

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014156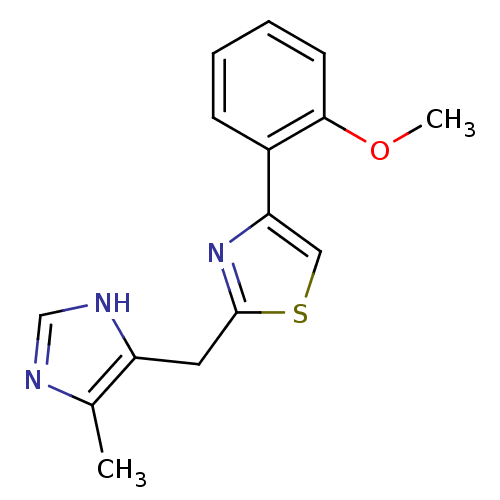 (4-(2-Methoxy-phenyl)-2-(5-methyl-1H-imidazol-4-ylm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 0.420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50014174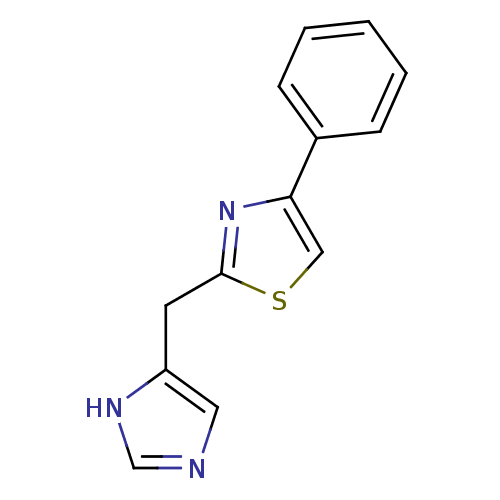 (2-(1H-Imidazol-4-ylmethyl)-4-phenyl-thiazole | CHE...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 0.990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014164 (8-[2-(5-Methyl-1H-imidazol-4-ylmethyl)-thiazol-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description Antibacterial activity against Escherichia coli DHFR | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50014159 (4-(2-Fluoro-phenyl)-2-(5-methyl-1H-imidazol-4-ylme...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50108392 ((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50108392 ((3-ENDO)-8-METHYL-8-AZABICYCLO[3.2.1]OCT-3-YL 1H-I...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 1A (Homo sapiens (Human)) | BDBM50081081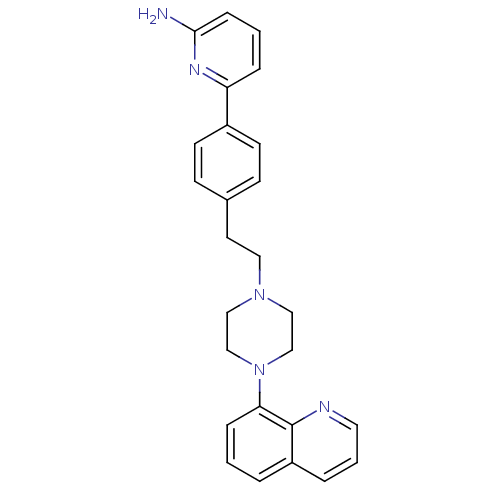 (6-{4-[2-(4-Quinolin-8-yl-piperazin-1-yl)-ethyl]-ph...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Groton Curated by ChEMBL | Assay Description Binding affinity of compound to human 5-hydroxytryptamine 1A receptor | Bioorg Med Chem Lett 9: 2569-72 (1999) BindingDB Entry DOI: 10.7270/Q2BC3XRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50013039 (CHEMBL40260 | N-[4-(1H-Indol-3-yl)-thiazol-2-ylmet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of dihydrofolate reductase of Escherichia coli | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50013043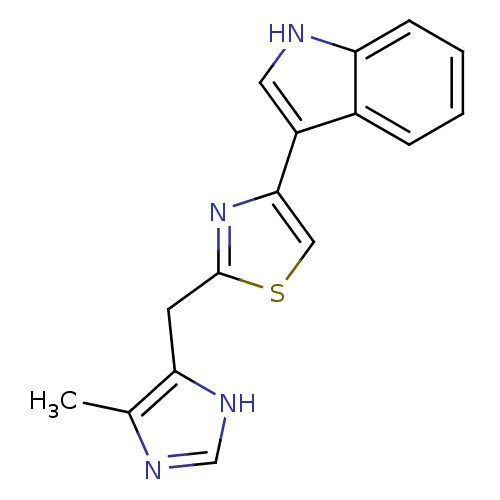 (3-[2-(5-Methyl-1H-imidazol-4-ylmethyl)-thiazol-4-y...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50081081 (6-{4-[2-(4-Quinolin-8-yl-piperazin-1-yl)-ethyl]-ph...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Groton Curated by ChEMBL | Assay Description Binding ability of compound to human Dopamine receptor D2 | Bioorg Med Chem Lett 9: 2569-72 (1999) BindingDB Entry DOI: 10.7270/Q2BC3XRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM50013043 (3-[2-(5-Methyl-1H-imidazol-4-ylmethyl)-thiazol-4-y...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50014600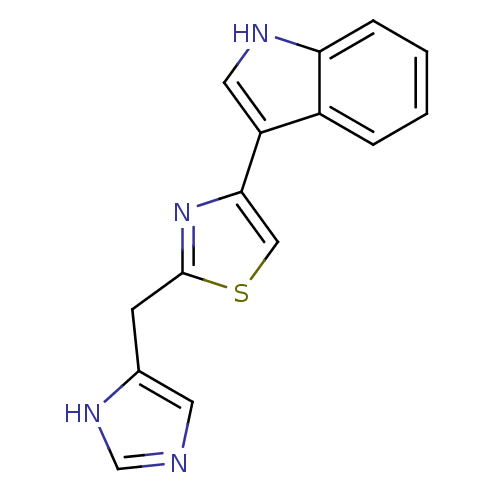 (3-[2-(1H-Imidazol-4-ylmethyl)-thiazol-4-yl]-1H-ind...