 Found 139 hits with Last Name = 'ning' and Initial = 'z'
Found 139 hits with Last Name = 'ning' and Initial = 'z' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM402042
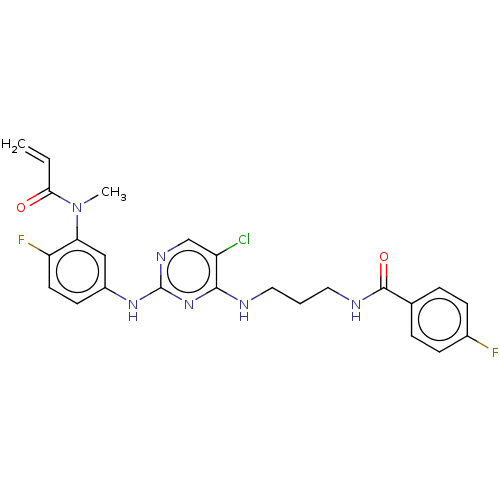
(Preparation of N-(3-(5-chloro-2-(4-fluoro-3-(N-met...)Show SMILES CN(C(=O)C=C)c1cc(Nc2ncc(Cl)c(NCCCNC(=O)c3ccc(F)cc3)n2)ccc1F Show InChI InChI=1S/C24H23ClF2N6O2/c1-3-21(34)33(2)20-13-17(9-10-19(20)27)31-24-30-14-18(25)22(32-24)28-11-4-12-29-23(35)15-5-7-16(26)8-6-15/h3,5-10,13-14H,1,4,11-12H2,2H3,(H,29,35)(H2,28,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Briefly, according to the requirements of different kinase reactions, 0.2 μL test compound (50 μM, dissolved in dimethyl sulfoxide (DMSO)) ... |
J Med Chem 52: 7081-9 (2009)
BindingDB Entry DOI: 10.7270/Q2D22106 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM402040
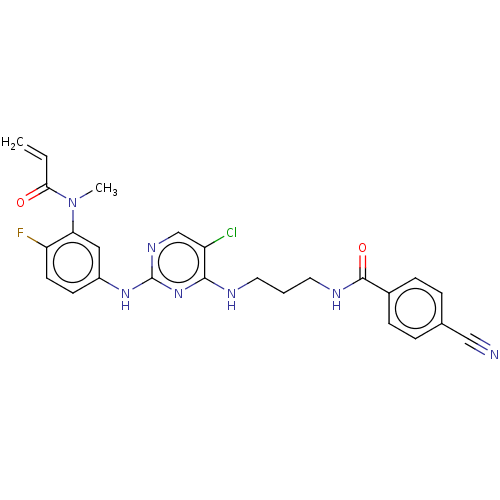
(Preparation of N-(3-(5-chloro-2-(4-fluoro-3-(N-met...)Show SMILES CN(C(=O)C=C)c1cc(Nc2ncc(Cl)c(NCCCNC(=O)c3ccc(cc3)C#N)n2)ccc1F Show InChI InChI=1S/C25H23ClFN7O2/c1-3-22(35)34(2)21-13-18(9-10-20(21)27)32-25-31-15-19(26)23(33-25)29-11-4-12-30-24(36)17-7-5-16(14-28)6-8-17/h3,5-10,13,15H,1,4,11-12H2,2H3,(H,30,36)(H2,29,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Briefly, according to the requirements of different kinase reactions, 0.2 μL test compound (50 μM, dissolved in dimethyl sulfoxide (DMSO)) ... |
J Med Chem 52: 7081-9 (2009)
BindingDB Entry DOI: 10.7270/Q2D22106 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK3
(Homo sapiens (Human)) | BDBM402041
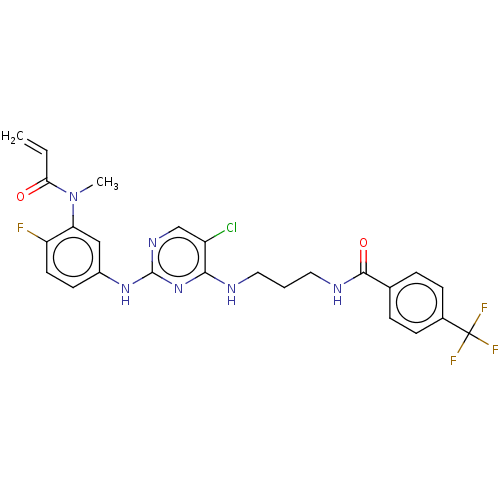
(Preparation of N-(3-(5-chloro-2-(4-fluoro-3-(N-met...)Show SMILES CN(C(=O)C=C)c1cc(Nc2ncc(Cl)c(NCCCNC(=O)c3ccc(cc3)C(F)(F)F)n2)ccc1F Show InChI InChI=1S/C25H23ClF4N6O2/c1-3-21(37)36(2)20-13-17(9-10-19(20)27)34-24-33-14-18(26)22(35-24)31-11-4-12-32-23(38)15-5-7-16(8-6-15)25(28,29)30/h3,5-10,13-14H,1,4,11-12H2,2H3,(H,32,38)(H2,31,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Briefly, according to the requirements of different kinase reactions, 0.2 μL test compound (50 μM, dissolved in dimethyl sulfoxide (DMSO)) ... |
J Med Chem 52: 7081-9 (2009)
BindingDB Entry DOI: 10.7270/Q2D22106 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM402042

