 Found 43972 hits with Last Name = 'oh' and Initial = 'm'
Found 43972 hits with Last Name = 'oh' and Initial = 'm' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50214974
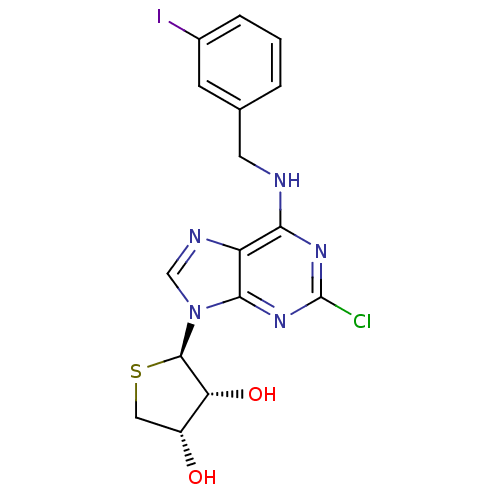
((2R,3R,4S)-2-(2-chloro-6-(3-iodobenzylamino)-9H-pu...)Show SMILES O[C@@H]1CS[C@H]([C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C16H15ClIN5O2S/c17-16-21-13(19-5-8-2-1-3-9(18)4-8)11-14(22-16)23(7-20-11)15-12(25)10(24)6-26-15/h1-4,7,10,12,15,24-25H,5-6H2,(H,19,21,22)/t10-,12-,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00483 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University
Curated by ChEMBL
| Assay Description
Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARdelta LBD by TR-FRET assay |
J Med Chem 60: 7459-7475 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00805
BindingDB Entry DOI: 10.7270/Q2XK8HQH |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 2A
(Rattus norvegicus (rat)) | BDBM84928
(CAS_125368 | FK 1052 | NSC_125368)Show InChI InChI=1S/C18H19N3O/c1-11-14-5-3-4-6-17(14)21-16(11)8-7-13(18(21)22)9-15-12(2)19-10-20-15/h3-6,10,13H,7-9H2,1-2H3,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 752-8 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4G73 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM84928

(CAS_125368 | FK 1052 | NSC_125368)Show InChI InChI=1S/C18H19N3O/c1-11-14-5-3-4-6-17(14)21-16(11)8-7-13(18(21)22)9-15-12(2)19-10-20-15/h3-6,10,13H,7-9H2,1-2H3,(H,19,20) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Fujisawa Pharmaceutical Co., Ltd.
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 265: 752-8 (1993)
BindingDB Entry DOI: 10.7270/Q2CV4G73 |
More data for this
Ligand-Target Pair | |
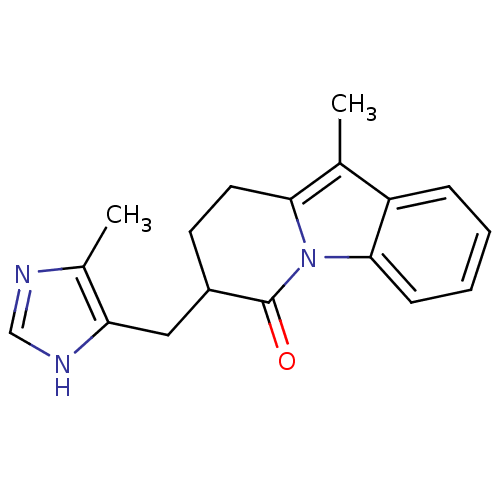
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50095105
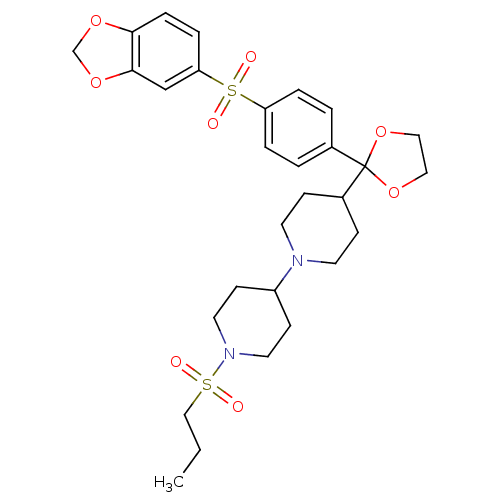
