

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50140241 (Allopurinol | Aloral | Aluline 100 | Aluline 300 |...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Medicinal Materials Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4625-8 (2012) Article DOI: 10.1016/j.bmcl.2012.05.051 BindingDB Entry DOI: 10.7270/Q2S46VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50203065 (3-phenylacrylaldehyde | 3-phenylprop-2-enal | CHEM...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 5.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Medicinal Materials Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4625-8 (2012) Article DOI: 10.1016/j.bmcl.2012.05.051 BindingDB Entry DOI: 10.7270/Q2S46VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50485313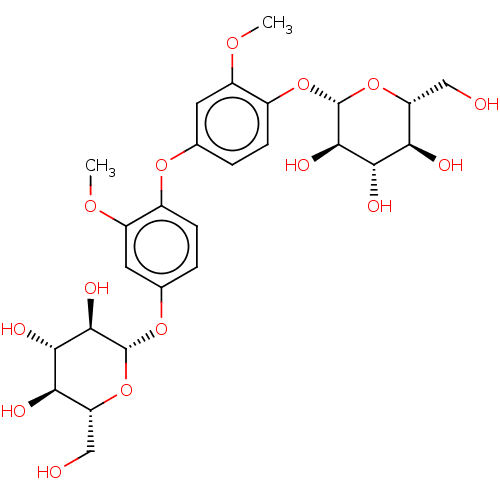 (Cinnacasolide C) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | >8.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Medicinal Materials Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4625-8 (2012) Article DOI: 10.1016/j.bmcl.2012.05.051 BindingDB Entry DOI: 10.7270/Q2S46VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50240642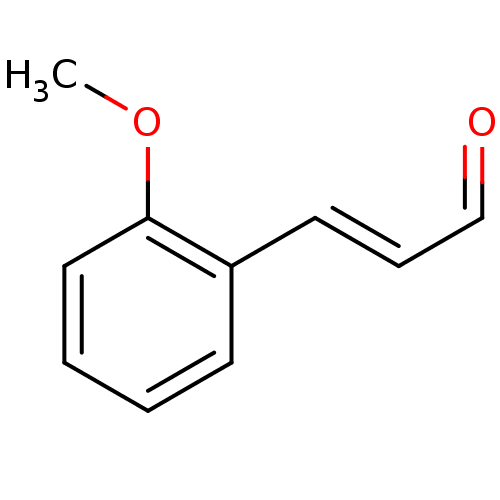 ((E)-3-(2-Methoxy-phenyl)-propenal | 2-methoxy cinn...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Medicinal Materials Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4625-8 (2012) Article DOI: 10.1016/j.bmcl.2012.05.051 BindingDB Entry DOI: 10.7270/Q2S46VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM7568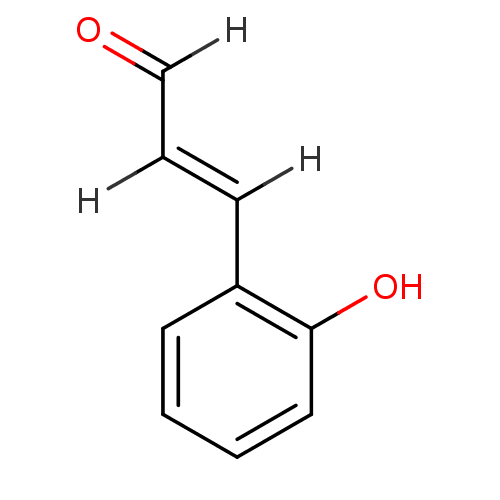 ((2E)-3-(2-hydroxyphenyl)prop-2-enal | Cinnamaldehy...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.01E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Medicinal Materials Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4625-8 (2012) Article DOI: 10.1016/j.bmcl.2012.05.051 BindingDB Entry DOI: 10.7270/Q2S46VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50485315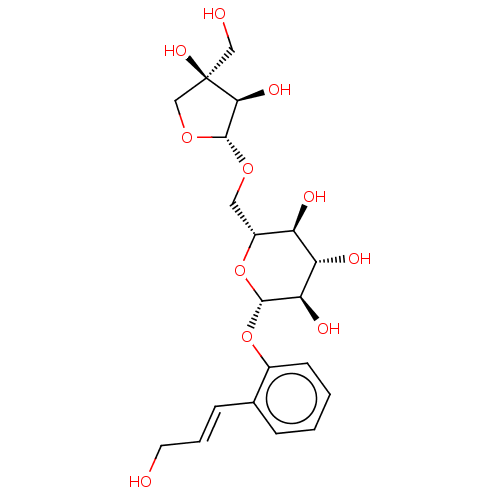 (Cinnacasolide B) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Medicinal Materials Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4625-8 (2012) Article DOI: 10.1016/j.bmcl.2012.05.051 BindingDB Entry DOI: 10.7270/Q2S46VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50485314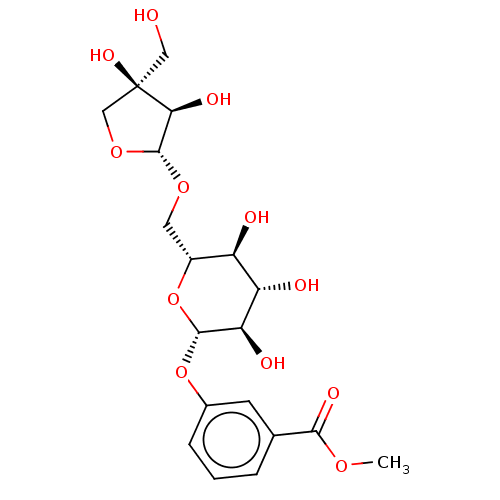 (Cinnacasolide A) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.12E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Medicinal Materials Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4625-8 (2012) Article DOI: 10.1016/j.bmcl.2012.05.051 BindingDB Entry DOI: 10.7270/Q2S46VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50310452 (CHEMBL1083511 | rosavin) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.16E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Medicinal Materials Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4625-8 (2012) Article DOI: 10.1016/j.bmcl.2012.05.051 BindingDB Entry DOI: 10.7270/Q2S46VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50310450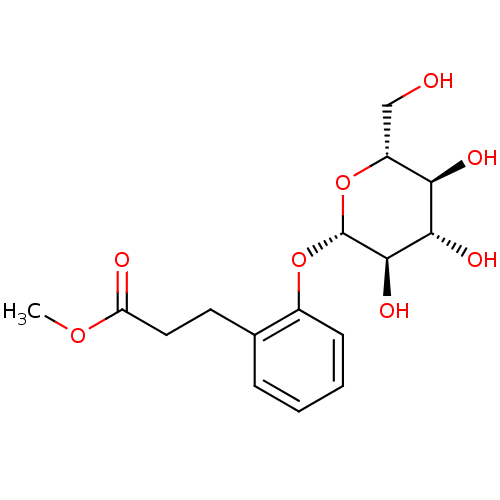 (CHEMBL1087939 | methyl dihydromelilotoside) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.46E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Medicinal Materials Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4625-8 (2012) Article DOI: 10.1016/j.bmcl.2012.05.051 BindingDB Entry DOI: 10.7270/Q2S46VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50310449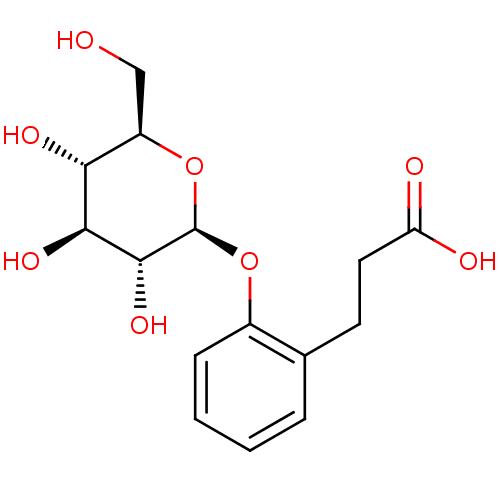 (CHEMBL1087938 | dihydromelilotoside) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.52E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Medicinal Materials Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4625-8 (2012) Article DOI: 10.1016/j.bmcl.2012.05.051 BindingDB Entry DOI: 10.7270/Q2S46VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM16430 ((2E)-3-phenylprop-2-enoic acid | CHEMBL27246 | Cin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents | Article PubMed | n/a | n/a | 1.75E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Medicinal Materials Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4625-8 (2012) Article DOI: 10.1016/j.bmcl.2012.05.051 BindingDB Entry DOI: 10.7270/Q2S46VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50146462 ((2E)-3-(2-hydroxyphenyl)-2-propenoic acid | (2E)-3...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.94E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Medicinal Materials Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4625-8 (2012) Article DOI: 10.1016/j.bmcl.2012.05.051 BindingDB Entry DOI: 10.7270/Q2S46VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50211193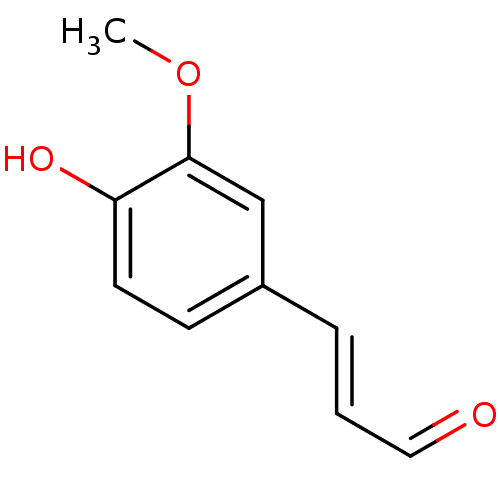 (3-(4-hydroxy-3-methoxyphenyl)prop-2-enal | CHEMBL2...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 2.02E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Medicinal Materials Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4625-8 (2012) Article DOI: 10.1016/j.bmcl.2012.05.051 BindingDB Entry DOI: 10.7270/Q2S46VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Xanthine dehydrogenase/oxidase (Homo sapiens (Human)) | BDBM50310446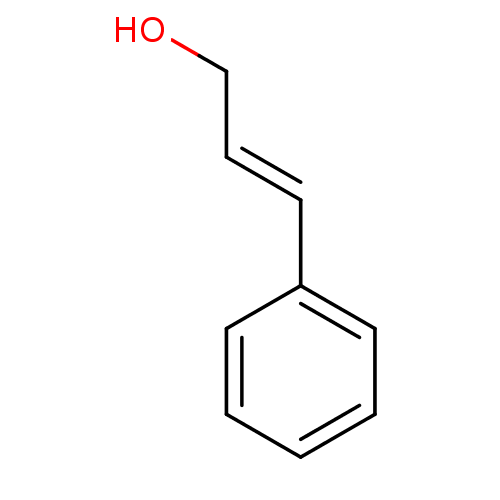 ((E)-3-Phenyl-prop-2-en-1-ol | 3-phenylprop-2-en-1-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | >3.72E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