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Homo sapiens (Human)) | BDBM50081081 (6-{4-[2-(4-Quinolin-8-yl-piperazin-1-yl)-ethyl]-ph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Groton Curated by ChEMBL | Assay Description Binding affinity of compound to human 5-hydroxytryptamine 2A receptor | Bioorg Med Chem Lett 9: 2569-72 (1999) BindingDB Entry DOI: 10.7270/Q2BC3XRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A (Homo sapiens (Human)) | BDBM85330 (CAS_68647 | NSC_68647 | ONDANSETRON | Ondansetron ...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | DrugBank PDB PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity towards 5-hydroxytryptamine 3 receptor by displacement of [3H]2 in Neuroblastoma-Glioma NG-108-15 cells | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM85330 (CAS_68647 | NSC_68647 | ONDANSETRON | Ondansetron ...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase DrugBank MCE KEGG PC cid PC sid PDB UniChem Similars | PDB PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50014601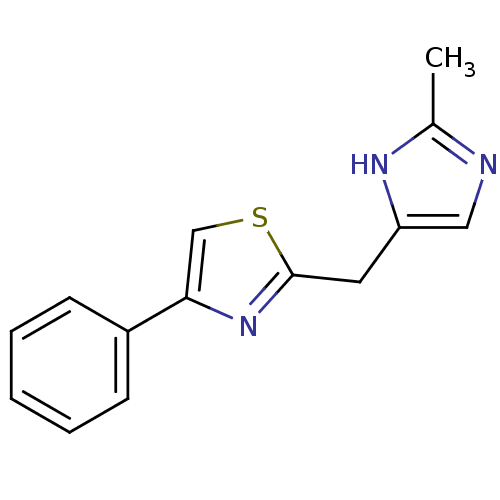 (2-(2-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 226 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of Escherichia coli dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M2 (Homo sapiens (Human)) | BDBM50081082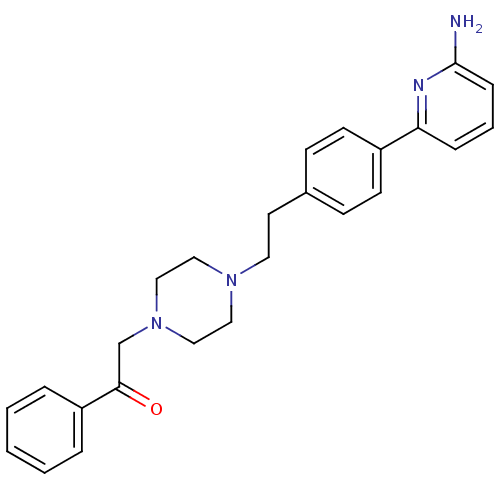 (2-(4-{2-[4-(6-Amino-pyridin-2-yl)-phenyl]-ethyl}-p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Groton Curated by ChEMBL | Assay Description Binding affinity of compound to m2 muscarinic receptor | Bioorg Med Chem Lett 9: 2569-72 (1999) BindingDB Entry DOI: 10.7270/Q2BC3XRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M4 (Homo sapiens (Human)) | BDBM50081082 (2-(4-{2-[4-(6-Amino-pyridin-2-yl)-phenyl]-ethyl}-p...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 590 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Groton Curated by ChEMBL | Assay Description Binding ability of compound to m4 muscarinic receptor | Bioorg Med Chem Lett 9: 2569-72 (1999) BindingDB Entry DOI: 10.7270/Q2BC3XRP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Mus musculus (Mouse)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Dopamine receptor D1 was determined by using [3H]SCH-23390 as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2C (Rattus norvegicus (Rat)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB KEGG UniProtKB/SwissProt DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1C receptor was determined by using [3H]- mesulergine as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1B (Homo sapiens (Human)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1B receptor was determined by using [3H]5-HT as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Muscarinic acetylcholine receptor M3 (RAT) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Muscarinic acetylcholine receptor was determined by using [3H]QNB as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Rattus norvegicus) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Alpha-2 adrenergic receptor was determined by using [3H]p-aminoclonidine as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Beta-2 adrenergic receptor (Canis familiaris) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Beta adrenergic receptor was determined by using [3H]dihydroalprenolol as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1A (Mus musculus (Mouse)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1A receptor was determined by using [3H]8-OH-DPAT as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sigma non-opioid intracellular receptor 1 (Homo sapiens (Human)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Sigma opioid receptor was determined by using [3H](+)-3-(3-hydroxyphenyl)N-1-propyl-piperdine((+)-3-PPP) as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Alpha-2A adrenergic receptor [16-465] (Rattus norvegicus (rat)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Alpha-1 adrenergic receptor was determined by using [3H]prazosin as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50013045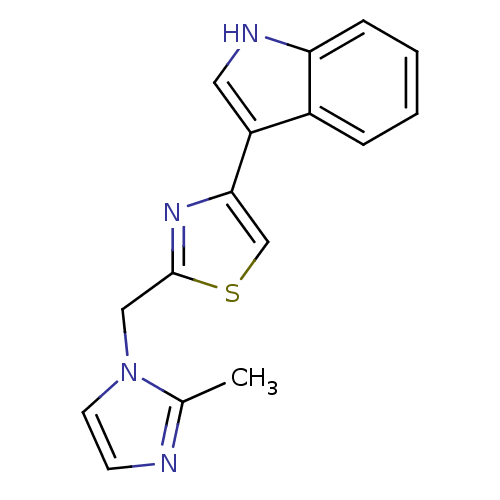 (3-[2-(2-Methyl-imidazol-1-ylmethyl)-thiazol-4-yl]-...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of rat liver dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (BOVINE) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 2 receptor was determined by using [3H]ketanserin as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Homo sapiens (Human)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Dopamine receptor D2 was determined by using [3H]spiperone as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for Opioid receptor mu 1 was determined by using [3H]naloxone as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 1D (Homo sapiens (Human)) | BDBM50014154 (2-(5-Methyl-1H-imidazol-4-ylmethyl)-4-phenyl-thiaz...