

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4227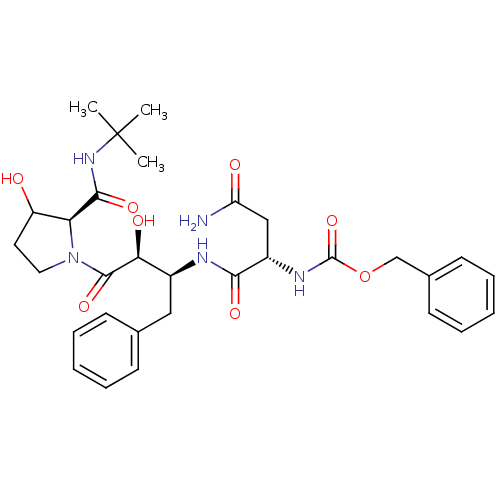 (AHPBA 35a | Z-Asn.(2S,3S)-AHPBA-[3(R)-hydroxy]Pro ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 0.100 | -59.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM126701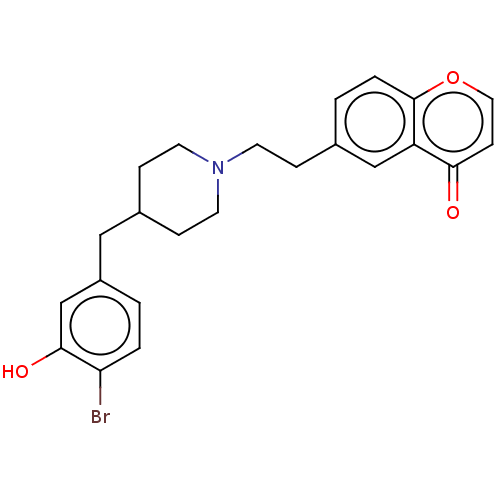 (US8778970, 5-9) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.270 | -54.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Dainippon Sumitomo Pharma Co., Ltd US Patent | Assay Description The experiment was carried out according to the method of Yabuuchi et al. [Yabuuchi K. et al., Biogenic Amines, 18, 319-328 (2004)]. 50 ul of [3H] 8-... | US Patent US8778970 (2014) BindingDB Entry DOI: 10.7270/Q2N878GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM126698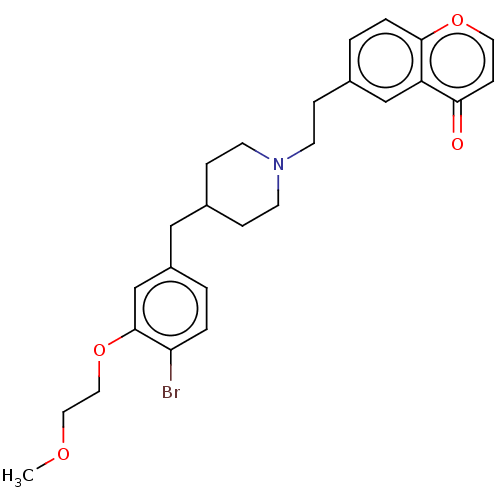 (US8778970, 5-6) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.270 | -54.6 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Dainippon Sumitomo Pharma Co., Ltd US Patent | Assay Description [3H] citalopram binding was assayed according to the method of Owens et al. [Owens M. J. et al., J. Pharm. Exp. Ther., 283, 1305-1322 (1997)]. Specif... | US Patent US8778970 (2014) BindingDB Entry DOI: 10.7270/Q2N878GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50460160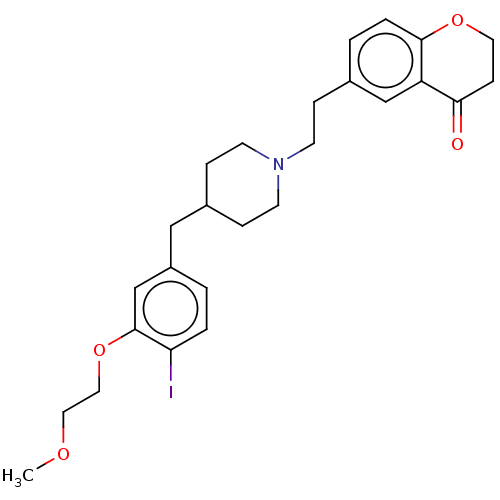 (CHEMBL4229131) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma CO., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram from human SERT expressed in HEK293 cell membranes after 60 mins by liquid scintillation counting | Bioorg Med Chem 26: 1614-1627 (2018) Article DOI: 10.1016/j.bmc.2018.02.008 BindingDB Entry DOI: 10.7270/Q2CN76HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50126916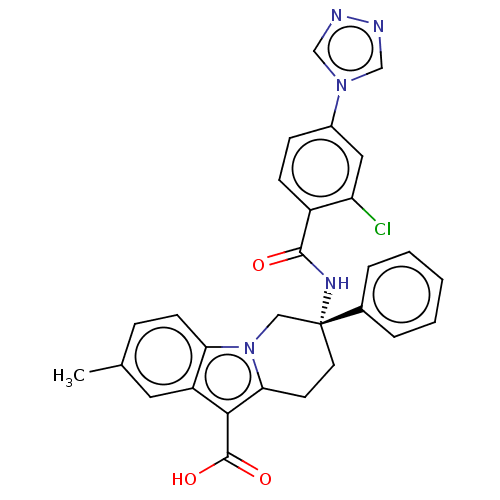 (CHEMBL3629111 | US10351558, Example 139) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay | Bioorg Med Chem Lett 25: 5437-43 (2015) Article DOI: 10.1016/j.bmcl.2015.07.078 BindingDB Entry DOI: 10.7270/Q2B859X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50126919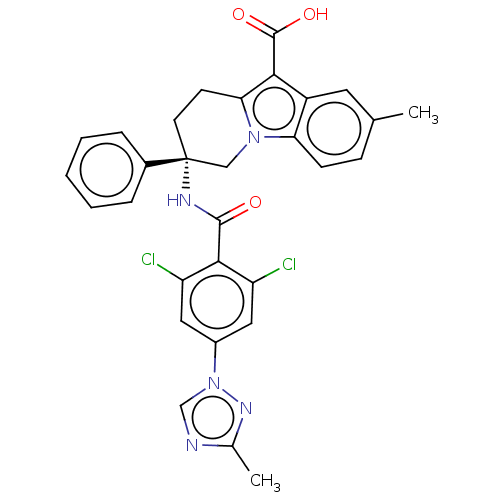 (CHEMBL3629114) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay | Bioorg Med Chem Lett 25: 5437-43 (2015) Article DOI: 10.1016/j.bmcl.2015.07.078 BindingDB Entry DOI: 10.7270/Q2B859X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50460148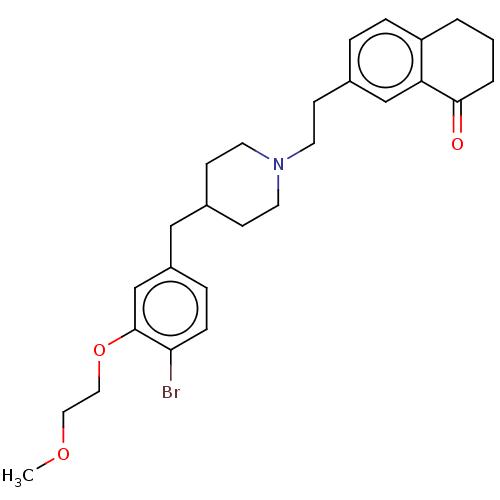 (CHEMBL4226053) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma CO., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram from human SERT expressed in HEK293 cell membranes after 60 mins by liquid scintillation counting | Bioorg Med Chem 26: 1614-1627 (2018) Article DOI: 10.1016/j.bmc.2018.02.008 BindingDB Entry DOI: 10.7270/Q2CN76HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50126872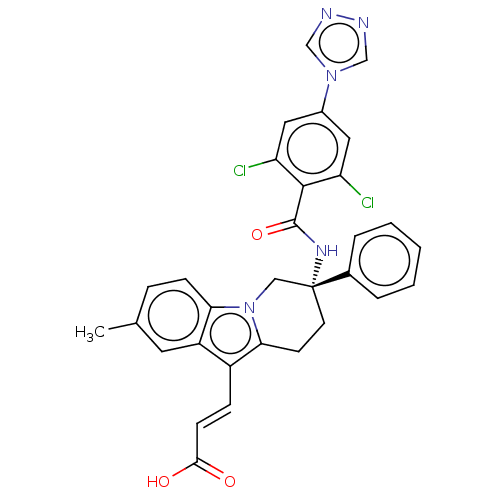 (CHEMBL3628964) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay | Bioorg Med Chem Lett 25: 5437-43 (2015) Article DOI: 10.1016/j.bmcl.2015.07.078 BindingDB Entry DOI: 10.7270/Q2B859X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM126709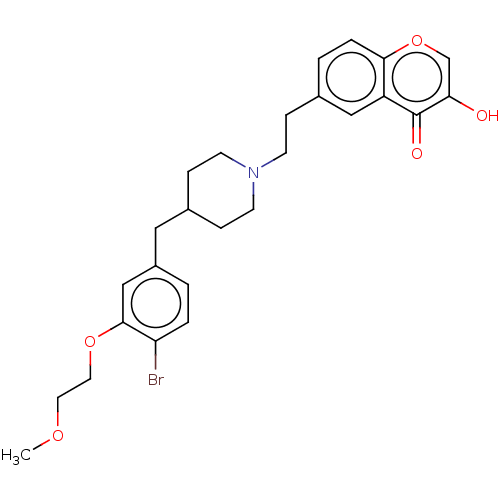 (US8778970, 5-14) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.880 | -51.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Dainippon Sumitomo Pharma Co., Ltd US Patent | Assay Description [3H] citalopram binding was assayed according to the method of Owens et al. [Owens M. J. et al., J. Pharm. Exp. Ther., 283, 1305-1322 (1997)]. Specif... | US Patent US8778970 (2014) BindingDB Entry DOI: 10.7270/Q2N878GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50460159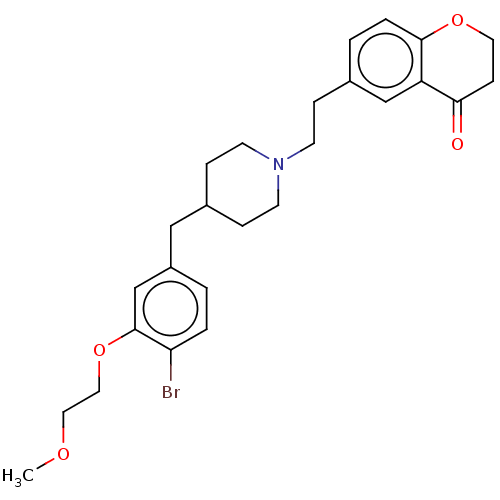 (CHEMBL4226281) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma CO., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram from human SERT expressed in HEK293 cell membranes after 60 mins by liquid scintillation counting | Bioorg Med Chem 26: 1614-1627 (2018) Article DOI: 10.1016/j.bmc.2018.02.008 BindingDB Entry DOI: 10.7270/Q2CN76HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4224 (AHPBA 32a | Z.Asn.( 2S,3S).AHPBA. [ 4( S)-morpholi...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -53.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dimer of Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM4216 (AHPBA 24a | Z.Asn-(2S,3S)-AHPBA-[4(S)-hydroxy]Pro ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1 | -53.4 | n/a | n/a | n/a | n/a | n/a | 4.7 | 37 |
Sankyo Co. Ltd. | Assay Description The inhibitory activities of the compounds toward HIV-1 PR were determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as... | Bioorg Med Chem 4: 1365-77 (1996) Article DOI: 10.1016/0968-0896(96)00130-7 BindingDB Entry DOI: 10.7270/Q2V9868K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50125979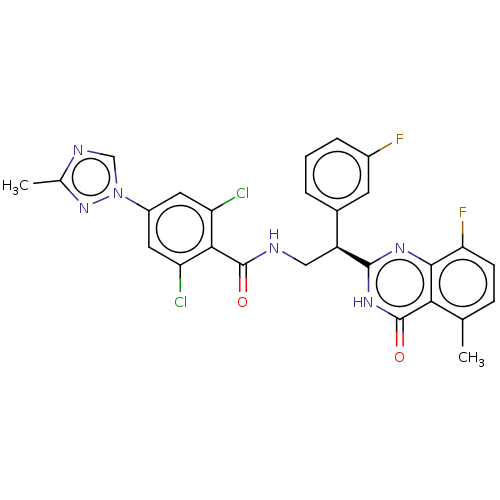 (CHEMBL3627899) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate | Bioorg Med Chem Lett 25: 4945-9 (2015) Article DOI: 10.1016/j.bmcl.2015.04.057 BindingDB Entry DOI: 10.7270/Q20Z753B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM126697 (US8778970, 5-5) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.10 | -51.