(Preparation of N-(3-(5-chloro-2-(4-fluoro-3-(N-met...)Show SMILES CN(C(=O)C=C)c1cc(Nc2ncc(Cl)c(NCCCNC(=O)c3ccc(F)cc3)n2)ccc1F Show InChI InChI=1S/C24H23ClF2N6O2/c1-3-21(34)33(2)20-13-17(9-10-19(20)27)31-24-30-14-18(25)22(32-24)28-11-4-12-29-23(35)15-5-7-16(26)8-6-15/h3,5-10,13-14H,1,4,11-12H2,2H3,(H,29,35)(H2,28,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Briefly, according to the requirements of different kinase reactions, 0.2 μL test compound (50 μM, dissolved in dimethyl sulfoxide (DMSO)) ... |
J Med Chem 52: 7081-9 (2009)
BindingDB Entry DOI: 10.7270/Q2D22106 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50334368

(CHEMBL1643325 | N-(2-aminophenyl)-3-(4-(2-(4'-cycl...)Show SMILES Nc1ccccc1NC(=O)\C=C\c1ccc(cc1)C(NCCN1CCCCC1)C(=O)Nc1ccc(cc1)C1CC1 Show InChI InChI=1S/C33H39N5O2/c34-29-6-2-3-7-30(29)37-31(39)19-10-24-8-11-27(12-9-24)32(35-20-23-38-21-4-1-5-22-38)33(40)36-28-17-15-26(16-18-28)25-13-14-25/h2-3,6-12,15-19,25,32,35H,1,4-5,13-14,20-23,34H2,(H,36,40)(H,37,39)/b19-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 21: 110-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.063
BindingDB Entry DOI: 10.7270/Q2S182S8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50399006
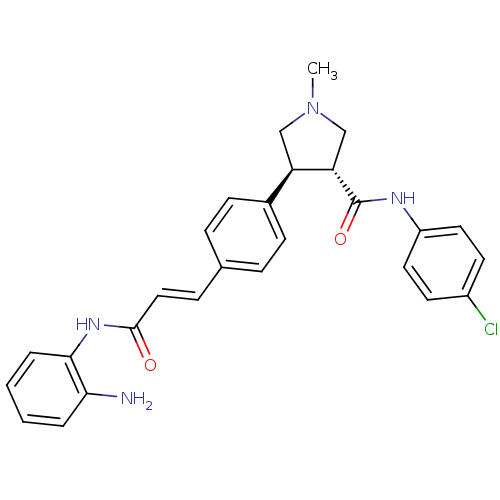
(CHEMBL2177587)Show SMILES CN1C[C@@H]([C@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H27ClN4O2/c1-32-16-22(23(17-32)27(34)30-21-13-11-20(28)12-14-21)19-9-6-18(7-10-19)8-15-26(33)31-25-5-3-2-4-24(25)29/h2-15,22-23H,16-17,29H2,1H3,(H,30,34)(H,31,33)/b15-8+/t22-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50334366
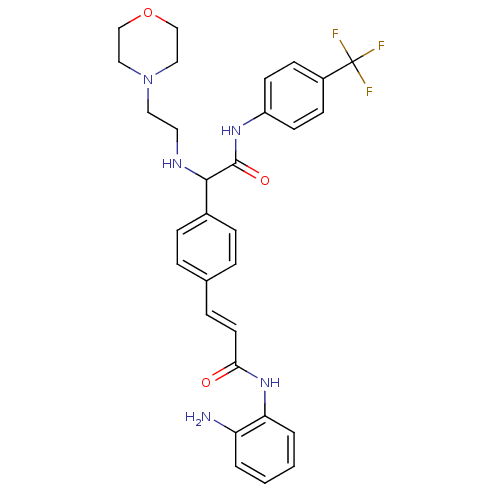
(CHEMBL1643308 | N-(2-aminophenyl)-3-(4-(1-(2-morph...)Show SMILES Nc1ccccc1NC(=O)\C=C\c1ccc(cc1)C(NCCN1CCOCC1)C(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C30H32F3N5O3/c31-30(32,33)23-10-12-24(13-11-23)36-29(40)28(35-15-16-38-17-19-41-20-18-38)22-8-5-21(6-9-22)7-14-27(39)37-26-4-2-1-3-25(26)34/h1-14,28,35H,15-20,34H2,(H,36,40)(H,37,39)/b14-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 21: 110-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.063
BindingDB Entry DOI: 10.7270/Q2S182S8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50334366

(CHEMBL1643308 | N-(2-aminophenyl)-3-(4-(1-(2-morph...)Show SMILES Nc1ccccc1NC(=O)\C=C\c1ccc(cc1)C(NCCN1CCOCC1)C(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C30H32F3N5O3/c31-30(32,33)23-10-12-24(13-11-23)36-29(40)28(35-15-16-38-17-19-41-20-18-38)22-8-5-21(6-9-22)7-14-27(39)37-26-4-2-1-3-25(26)34/h1-14,28,35H,15-20,34H2,(H,36,40)(H,37,39)/b14-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50399004
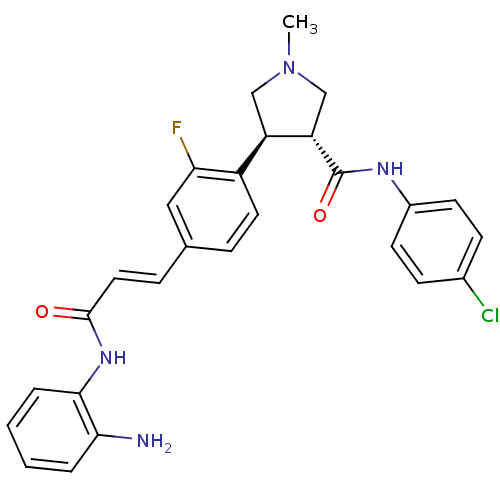
(CHEMBL2177582)Show SMILES CN1C[C@@H]([C@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1F)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H26ClFN4O2/c1-33-15-21(22(16-33)27(35)31-19-10-8-18(28)9-11-19)20-12-6-17(14-23(20)29)7-13-26(34)32-25-5-3-2-4-24(25)30/h2-14,21-22H,15-16,30H2,1H3,(H,31,35)(H,32,34)/b13-7+/t21-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM402040