National Institute of Medicinal Materials Curated by ChEMBL | Assay Description Inhibition of xanthine oxidase- mediated uric acid formation after 5 mins by spectrophotometry | Bioorg Med Chem Lett 22: 4625-8 (2012) Article DOI: 10.1016/j.bmcl.2012.05.051 BindingDB Entry DOI: 10.7270/Q2S46VVZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50394342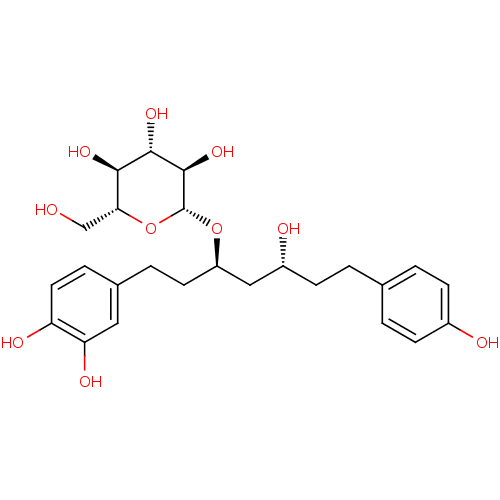 (CHEMBL458899) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.29E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARalpha ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50394343 (CHEMBL516827) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARalpha ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50394344 (CHEMBL2159607) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARalpha ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50394345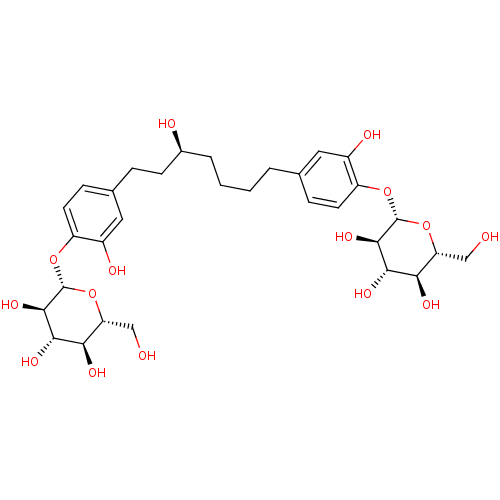 (CHEMBL2159606) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARalpha ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50394346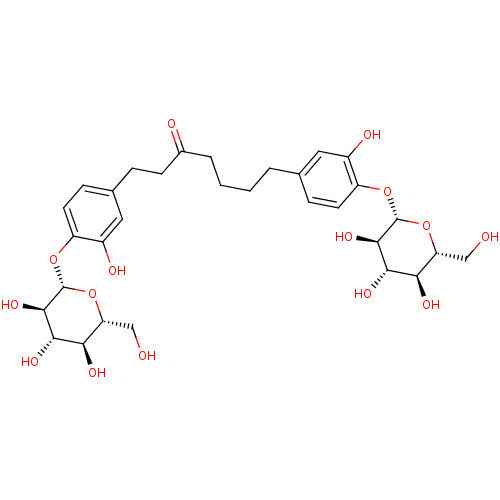 (CHEMBL2159605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 4.71E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARalpha ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50394344 (CHEMBL2159607) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.01E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARbeta (delta) ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50394341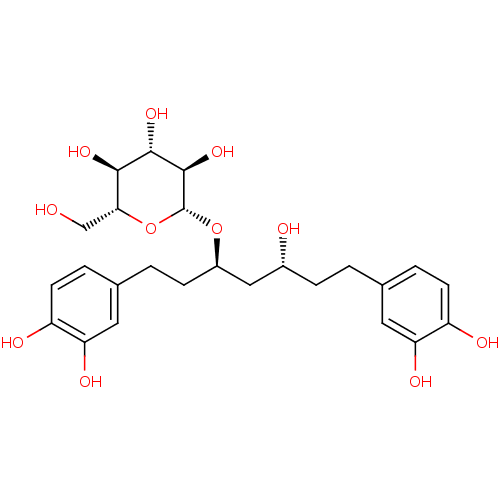 (CHEMBL465800) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARalpha ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor alpha (Homo sapiens (Human)) | BDBM50371235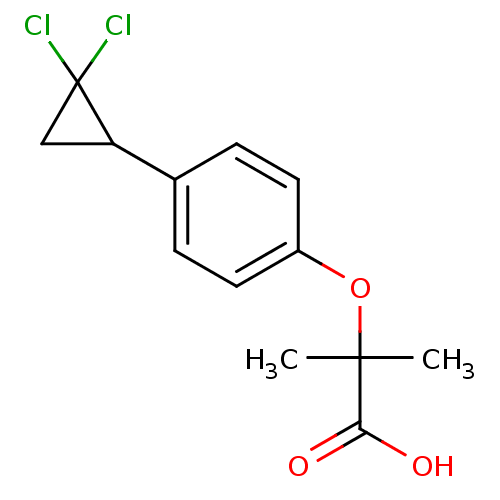 (CIPROFIBRATE) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | n/a | n/a | 900 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARalpha ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50394346 (CHEMBL2159605) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARgamma ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50394345 (CHEMBL2159606) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 