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Dainippon Sumitomo Pharma Co., Ltd US Patent | Assay Description The experiment was carried out according to the method of Yabuuchi et al. [Yabuuchi K. et al., Biogenic Amines, 18, 319-328 (2004)]. 50 ul of [3H] 8-... | US Patent US8778970 (2014) BindingDB Entry DOI: 10.7270/Q2N878GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50126956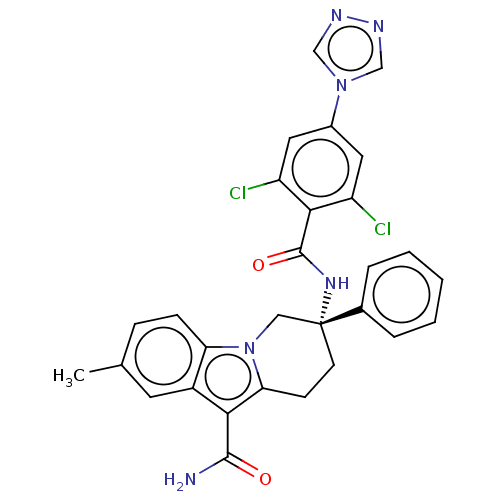 (CHEMBL3628961 | US10351558, Example 174) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay | Bioorg Med Chem Lett 25: 5437-43 (2015) Article DOI: 10.1016/j.bmcl.2015.07.078 BindingDB Entry DOI: 10.7270/Q2B859X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50460149 (CHEMBL4228477) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma CO., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram from human SERT expressed in HEK293 cell membranes after 60 mins by liquid scintillation counting | Bioorg Med Chem 26: 1614-1627 (2018) Article DOI: 10.1016/j.bmc.2018.02.008 BindingDB Entry DOI: 10.7270/Q2CN76HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM126700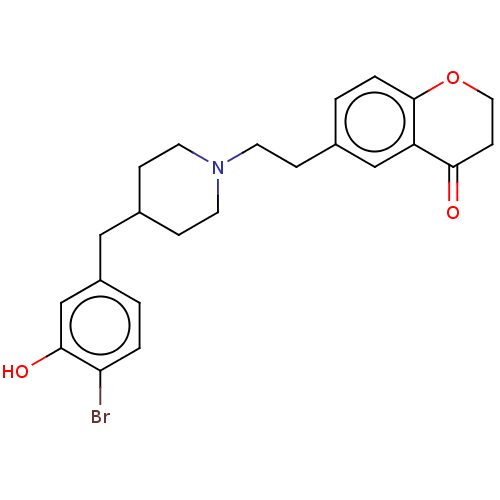 (US8778970, 5-8) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.30 | -50.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Dainippon Sumitomo Pharma Co., Ltd US Patent | Assay Description The experiment was carried out according to the method of Yabuuchi et al. [Yabuuchi K. et al., Biogenic Amines, 18, 319-328 (2004)]. 50 ul of [3H] 8-... | US Patent US8778970 (2014) BindingDB Entry DOI: 10.7270/Q2N878GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM126702 (US8778970, 5-10) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.30 | -50.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Dainippon Sumitomo Pharma Co., Ltd US Patent | Assay Description [3H] citalopram binding was assayed according to the method of Owens et al. [Owens M. J. et al., J. Pharm. Exp. Ther., 283, 1305-1322 (1997)]. Specif... | US Patent US8778970 (2014) BindingDB Entry DOI: 10.7270/Q2N878GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50460147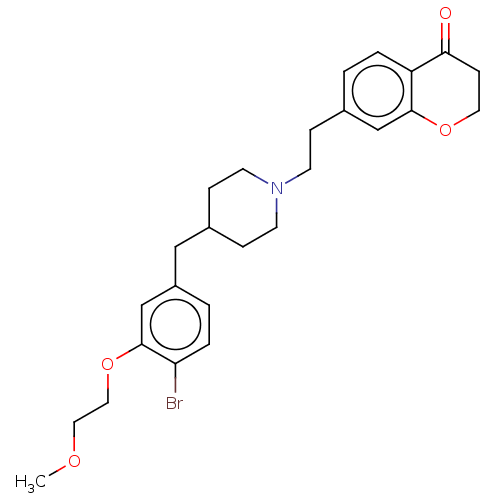 (CHEMBL4226031) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma CO., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram from human SERT expressed in HEK293 cell membranes after 60 mins by liquid scintillation counting | Bioorg Med Chem 26: 1614-1627 (2018) Article DOI: 10.1016/j.bmc.2018.02.008 BindingDB Entry DOI: 10.7270/Q2CN76HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50126913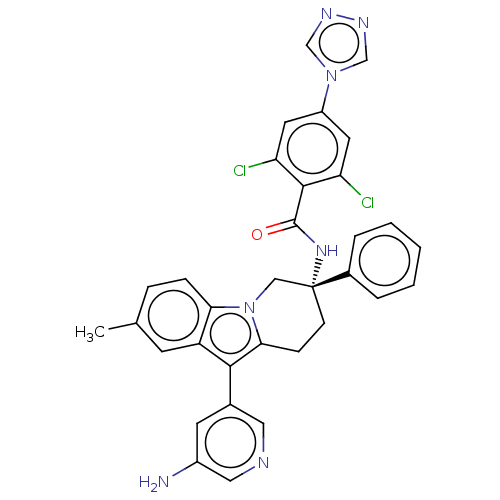 (CHEMBL3628966) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay | Bioorg Med Chem Lett 25: 5437-43 (2015) Article DOI: 10.1016/j.bmcl.2015.07.078 BindingDB Entry DOI: 10.7270/Q2B859X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM126701 (US8778970, 5-9) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.70 | -50.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Dainippon Sumitomo Pharma Co., Ltd US Patent | Assay Description [3H] citalopram binding was assayed according to the method of Owens et al. [Owens M. J. et al., J. Pharm. Exp. Ther., 283, 1305-1322 (1997)]. Specif... | US Patent US8778970 (2014) BindingDB Entry DOI: 10.7270/Q2N878GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50460146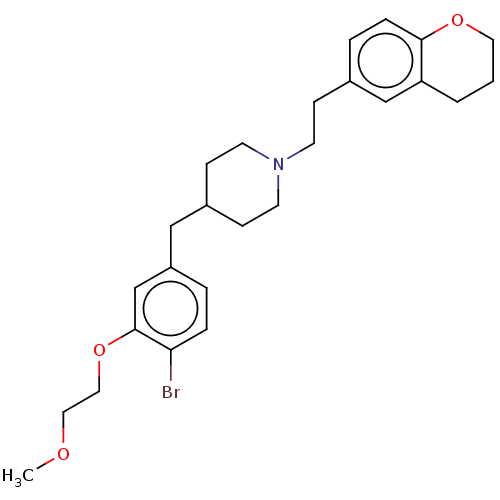 (CHEMBL4226546) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma CO., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram from human SERT expressed in HEK293 cell membranes after 60 mins by liquid scintillation counting | Bioorg Med Chem 26: 1614-1627 (2018) Article DOI: 10.1016/j.bmc.2018.02.008 BindingDB Entry DOI: 10.7270/Q2CN76HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50126949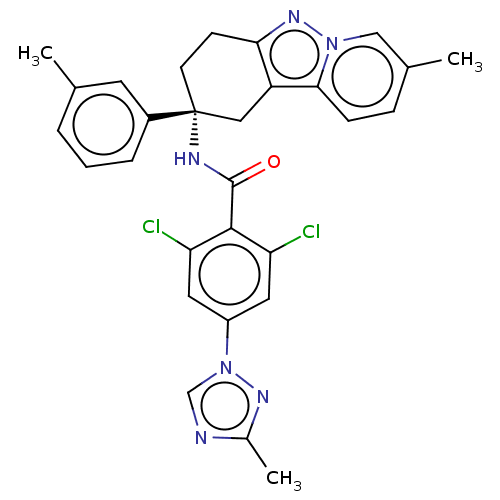 (CHEMBL3628954) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay | Bioorg Med Chem Lett 25: 5437-43 (2015) Article DOI: 10.1016/j.bmcl.2015.07.078 BindingDB Entry DOI: 10.7270/Q2B859X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM126698 (US8778970, 5-6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2 | -49.7 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Dainippon Sumitomo Pharma Co., Ltd US Patent | Assay Description The experiment was carried out according to the method of Yabuuchi et al. [Yabuuchi K. et al., Biogenic Amines, 18, 319-328 (2004)]. 