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer central Research Curated by ChEMBL | Assay Description Binding affinity for 5-hydroxytryptamine 1D receptor was determined by using [3H]5-HT as radioligand | J Med Chem 33: 2715-20 (1990) BindingDB Entry DOI: 10.7270/Q2H995S5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 3A/3B (Rattus norvegicus-RAT) | BDBM50014406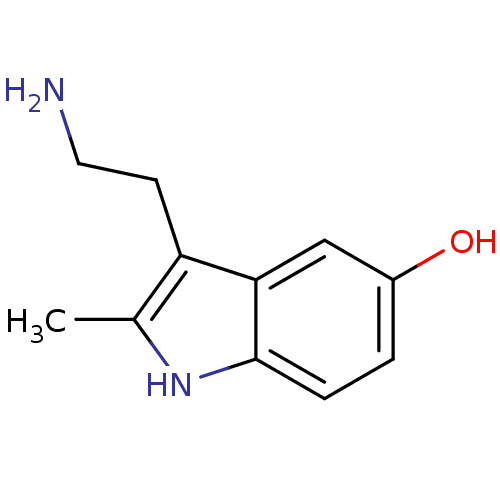 (2-Me 5-HT | 2-Methyl-5-hydroxytryptamine | 2-methy...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of Escherichia coli dihydrofolate reductase. | J Med Chem 33: 13-6 (1990) BindingDB Entry DOI: 10.7270/Q29S1RNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50363465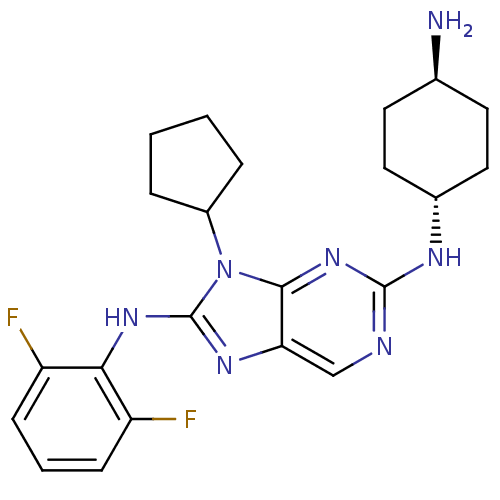 (CHEMBL1946646) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 1427-32 (2012) Article DOI: 10.1016/j.bmcl.2011.12.028 BindingDB Entry DOI: 10.7270/Q2513ZP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50363455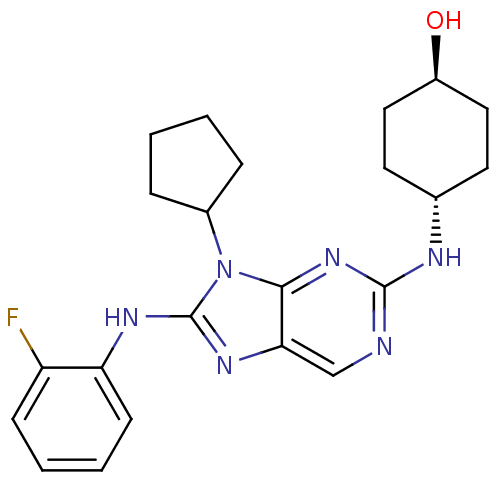 (CHEMBL1946333) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 1427-32 (2012) Article DOI: 10.1016/j.bmcl.2011.12.028 BindingDB Entry DOI: 10.7270/Q2513ZP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50363466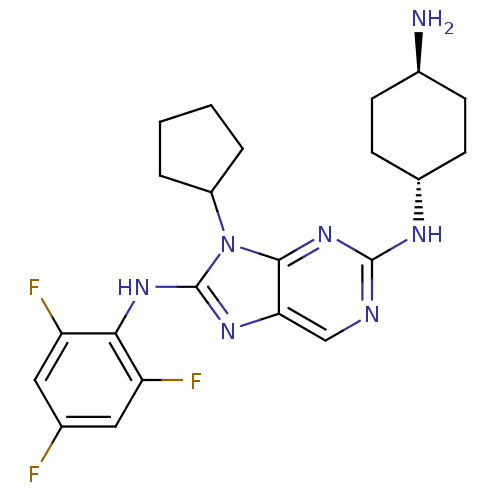 (CHEMBL1946647) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 1427-32 (2012) Article DOI: 10.1016/j.bmcl.2011.12.028 BindingDB Entry DOI: 10.7270/Q2513ZP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50363455 (CHEMBL1946333) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 1427-32 (2012) Article DOI: 10.1016/j.bmcl.2011.12.028 BindingDB Entry DOI: 10.7270/Q2513ZP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50363465 (CHEMBL1946646) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 1427-32 (2012) Article DOI: 10.1016/j.bmcl.2011.12.028 BindingDB Entry DOI: 10.7270/Q2513ZP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50363462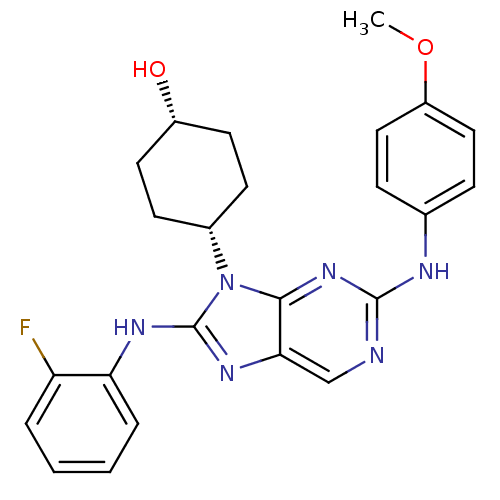 (CHEMBL1946340) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 1427-32 (2012) Article DOI: 10.1016/j.bmcl.2011.12.028 BindingDB Entry DOI: 10.7270/Q2513ZP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50363466 (CHEMBL1946647) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 1427-32 (2012) Article DOI: 10.1016/j.bmcl.2011.12.028 BindingDB Entry DOI: 