(Preparation of N-(3-(5-chloro-2-(4-fluoro-3-(N-met...)Show SMILES CN(C(=O)C=C)c1cc(Nc2ncc(Cl)c(NCCCNC(=O)c3ccc(cc3)C#N)n2)ccc1F Show InChI InChI=1S/C25H23ClFN7O2/c1-3-22(35)34(2)21-13-18(9-10-20(21)27)32-25-31-15-19(26)23(33-25)29-11-4-12-30-24(36)17-7-5-16(14-28)6-8-17/h3,5-10,13,15H,1,4,11-12H2,2H3,(H,30,36)(H2,29,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 105 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Briefly, according to the requirements of different kinase reactions, 0.2 μL test compound (50 μM, dissolved in dimethyl sulfoxide (DMSO)) ... |
J Med Chem 52: 7081-9 (2009)
BindingDB Entry DOI: 10.7270/Q2D22106 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 3
(Homo sapiens (Human)) | BDBM402040

(Preparation of N-(3-(5-chloro-2-(4-fluoro-3-(N-met...)Show SMILES CN(C(=O)C=C)c1cc(Nc2ncc(Cl)c(NCCCNC(=O)c3ccc(cc3)C#N)n2)ccc1F Show InChI InChI=1S/C25H23ClFN7O2/c1-3-22(35)34(2)21-13-18(9-10-20(21)27)32-25-31-15-19(26)23(33-25)29-11-4-12-30-24(36)17-7-5-16(14-28)6-8-17/h3,5-10,13,15H,1,4,11-12H2,2H3,(H,30,36)(H2,29,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 107 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Briefly, according to the requirements of different kinase reactions, 0.2 μL test compound (50 μM, dissolved in dimethyl sulfoxide (DMSO)) ... |
J Med Chem 52: 7081-9 (2009)
BindingDB Entry DOI: 10.7270/Q2D22106 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50399005
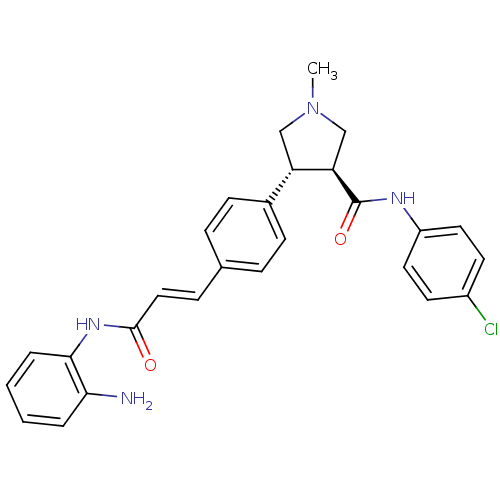
(CHEMBL2177588)Show SMILES CN1C[C@H]([C@@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H27ClN4O2/c1-32-16-22(23(17-32)27(34)30-21-13-11-20(28)12-14-21)19-9-6-18(7-10-19)8-15-26(33)31-25-5-3-2-4-24(25)29/h2-15,22-23H,16-17,29H2,1H3,(H,30,34)(H,31,33)/b15-8+/t22-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM402040

(Preparation of N-(3-(5-chloro-2-(4-fluoro-3-(N-met...)Show SMILES CN(C(=O)C=C)c1cc(Nc2ncc(Cl)c(NCCCNC(=O)c3ccc(cc3)C#N)n2)ccc1F Show InChI InChI=1S/C25H23ClFN7O2/c1-3-22(35)34(2)21-13-18(9-10-20(21)27)32-25-31-15-19(26)23(33-25)29-11-4-12-30-24(36)17-7-5-16(14-28)6-8-17/h3,5-10,13,15H,1,4,11-12H2,2H3,(H,30,36)(H2,29,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 162 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Briefly, according to the requirements of different kinase reactions, 0.2 μL test compound (50 μM, dissolved in dimethyl sulfoxide (DMSO)) ... |
J Med Chem 52: 7081-9 (2009)
BindingDB Entry DOI: 10.7270/Q2D22106 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM402042