50 ul of [3H] 8-... | US Patent US8778970 (2014) BindingDB Entry DOI: 10.7270/Q2N878GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50126941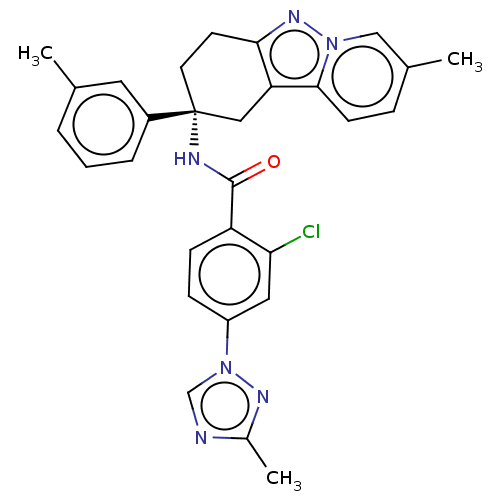 (CHEMBL3628838) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay | Bioorg Med Chem Lett 25: 5437-43 (2015) Article DOI: 10.1016/j.bmcl.2015.07.078 BindingDB Entry DOI: 10.7270/Q2B859X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM126699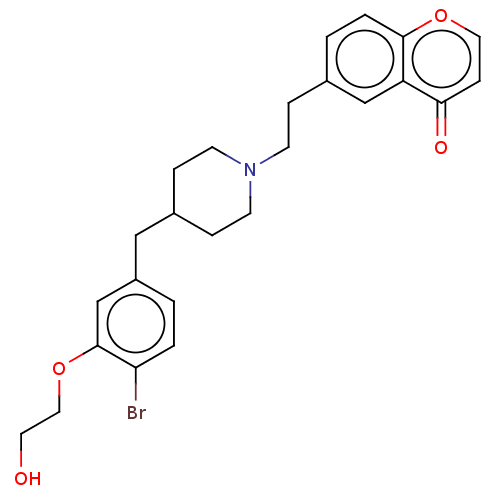 (US8778970, 5-7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.10 | -49.5 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Dainippon Sumitomo Pharma Co., Ltd US Patent | Assay Description The experiment was carried out according to the method of Yabuuchi et al. [Yabuuchi K. et al., Biogenic Amines, 18, 319-328 (2004)]. 50 ul of [3H] 8-... | US Patent US8778970 (2014) BindingDB Entry DOI: 10.7270/Q2N878GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM126703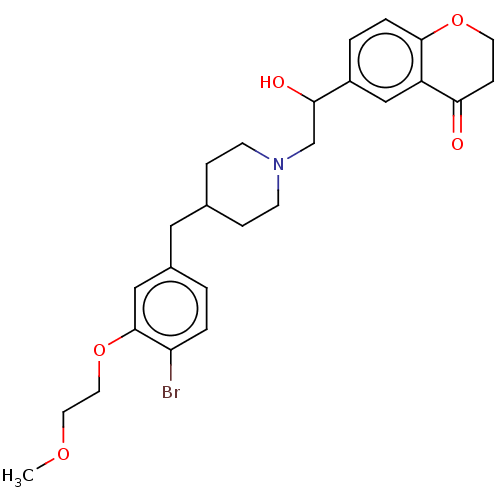 (US8778970, 5-11) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.20 | -49.4 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Dainippon Sumitomo Pharma Co., Ltd US Patent | Assay Description [3H] citalopram binding was assayed according to the method of Owens et al. [Owens M. J. et al., J. Pharm. Exp. Ther., 283, 1305-1322 (1997)]. Specif... | US Patent US8778970 (2014) BindingDB Entry DOI: 10.7270/Q2N878GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50460158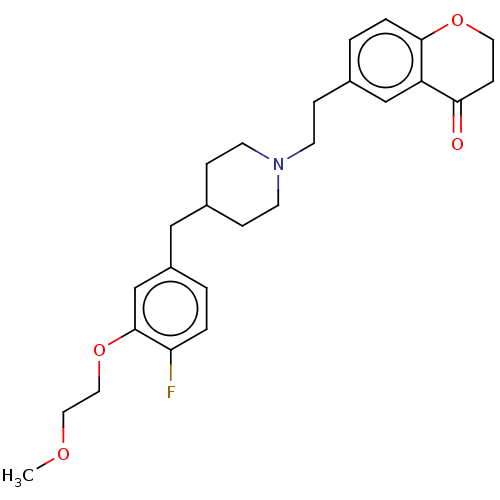 (CHEMBL4228366) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma CO., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cell membranes after 30 mins by liquid scintillation counting | Bioorg Med Chem 26: 1614-1627 (2018) Article DOI: 10.1016/j.bmc.2018.02.008 BindingDB Entry DOI: 10.7270/Q2CN76HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50099386 ((1-Benzyl-piperidin-4-yl)-[6-chloro-5-(4-chloro-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Pharmaceuticals Curated by ChEMBL | Assay Description Binding affinity of the compound to Dopamine receptor D4 was determined | Bioorg Med Chem Lett 11: 1141-4 (2001) BindingDB Entry DOI: 10.7270/Q2QJ7GJ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50460150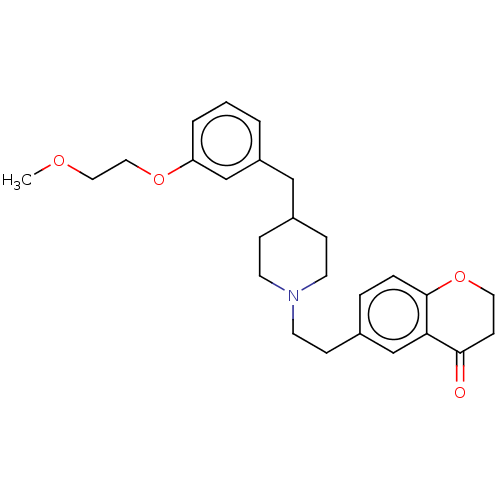 (CHEMBL4227376) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma CO., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cell membranes after 30 mins by liquid scintillation counting | Bioorg Med Chem 26: 1614-1627 (2018) Article DOI: 10.1016/j.bmc.2018.02.008 BindingDB Entry DOI: 10.7270/Q2CN76HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50126912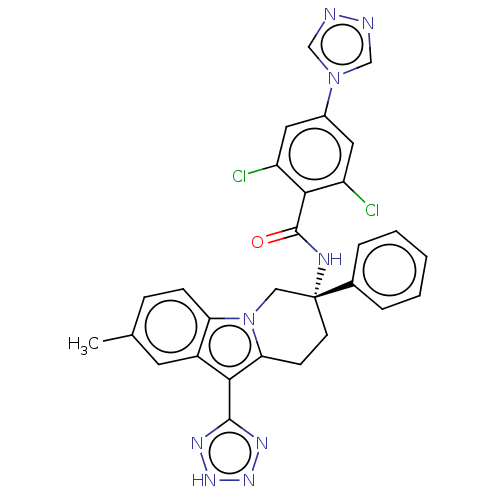 (CHEMBL3628965) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay | Bioorg Med Chem Lett 25: 5437-43 (2015) Article DOI: 10.1016/j.bmcl.2015.07.078 BindingDB Entry DOI: 10.7270/Q2B859X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50460154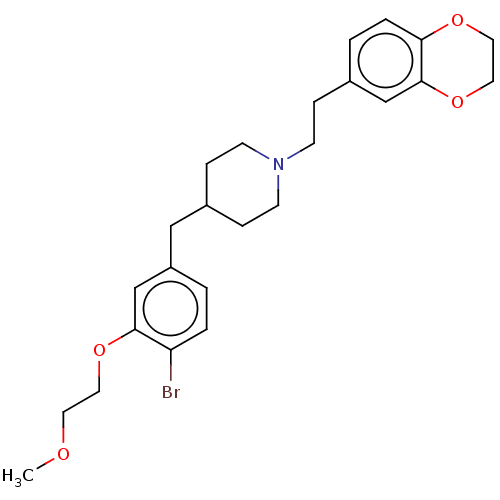 (CHEMBL4227238) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma CO., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram from human SERT expressed in HEK293 cell membranes after 60 mins by liquid scintillation counting | Bioorg Med Chem 26: 1614-1627 (2018) Article DOI: 10.1016/j.bmc.2018.02.008 BindingDB Entry DOI: 10.7270/Q2CN76HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50126855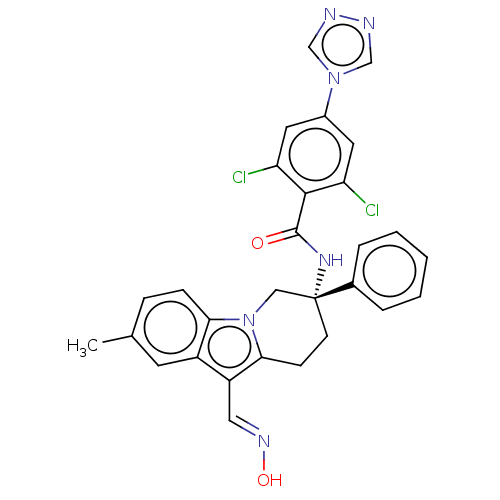 (CHEMBL3628962 | US10351558, Example 173) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay | Bioorg Med Chem Lett 25: 5437-43 (2015) Article DOI: 10.1016/j.bmcl.2015.07.078 BindingDB Entry DOI: 10.7270/Q2B859X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM126709 (US8778970, 5-14) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.5 | -49.1 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Dainippon Sumitomo Pharma Co., Ltd US Patent | Assay Description The experiment was carried out according to the method of Yabuuchi et al. [Yabuuchi K. et al., Biogenic Amines, 18, 319-328 (2004)]. 50 ul of [3H] 8-... | US Patent US8778970 (2014) BindingDB Entry DOI: 10.7270/Q2N878GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50126917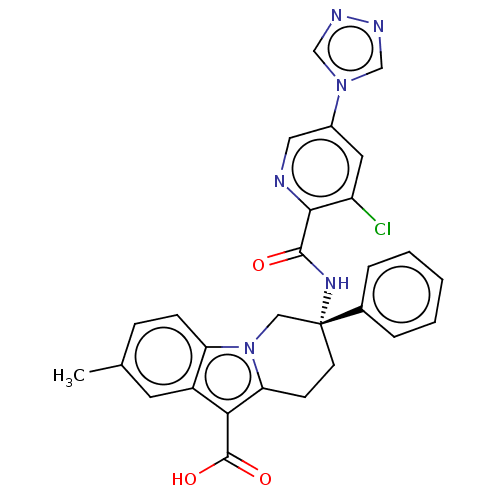 (CHEMBL3629112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay | Bioorg Med Chem Lett 25: 5437-43 (2015) Article DOI: 10.1016/j.bmcl.2015.07.078 BindingDB Entry DOI: 10.7270/Q2B859X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50126915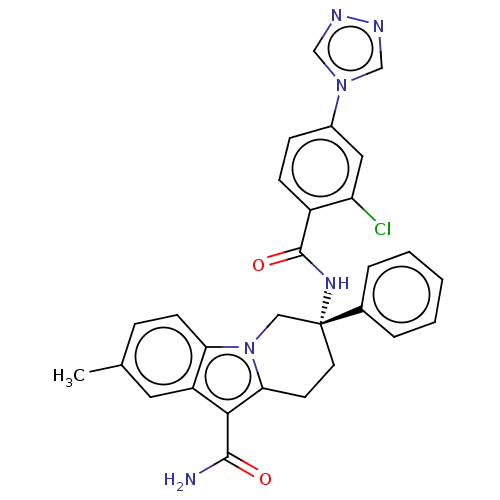 (CHEMBL3629110 | US10351558, Example 138) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay | Bioorg Med Chem Lett 25: 5437-43 (2015) Article DOI: 10.1016/j.bmcl.2015.07.078 BindingDB Entry DOI: 10.7270/Q2B859X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50460143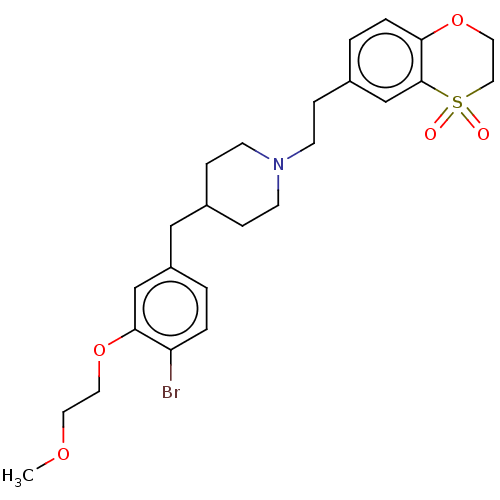 (CHEMBL4227642) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma CO., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram from human SERT expressed in HEK293 cell membranes after 60 mins by liquid scintillation counting | Bioorg Med Chem 26: 1614-1627 (2018) Article DOI: 10.1016/j.bmc.2018.02.008 BindingDB Entry DOI: 