2011.01.048
BindingDB Entry DOI: 10.7270/Q2JD50N4 |
More data for this
Ligand-Target Pair | |
Beta-secretase 1
(Homo sapiens (Human)) | BDBM50372558
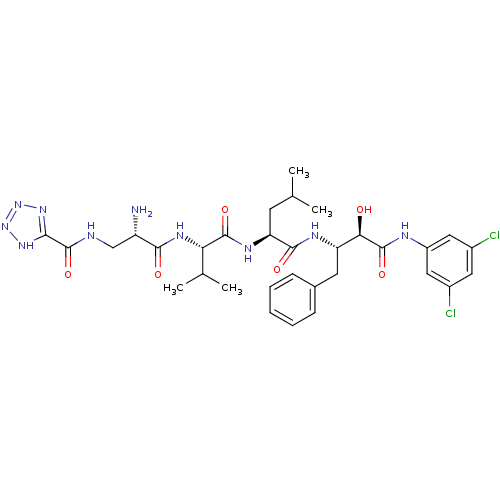
(CHEMBL257587)Show SMILES CC(C)C[C@H](NC(=O)[C@@H](NC(=O)[C@@H](N)CNC(=O)c1nnn[nH]1)C(C)C)C(=O)N[C@@H](Cc1ccccc1)[C@@H](O)C(=O)Nc1cc(Cl)cc(Cl)c1 Show InChI InChI=1S/C32H42Cl2N10O6/c1-16(2)10-24(39-30(48)25(17(3)4)40-28(46)22(35)15-36-32(50)27-41-43-44-42-27)29(47)38-23(11-18-8-6-5-7-9-18)26(45)31(49)37-21-13-19(33)12-20(34)14-21/h5-9,12-14,16-17,22-26,45H,10-11,15,35H2,1-4H3,(H,36,50)(H,37,49)(H,38,47)(H,39,48)(H,40,46)(H,41,42,43,44)/t22-,23-,24-,25-,26+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant BACE1 |
Bioorg Med Chem Lett 18: 1649-53 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.058
BindingDB Entry DOI: 10.7270/Q2PV6M6V |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data