(Preparation of N-(3-(5-chloro-2-(4-fluoro-3-(N-met...)Show SMILES CN(C(=O)C=C)c1cc(Nc2ncc(Cl)c(NCCCNC(=O)c3ccc(F)cc3)n2)ccc1F Show InChI InChI=1S/C24H23ClF2N6O2/c1-3-21(34)33(2)20-13-17(9-10-19(20)27)31-24-30-14-18(25)22(32-24)28-11-4-12-29-23(35)15-5-7-16(26)8-6-15/h3,5-10,13-14H,1,4,11-12H2,2H3,(H,29,35)(H2,28,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 173 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Briefly, according to the requirements of different kinase reactions, 0.2 μL test compound (50 μM, dissolved in dimethyl sulfoxide (DMSO)) ... |
J Med Chem 52: 7081-9 (2009)
BindingDB Entry DOI: 10.7270/Q2D22106 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50334367
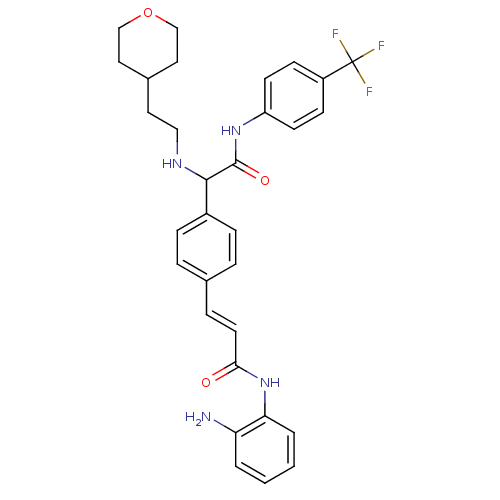
(CHEMBL1643315 | N-(2-aminophenyl)-3-(4-(2-oxo-1-(2...)Show SMILES Nc1ccccc1NC(=O)\C=C\c1ccc(cc1)C(NCCC1CCOCC1)C(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C31H33F3N4O3/c32-31(33,34)24-10-12-25(13-11-24)37-30(40)29(36-18-15-22-16-19-41-20-17-22)23-8-5-21(6-9-23)7-14-28(39)38-27-4-2-1-3-26(27)35/h1-14,22,29,36H,15-20,35H2,(H,37,40)(H,38,39)/b14-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 21: 110-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.063
BindingDB Entry DOI: 10.7270/Q2S182S8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50399005

(CHEMBL2177588)Show SMILES CN1C[C@H]([C@@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H27ClN4O2/c1-32-16-22(23(17-32)27(34)30-21-13-11-20(28)12-14-21)19-9-6-18(7-10-19)8-15-26(33)31-25-5-3-2-4-24(25)29/h2-15,22-23H,16-17,29H2,1H3,(H,30,34)(H,31,33)/b15-8+/t22-,23+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50399004

(CHEMBL2177582)Show SMILES CN1C[C@@H]([C@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1F)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H26ClFN4O2/c1-33-15-21(22(16-33)27(35)31-19-10-8-18(28)9-11-19)20-12-6-17(14-23(20)29)7-13-26(34)32-25-5-3-2-4-24(25)30/h2-14,21-22H,15-16,30H2,1H3,(H,31,35)(H,32,34)/b13-7+/t21-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50399004

(CHEMBL2177582)Show SMILES CN1C[C@@H]([C@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1F)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H26ClFN4O2/c1-33-15-21(22(16-33)27(35)31-19-10-8-18(28)9-11-19)20-12-6-17(14-23(20)29)7-13-26(34)32-25-5-3-2-4-24(25)30/h2-14,21-22H,15-16,30H2,1H3,(H,31,35)(H,32,34)/b13-7+/t21-,22+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50399006

(CHEMBL2177587)Show SMILES CN1C[C@@H]([C@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H27ClN4O2/c1-32-16-22(23(17-32)27(34)30-21-13-11-20(28)12-14-21)19-9-6-18(7-10-19)8-15-26(33)31-25-5-3-2-4-24(25)29/h2-15,22-23H,16-17,29H2,1H3,(H,30,34)(H,31,33)/b15-8+/t22-,23+/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50334368

(CHEMBL1643325 | N-(2-aminophenyl)-3-(4-(2-(4'-cycl...)Show SMILES Nc1ccccc1NC(=O)\C=C\c1ccc(cc1)C(NCCN1CCCCC1)C(=O)Nc1ccc(cc1)C1CC1 Show InChI InChI=1S/C33H39N5O2/c34-29-6-2-3-7-30(29)37-31(39)19-10-24-8-11-27(12-9-24)32(35-20-23-38-21-4-1-5-22-38)33(40)36-28-17-15-26(16-18-28)25-13-14-25/h2-3,6-12,15-19,25,32,35H,1,4-5,13-14,20-23,34H2,(H,36,40)(H,37,39)/b19-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 |
Bioorg Med Chem Lett 21: 110-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.063
BindingDB Entry DOI: 10.7270/Q2S182S8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50399006

(CHEMBL2177587)Show SMILES CN1C[C@@H]([C@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H27ClN4O2/c1-32-16-22(23(17-32)27(34)30-21-13-11-20(28)12-14-21)19-9-6-18(7-10-19)8-15-26(33)31-25-5-3-2-4-24(25)29/h2-15,22-23H,16-17,29H2,1H3,(H,30,34)(H,31,33)/b15-8+/t22-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50399005

(CHEMBL2177588)Show SMILES CN1C[C@H]([C@@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H27ClN4O2/c1-32-16-22(23(17-32)27(34)30-21-13-11-20(28)12-14-21)19-9-6-18(7-10-19)8-15-26(33)31-25-5-3-2-4-24(25)29/h2-15,22-23H,16-17,29H2,1H3,(H,30,34)(H,31,33)/b15-8+/t22-,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK1
(Homo sapiens (Human)) | BDBM402041