10.7270/Q2CN76HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM126695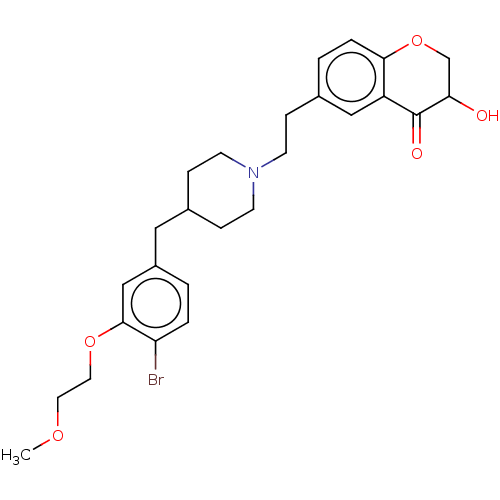 (US8778970, 5-3) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.70 | -48.9 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Dainippon Sumitomo Pharma Co., Ltd US Patent | Assay Description [3H] citalopram binding was assayed according to the method of Owens et al. [Owens M. J. et al., J. Pharm. Exp. Ther., 283, 1305-1322 (1997)]. Specif... | US Patent US8778970 (2014) BindingDB Entry DOI: 10.7270/Q2N878GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50126926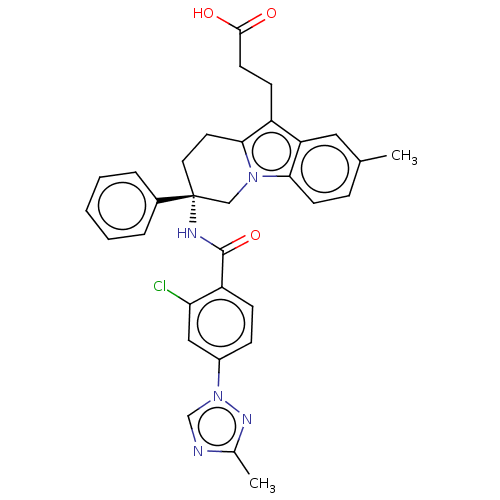 (CHEMBL3628835 | US10351558, Example 183) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay | Bioorg Med Chem Lett 25: 5437-43 (2015) Article DOI: 10.1016/j.bmcl.2015.07.078 BindingDB Entry DOI: 10.7270/Q2B859X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50460151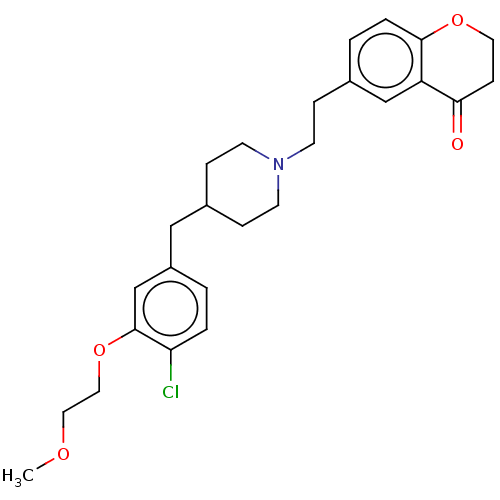 (CHEMBL4228158) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma CO., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]8-OH-DPAT from human 5-HT1A receptor expressed in CHOK1 cell membranes after 30 mins by liquid scintillation counting | Bioorg Med Chem 26: 1614-1627 (2018) Article DOI: 10.1016/j.bmc.2018.02.008 BindingDB Entry DOI: 10.7270/Q2CN76HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50125972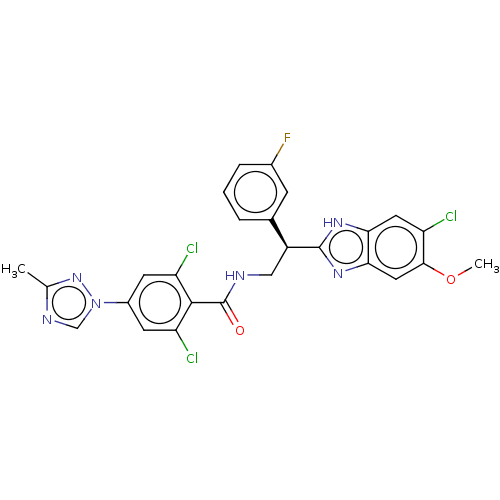 (CHEMBL3627894) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate | Bioorg Med Chem Lett 25: 4945-9 (2015) Article DOI: 10.1016/j.bmcl.2015.04.057 BindingDB Entry DOI: 10.7270/Q20Z753B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50126856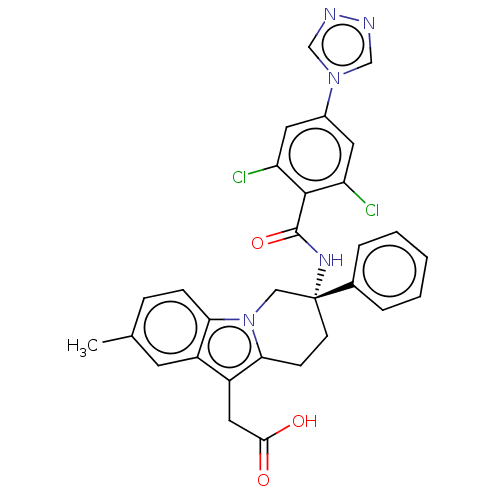 (CHEMBL3628963) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay | Bioorg Med Chem Lett 25: 5437-43 (2015) Article DOI: 10.1016/j.bmcl.2015.07.078 BindingDB Entry DOI: 10.7270/Q2B859X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50126942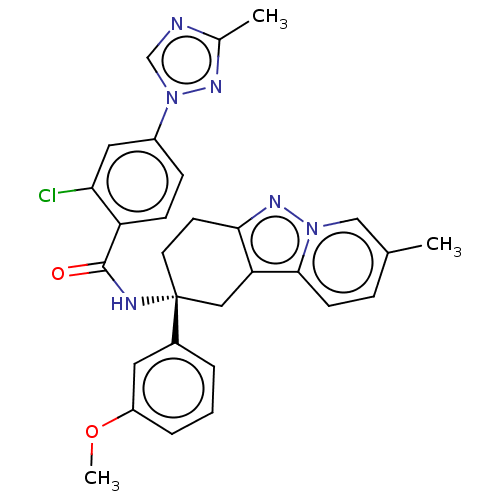 (CHEMBL3628947) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay | Bioorg Med Chem Lett 25: 5437-43 (2015) Article DOI: 10.1016/j.bmcl.2015.07.078 BindingDB Entry DOI: 10.7270/Q2B859X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50126944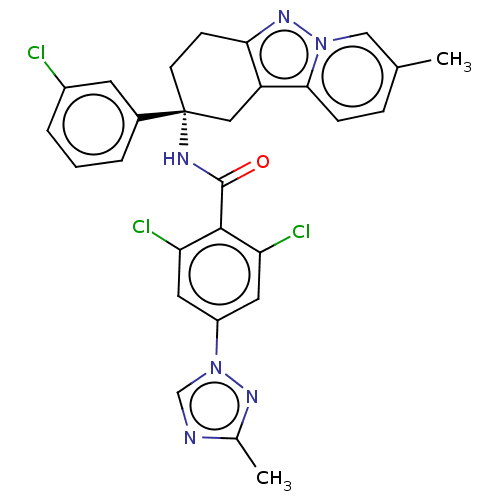 (CHEMBL3628949) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay | Bioorg Med Chem Lett 25: 5437-43 (2015) Article DOI: 10.1016/j.bmcl.2015.07.078 BindingDB Entry DOI: 