10.7270/Q2513ZP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50363462 (CHEMBL1946340) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 1427-32 (2012) Article DOI: 10.1016/j.bmcl.2011.12.028 BindingDB Entry DOI: 10.7270/Q2513ZP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50363464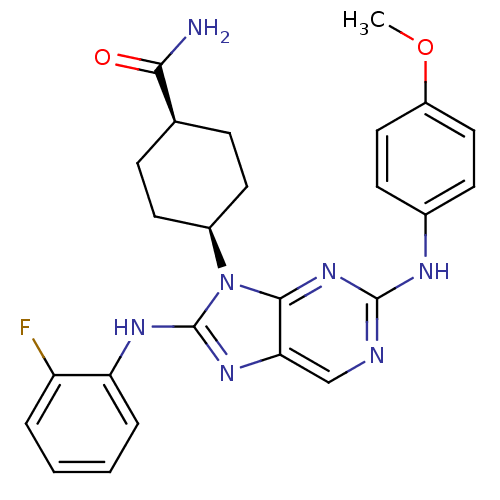 (CHEMBL1946495) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 1427-32 (2012) Article DOI: 10.1016/j.bmcl.2011.12.028 BindingDB Entry DOI: 10.7270/Q2513ZP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50363464 (CHEMBL1946495) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 1427-32 (2012) Article DOI: 10.1016/j.bmcl.2011.12.028 BindingDB Entry DOI: 10.7270/Q2513ZP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50363467 (CHEMBL1946837) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 1427-32 (2012) Article DOI: 10.1016/j.bmcl.2011.12.028 BindingDB Entry DOI: 10.7270/Q2513ZP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50363467 (CHEMBL1946837) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 1427-32 (2012) Article DOI: 10.1016/j.bmcl.2011.12.028 BindingDB Entry DOI: 10.7270/Q2513ZP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50363456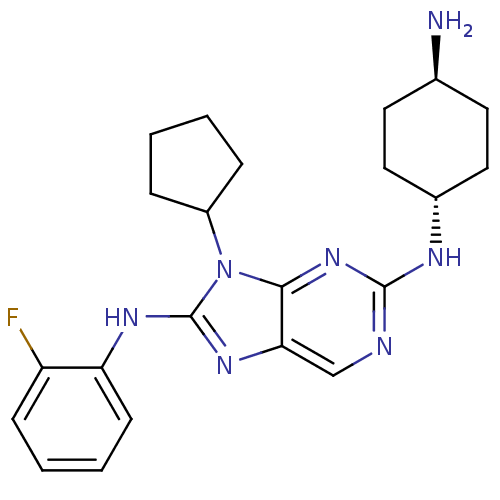 (CHEMBL1946334) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 1427-32 (2012) Article DOI: 10.1016/j.bmcl.2011.12.028 BindingDB Entry DOI: 10.7270/Q2513ZP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 10 (Homo sapiens (Human)) | BDBM50363456 (CHEMBL1946334) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of full length Hexa-His-tagged JNK3 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 1427-32 (2012) Article DOI: 10.1016/j.bmcl.2011.12.028 BindingDB Entry DOI: 10.7270/Q2513ZP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mitogen-activated protein kinase 9 (Homo sapiens (Human)) | BDBM50363450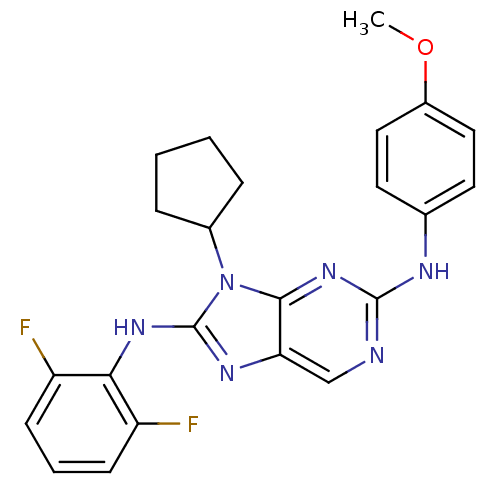 (CHEMBL1946328) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Celgene Corporation Curated by ChEMBL | Assay Description Inhibition of full length Hexa-His-tagged JNK2 using GST-tagged cJun and [gamma33P]ATP as substrate after 60 mins by scintillation counting | Bioorg Med Chem Lett 22: 1427-32 (2012) Article DOI: 10.1016/j.bmcl.2011.12.028 BindingDB Entry DOI: 10.7270/Q2513ZP6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 145 total ) | Next | Last >> |