(Preparation of N-(3-(5-chloro-2-(4-fluoro-3-(N-met...)Show SMILES CN(C(=O)C=C)c1cc(Nc2ncc(Cl)c(NCCCNC(=O)c3ccc(cc3)C(F)(F)F)n2)ccc1F Show InChI InChI=1S/C25H23ClF4N6O2/c1-3-21(37)36(2)20-13-17(9-10-19(20)27)34-24-33-14-18(26)22(35-24)31-11-4-12-32-23(38)15-5-7-16(8-6-15)25(28,29)30/h3,5-10,13-14H,1,4,11-12H2,2H3,(H,32,38)(H2,31,33,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 279 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Briefly, according to the requirements of different kinase reactions, 0.2 μL test compound (50 μM, dissolved in dimethyl sulfoxide (DMSO)) ... |
J Med Chem 52: 7081-9 (2009)
BindingDB Entry DOI: 10.7270/Q2D22106 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM402040

(Preparation of N-(3-(5-chloro-2-(4-fluoro-3-(N-met...)Show SMILES CN(C(=O)C=C)c1cc(Nc2ncc(Cl)c(NCCCNC(=O)c3ccc(cc3)C#N)n2)ccc1F Show InChI InChI=1S/C25H23ClFN7O2/c1-3-22(35)34(2)21-13-18(9-10-20(21)27)32-25-31-15-19(26)23(33-25)29-11-4-12-30-24(36)17-7-5-16(14-28)6-8-17/h3,5-10,13,15H,1,4,11-12H2,2H3,(H,30,36)(H2,29,31,32,33) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 286 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Briefly, according to the requirements of different kinase reactions, 0.2 μL test compound (50 μM, dissolved in dimethyl sulfoxide (DMSO)) ... |
J Med Chem 52: 7081-9 (2009)
BindingDB Entry DOI: 10.7270/Q2D22106 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50334366

(CHEMBL1643308 | N-(2-aminophenyl)-3-(4-(1-(2-morph...)Show SMILES Nc1ccccc1NC(=O)\C=C\c1ccc(cc1)C(NCCN1CCOCC1)C(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C30H32F3N5O3/c31-30(32,33)23-10-12-24(13-11-23)36-29(40)28(35-15-16-38-17-19-41-20-18-38)22-8-5-21(6-9-22)7-14-27(39)37-26-4-2-1-3-25(26)34/h1-14,28,35H,15-20,34H2,(H,36,40)(H,37,39)/b14-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 |
Bioorg Med Chem Lett 21: 110-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.063
BindingDB Entry DOI: 10.7270/Q2S182S8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase ITK/TSK
(Homo sapiens (Human)) | BDBM402040

(Preparation of N-(3-(5-chloro-2-(4-fluoro-3-(N-met...)Show SMILES CN(C(=O)C=C)c1cc(Nc2ncc(Cl)c(NCCCNC(=O)c3ccc(cc3)C#N)n2)ccc1F Show InChI InChI=1S/C25H23ClFN7O2/c1-3-22(35)34(2)21-13-18(9-10-20(21)27)32-25-31-15-19(26)23(33-25)29-11-4-12-30-24(36)17-7-5-16(14-28)6-8-17/h3,5-10,13,15H,1,4,11-12H2,2H3,(H,30,36)(H2,29,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 319 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Briefly, according to the requirements of different kinase reactions, 0.2 μL test compound (50 μM, dissolved in dimethyl sulfoxide (DMSO)) ... |
J Med Chem 52: 7081-9 (2009)
BindingDB Entry DOI: 10.7270/Q2D22106 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50334368

(CHEMBL1643325 | N-(2-aminophenyl)-3-(4-(2-(4'-cycl...)Show SMILES Nc1ccccc1NC(=O)\C=C\c1ccc(cc1)C(NCCN1CCCCC1)C(=O)Nc1ccc(cc1)C1CC1 Show InChI InChI=1S/C33H39N5O2/c34-29-6-2-3-7-30(29)37-31(39)19-10-24-8-11-27(12-9-24)32(35-20-23-38-21-4-1-5-22-38)33(40)36-28-17-15-26(16-18-28)25-13-14-25/h2-3,6-12,15-19,25,32,35H,1,4-5,13-14,20-23,34H2,(H,36,40)(H,37,39)/b19-10+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 |
Bioorg Med Chem Lett 21: 110-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.063
BindingDB Entry DOI: 10.7270/Q2S182S8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50334366

(CHEMBL1643308 | N-(2-aminophenyl)-3-(4-(1-(2-morph...)Show SMILES Nc1ccccc1NC(=O)\C=C\c1ccc(cc1)C(NCCN1CCOCC1)C(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C30H32F3N5O3/c31-30(32,33)23-10-12-24(13-11-23)36-29(40)28(35-15-16-38-17-19-41-20-18-38)22-8-5-21(6-9-22)7-14-27(39)37-26-4-2-1-3-25(26)34/h1-14,28,35H,15-20,34H2,(H,36,40)(H,37,39)/b14-7+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 |
Bioorg Med Chem Lett 21: 110-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.063
BindingDB Entry DOI: 10.7270/Q2S182S8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 |
Bioorg Med Chem Lett 21: 110-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.063
BindingDB Entry DOI: 10.7270/Q2S182S8 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase TBK1
(Homo sapiens (Human)) | BDBM402042