3.31E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARgamma ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50394344 (CHEMBL2159607) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARgamma ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50394343 (CHEMBL516827) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARgamma ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50394342 (CHEMBL458899) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARgamma ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50394341 (CHEMBL465800) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARgamma ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50085045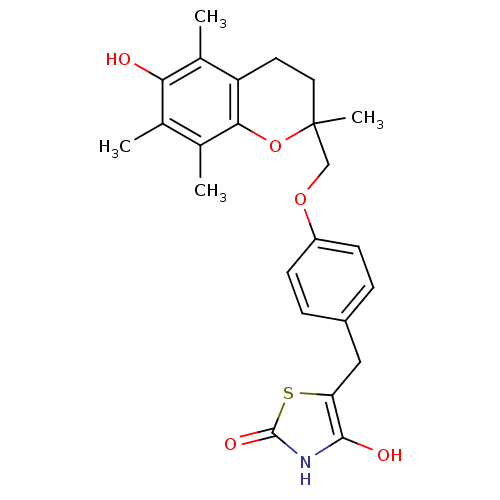 (5-((4-((6-hydroxy-2,5,7,8-tetramethyl-3,4-dihydro-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | n/a | n/a | 720 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARgamma ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50394346 (CHEMBL2159605) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARbeta (delta) ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50394345 (CHEMBL2159606) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.31E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARbeta (delta) ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50394343 (CHEMBL516827) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.93E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARbeta (delta) ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50394342 (CHEMBL458899) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 1.56E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARbeta (delta) ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50085041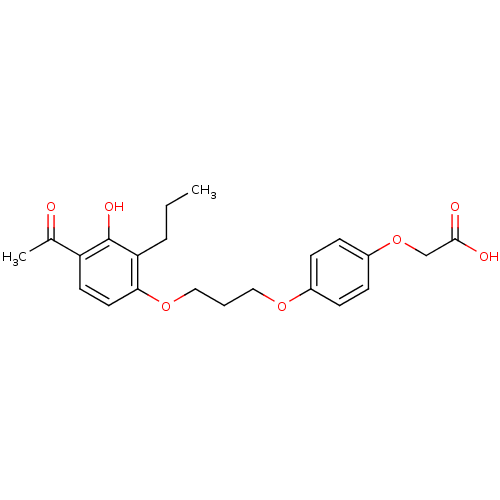 (2-(4-(3-(4-acetyl-3-hydroxy-2-propylphenoxy)propox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 650 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARbeta (delta) ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor delta (Homo sapiens (Human)) | BDBM50394341 (CHEMBL465800) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2.31E+4 | n/a | n/a | n/a | n/a |
Chungnam National University Curated by ChEMBL | Assay Description Transactivation of GAL4-fused PPARbeta (delta) ligand binding domain transfected in human HepG2 cells after 20 hrs by luciferase reporter gene assay | Bioorg Med Chem Lett 22: 6681-7 (2012) Article DOI: 10.1016/j.bmcl.2012.08.099 BindingDB Entry DOI: 10.7270/Q28C9XCD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||