10.7270/Q2B859X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50125980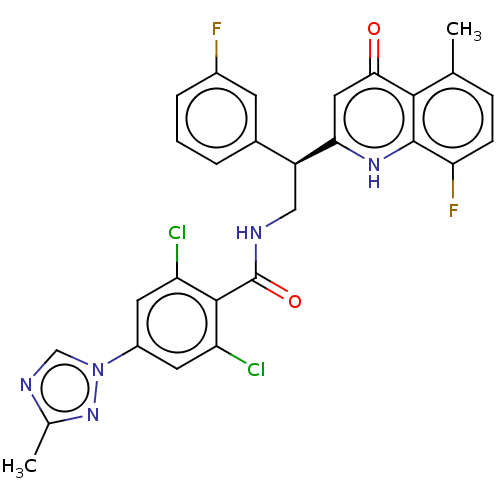 (CHEMBL3627900) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate | Bioorg Med Chem Lett 25: 4945-9 (2015) Article DOI: 10.1016/j.bmcl.2015.04.057 BindingDB Entry DOI: 10.7270/Q20Z753B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM50460151 (CHEMBL4228158) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sumitomo Dainippon Pharma CO., Ltd. Curated by ChEMBL | Assay Description Displacement of [3H]-citalopram from human SERT expressed in HEK293 cell membranes after 60 mins by liquid scintillation counting | Bioorg Med Chem 26: 1614-1627 (2018) Article DOI: 10.1016/j.bmc.2018.02.008 BindingDB Entry DOI: 10.7270/Q2CN76HF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sodium-dependent serotonin transporter (Homo sapiens (Human)) | BDBM126699 (US8778970, 5-7) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.5 | -48.3 | n/a | n/a | n/a | n/a | n/a | n/a | 25 |
Dainippon Sumitomo Pharma Co., Ltd US Patent | Assay Description [3H] citalopram binding was assayed according to the method of Owens et al. [Owens M. J. et al., J. Pharm. Exp. Ther., 283, 1305-1322 (1997)]. Specif... | US Patent US8778970 (2014) BindingDB Entry DOI: 10.7270/Q2N878GH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50125978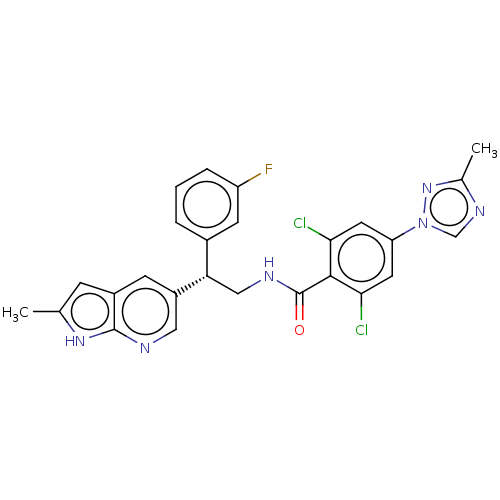 (CHEMBL3627898 | US10189819, Example 77) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 9a using fluorescent peptide CH3SO2-D-CHG-Gly-Arg-AFC-AcoH as substrate | Bioorg Med Chem Lett 25: 4945-9 (2015) Article DOI: 10.1016/j.bmcl.2015.04.057 BindingDB Entry DOI: 10.7270/Q20Z753B | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50126946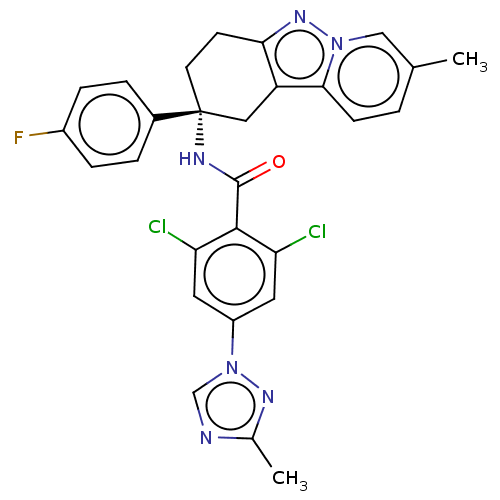 (CHEMBL3628951) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay | Bioorg Med Chem Lett 25: 5437-43 (2015) Article DOI: 10.1016/j.bmcl.2015.07.078 BindingDB Entry DOI: 10.7270/Q2B859X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor IX (Homo sapiens (Human)) | BDBM50126945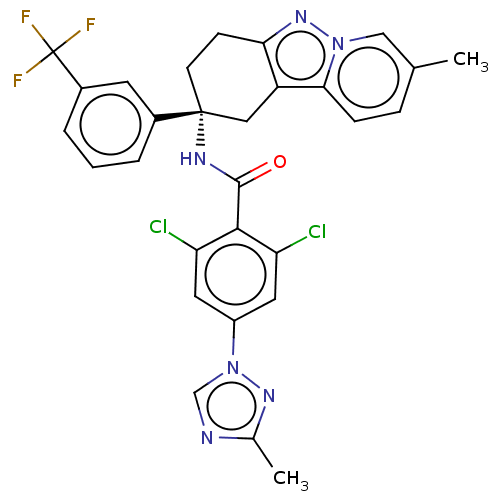 (CHEMBL3628950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of human F9a using CH3SO2-D-CHG-Gly-Arg-AFC.AcOH as substrate by fluorescence assay | Bioorg Med Chem Lett 25: 5437-43 (2015) Article DOI: 10.1016/j.bmcl.2015.07.078 BindingDB Entry DOI: 10.7270/Q2B859X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5357 total ) | Next | Last >> |