(Preparation of N-(3-(5-chloro-2-(4-fluoro-3-(N-met...)Show SMILES CN(C(=O)C=C)c1cc(Nc2ncc(Cl)c(NCCCNC(=O)c3ccc(F)cc3)n2)ccc1F Show InChI InChI=1S/C24H23ClF2N6O2/c1-3-21(34)33(2)20-13-17(9-10-19(20)27)31-24-30-14-18(25)22(32-24)28-11-4-12-29-23(35)15-5-7-16(26)8-6-15/h3,5-10,13-14H,1,4,11-12H2,2H3,(H,29,35)(H2,28,30,31,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 664 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Briefly, according to the requirements of different kinase reactions, 0.2 μL test compound (50 μM, dissolved in dimethyl sulfoxide (DMSO)) ... |
J Med Chem 52: 7081-9 (2009)
BindingDB Entry DOI: 10.7270/Q2D22106 |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM402040

(Preparation of N-(3-(5-chloro-2-(4-fluoro-3-(N-met...)Show SMILES CN(C(=O)C=C)c1cc(Nc2ncc(Cl)c(NCCCNC(=O)c3ccc(cc3)C#N)n2)ccc1F Show InChI InChI=1S/C25H23ClFN7O2/c1-3-22(35)34(2)21-13-18(9-10-20(21)27)32-25-31-15-19(26)23(33-25)29-11-4-12-30-24(36)17-7-5-16(14-28)6-8-17/h3,5-10,13,15H,1,4,11-12H2,2H3,(H,30,36)(H2,29,31,32,33) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 698 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Briefly, according to the requirements of different kinase reactions, 0.2 μL test compound (50 μM, dissolved in dimethyl sulfoxide (DMSO)) ... |
J Med Chem 52: 7081-9 (2009)
BindingDB Entry DOI: 10.7270/Q2D22106 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50399008
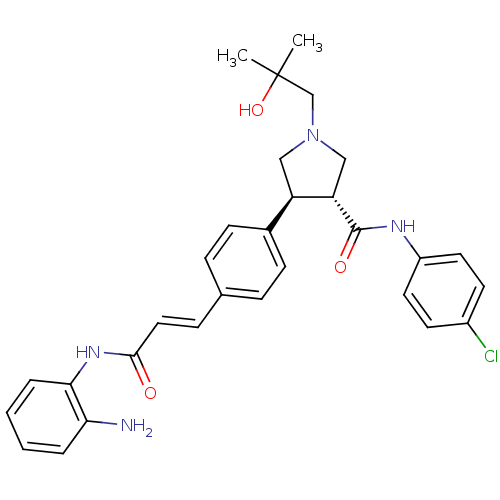
(CHEMBL2178223)Show SMILES CC(C)(O)CN1C[C@@H]([C@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C30H33ClN4O3/c1-30(2,38)19-35-17-24(25(18-35)29(37)33-23-14-12-22(31)13-15-23)21-10-7-20(8-11-21)9-16-28(36)34-27-6-4-3-5-26(27)32/h3-16,24-25,38H,17-19,32H2,1-2H3,(H,33,37)(H,34,36)/b16-9+/t24-,25+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes in presence of NADPH by LC-MS/MS method |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50334367

(CHEMBL1643315 | N-(2-aminophenyl)-3-(4-(2-oxo-1-(2...)Show SMILES Nc1ccccc1NC(=O)\C=C\c1ccc(cc1)C(NCCC1CCOCC1)C(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C31H33F3N4O3/c32-31(33,34)24-10-12-25(13-11-24)37-30(40)29(36-18-15-22-16-19-41-20-17-22)23-8-5-21(6-9-23)7-14-28(39)38-27-4-2-1-3-26(27)35/h1-14,22,29,36H,15-20,35H2,(H,37,40)(H,38,39)/b14-7+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 |
Bioorg Med Chem Lett 21: 110-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.063
BindingDB Entry DOI: 10.7270/Q2S182S8 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM402042

(Preparation of N-(3-(5-chloro-2-(4-fluoro-3-(N-met...)Show SMILES CN(C(=O)C=C)c1cc(Nc2ncc(Cl)c(NCCCNC(=O)c3ccc(F)cc3)n2)ccc1F Show InChI InChI=1S/C24H23ClF2N6O2/c1-3-21(34)33(2)20-13-17(9-10-19(20)27)31-24-30-14-18(25)22(32-24)28-11-4-12-29-23(35)15-5-7-16(26)8-6-15/h3,5-10,13-14H,1,4,11-12H2,2H3,(H,29,35)(H2,28,30,31,32) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 855 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Briefly, according to the requirements of different kinase reactions, 0.2 μL test compound (50 μM, dissolved in dimethyl sulfoxide (DMSO)) ... |
J Med Chem 52: 7081-9 (2009)
BindingDB Entry DOI: 10.7270/Q2D22106 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC2 |
Bioorg Med Chem Lett 21: 110-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.063
BindingDB Entry DOI: 10.7270/Q2S182S8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50334367

(CHEMBL1643315 | N-(2-aminophenyl)-3-(4-(2-oxo-1-(2...)Show SMILES Nc1ccccc1NC(=O)\C=C\c1ccc(cc1)C(NCCC1CCOCC1)C(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C31H33F3N4O3/c32-31(33,34)24-10-12-25(13-11-24)37-30(40)29(36-18-15-22-16-19-41-20-17-22)23-8-5-21(6-9-23)7-14-28(39)38-27-4-2-1-3-26(27)35/h1-14,22,29,36H,15-20,35H2,(H,37,40)(H,38,39)/b14-7+ | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 |
Bioorg Med Chem Lett 21: 110-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.063
BindingDB Entry DOI: 10.7270/Q2S182S8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM19410

(CHEMBL27759 | MS-275 | US11377423, MS-275 | US1167...)Show InChI InChI=1S/C21H20N4O3/c22-18-5-1-2-6-19(18)25-20(26)17-9-7-15(8-10-17)13-24-21(27)28-14-16-4-3-11-23-12-16/h1-12H,13-14,22H2,(H,24,27)(H,25,26) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center China Ltd
Curated by ChEMBL
| Assay Description
Inhibition of HDAC3 |
Bioorg Med Chem Lett 21: 110-6 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.063
BindingDB Entry DOI: 10.7270/Q2S182S8 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50399005

(CHEMBL2177588)Show SMILES CN1C[C@H]([C@@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H27ClN4O2/c1-32-16-22(23(17-32)27(34)30-21-13-11-20(28)12-14-21)19-9-6-18(7-10-19)8-15-26(33)31-25-5-3-2-4-24(25)29/h2-15,22-23H,16-17,29H2,1H3,(H,30,34)(H,31,33)/b15-8+/t22-,23+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC5 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Vascular endothelial growth factor receptor 1
(Homo sapiens (Human)) | BDBM402042

(Preparation of N-(3-(5-chloro-2-(4-fluoro-3-(N-met...)Show SMILES CN(C(=O)C=C)c1cc(Nc2ncc(Cl)c(NCCCNC(=O)c3ccc(F)cc3)n2)ccc1F Show InChI InChI=1S/C24H23ClF2N6O2/c1-3-21(34)33(2)20-13-17(9-10-19(20)27)31-24-30-14-18(25)22(32-24)28-11-4-12-29-23(35)15-5-7-16(26)8-6-15/h3,5-10,13-14H,1,4,11-12H2,2H3,(H,29,35)(H2,28,30,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Briefly, according to the requirements of different kinase reactions, 0.2 μL test compound (50 μM, dissolved in dimethyl sulfoxide (DMSO)) ... |
J Med Chem 52: 7081-9 (2009)
BindingDB Entry DOI: 10.7270/Q2D22106 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM402040

(Preparation of N-(3-(5-chloro-2-(4-fluoro-3-(N-met...)Show SMILES CN(C(=O)C=C)c1cc(Nc2ncc(Cl)c(NCCCNC(=O)c3ccc(cc3)C#N)n2)ccc1F Show InChI InChI=1S/C25H23ClFN7O2/c1-3-22(35)34(2)21-13-18(9-10-20(21)27)32-25-31-15-19(26)23(33-25)29-11-4-12-30-24(36)17-7-5-16(14-28)6-8-17/h3,5-10,13,15H,1,4,11-12H2,2H3,(H,30,36)(H2,29,31,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Briefly, according to the requirements of different kinase reactions, 0.2 μL test compound (50 μM, dissolved in dimethyl sulfoxide (DMSO)) ... |
J Med Chem 52: 7081-9 (2009)
BindingDB Entry DOI: 10.7270/Q2D22106 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50399005

(CHEMBL2177588)Show SMILES CN1C[C@H]([C@@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H27ClN4O2/c1-32-16-22(23(17-32)27(34)30-21-13-11-20(28)12-14-21)19-9-6-18(7-10-19)8-15-26(33)31-25-5-3-2-4-24(25)29/h2-15,22-23H,16-17,29H2,1H3,(H,30,34)(H,31,33)/b15-8+/t22-,23+/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase Blk
(Homo sapiens (Human)) | BDBM402041

(Preparation of N-(3-(5-chloro-2-(4-fluoro-3-(N-met...)Show SMILES CN(C(=O)C=C)c1cc(Nc2ncc(Cl)c(NCCCNC(=O)c3ccc(cc3)C(F)(F)F)n2)ccc1F Show InChI InChI=1S/C25H23ClF4N6O2/c1-3-21(37)36(2)20-13-17(9-10-19(20)27)34-24-33-14-18(26)22(35-24)31-11-4-12-32-23(38)15-5-7-16(8-6-15)25(28,29)30/h3,5-10,13-14H,1,4,11-12H2,2H3,(H,32,38)(H2,31,33,34,35) | Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Briefly, according to the requirements of different kinase reactions, 0.2 μL test compound (50 μM, dissolved in dimethyl sulfoxide (DMSO)) ... |
J Med Chem 52: 7081-9 (2009)
BindingDB Entry DOI: 10.7270/Q2D22106 |
More data for this
Ligand-Target Pair | |
Tyrosine-protein kinase JAK2
(Homo sapiens (Human)) | BDBM402042

(Preparation of N-(3-(5-chloro-2-(4-fluoro-3-(N-met...)Show SMILES CN(C(=O)C=C)c1cc(Nc2ncc(Cl)c(NCCCNC(=O)c3ccc(F)cc3)n2)ccc1F Show InChI InChI=1S/C24H23ClF2N6O2/c1-3-21(34)33(2)20-13-17(9-10-19(20)27)31-24-30-14-18(25)22(32-24)28-11-4-12-29-23(35)15-5-7-16(26)8-6-15/h3,5-10,13-14H,1,4,11-12H2,2H3,(H,29,35)(H2,28,30,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research
| Assay Description
Briefly, according to the requirements of different kinase reactions, 0.2 μL test compound (50 μM, dissolved in dimethyl sulfoxide (DMSO)) ... |
J Med Chem 52: 7081-9 (2009)
BindingDB Entry DOI: 10.7270/Q2D22106 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50399007
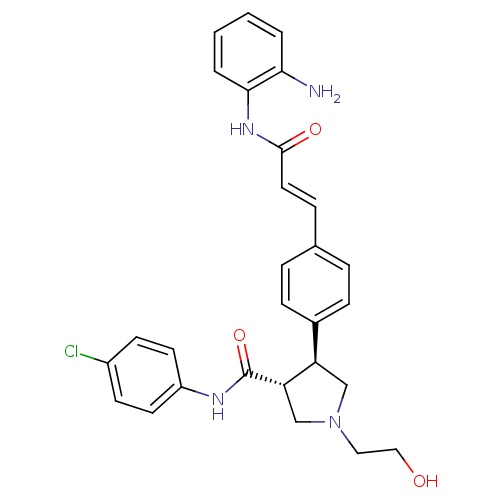
(CHEMBL2178221)Show SMILES Nc1ccccc1NC(=O)\C=C\c1ccc(cc1)[C@H]1CN(CCO)C[C@@H]1C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H29ClN4O3/c29-21-10-12-22(13-11-21)31-28(36)24-18-33(15-16-34)17-23(24)20-8-5-19(6-9-20)7-14-27(35)32-26-4-2-1-3-25(26)30/h1-14,23-24,34H,15-18,30H2,(H,31,36)(H,32,35)/b14-7+/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes in presence of NADPH by LC-MS/MS method |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50399007

(CHEMBL2178221)Show SMILES Nc1ccccc1NC(=O)\C=C\c1ccc(cc1)[C@H]1CN(CCO)C[C@@H]1C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H29ClN4O3/c29-21-10-12-22(13-11-21)31-28(36)24-18-33(15-16-34)17-23(24)20-8-5-19(6-9-20)7-14-27(35)32-26-4-2-1-3-25(26)30/h1-14,23-24,34H,15-18,30H2,(H,31,36)(H,32,35)/b14-7+/t23-,24+/m1/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 in human liver microsomes in presence of NADPH by LC-MS/MS method |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50399007

(CHEMBL2178221)Show SMILES Nc1ccccc1NC(=O)\C=C\c1ccc(cc1)[C@H]1CN(CCO)C[C@@H]1C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C28H29ClN4O3/c29-21-10-12-22(13-11-21)31-28(36)24-18-33(15-16-34)17-23(24)20-8-5-19(6-9-20)7-14-27(35)32-26-4-2-1-3-25(26)30/h1-14,23-24,34H,15-18,30H2,(H,31,36)(H,32,35)/b14-7+/t23-,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 in human liver microsomes in presence of NADPH by LC-MS/MS method |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50399008

(CHEMBL2178223)Show SMILES CC(C)(O)CN1C[C@@H]([C@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C30H33ClN4O3/c1-30(2,38)19-35-17-24(25(18-35)29(37)33-23-14-12-22(31)13-15-23)21-10-7-20(8-11-21)9-16-28(36)34-27-6-4-3-5-26(27)32/h3-16,24-25,38H,17-19,32H2,1-2H3,(H,33,37)(H,34,36)/b16-9+/t24-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes in presence of NADPH by LC-MS/MS method |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50399006

(CHEMBL2177587)Show SMILES CN1C[C@@H]([C@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H27ClN4O2/c1-32-16-22(23(17-32)27(34)30-21-13-11-20(28)12-14-21)19-9-6-18(7-10-19)8-15-26(33)31-25-5-3-2-4-24(25)29/h2-15,22-23H,16-17,29H2,1H3,(H,30,34)(H,31,33)/b15-8+/t22-,23+/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC10 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50399006

(CHEMBL2177587)Show SMILES CN1C[C@@H]([C@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H27ClN4O2/c1-32-16-22(23(17-32)27(34)30-21-13-11-20(28)12-14-21)19-9-6-18(7-10-19)8-15-26(33)31-25-5-3-2-4-24(25)29/h2-15,22-23H,16-17,29H2,1H3,(H,30,34)(H,31,33)/b15-8+/t22-,23+/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of HDAC5 using p53 (379 to 382 residues) based fluorogenic peptide substrate |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50399004

(CHEMBL2177582)Show SMILES CN1C[C@@H]([C@H](C1)c1ccc(\C=C\C(=O)Nc2ccccc2N)cc1F)C(=O)Nc1ccc(Cl)cc1 |r| Show InChI InChI=1S/C27H26ClFN4O2/c1-33-15-21(22(16-33)27(35)31-19-10-8-18(28)9-11-19)20-12-6-17(14-23(20)29)7-13-26(34)32-25-5-3-2-4-24(25)30/h2-14,21-22H,15-16,30H2,1H3,(H,31,35)(H,32,34)/b13-7+/t21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Roche R&D Center-China Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 in human liver microsomes in presence of NADPH by LC-MS/MS method |
J Med Chem 55: 8903-25 (2012)
Article DOI: 10.1021/jm3011838
BindingDB Entry DOI: 10.7270/Q2TT4S3Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data