(4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...)Show SMILES CCCS(=O)(=O)N1CCC(CC1)N1CCC(CC1)C1(OCCO1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H38N2O8S2/c1-2-19-40(32,33)31-15-11-24(12-16-31)30-13-9-23(10-14-30)29(38-17-18-39-29)22-3-5-25(6-4-22)41(34,35)26-7-8-27-28(20-26)37-21-36-27/h3-8,20,23-24H,2,9-19,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned acetylcholine receptor M2 in CHO cells. |
Bioorg Med Chem Lett 10: 2727-30 (2000)
BindingDB Entry DOI: 10.7270/Q2V40TGZ |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50000558
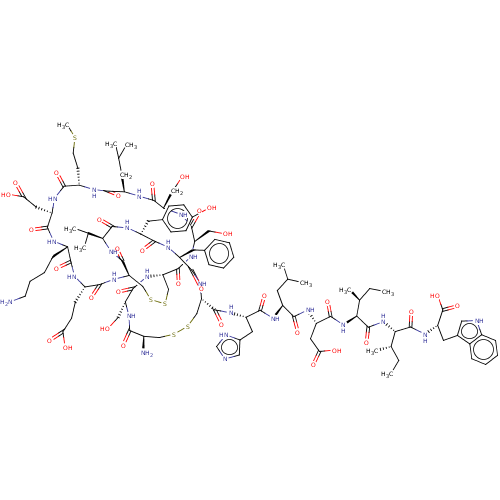
(CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(81)160/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66-,67-,68-,69+,70-,71-,72+,73-,74+,75-,76-,77+,78-,79+,80-,81-,82+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to Endothelin B receptor in the rat hippocampus |
J Med Chem 35: 1493-508 (1992)
BindingDB Entry DOI: 10.7270/Q27M08KX |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50095105

(4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...)Show SMILES CCCS(=O)(=O)N1CCC(CC1)N1CCC(CC1)C1(OCCO1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H38N2O8S2/c1-2-19-40(32,33)31-15-11-24(12-16-31)30-13-9-23(10-14-30)29(38-17-18-39-29)22-3-5-25(6-4-22)41(34,35)26-7-8-27-28(20-26)37-21-36-27/h3-8,20,23-24H,2,9-19,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Antagonistic activity of the compound against Muscarinic acetylcholine receptor M2 |
Bioorg Med Chem Lett 11: 2311-4 (2001)
BindingDB Entry DOI: 10.7270/Q28S4P7Z |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50214981
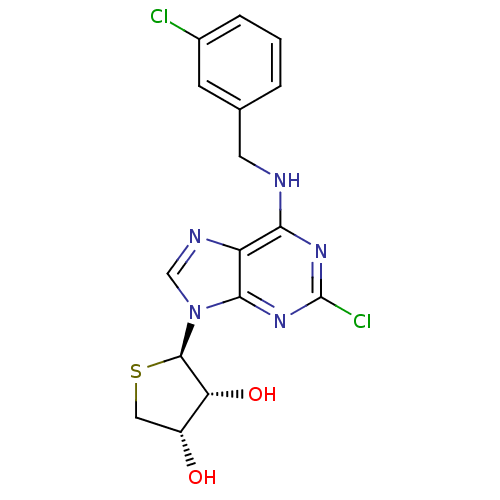
((2R,3R,4S)-2-(2-chloro-6-(3-chlorobenzylamino)-9H-...)Show SMILES O[C@@H]1CS[C@H]([C@@H]1O)n1cnc2c(NCc3cccc(Cl)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C16H15Cl2N5O2S/c17-9-3-1-2-8(4-9)5-19-13-11-14(22-16(18)21-13)23(7-20-11)15-12(25)10(24)6-26-15/h1-4,7,10,12,15,24-25H,5-6H2,(H,19,21,22)/t10-,12-,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University
Curated by ChEMBL
| Assay Description
Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARdelta LBD by TR-FRET assay |
J Med Chem 60: 7459-7475 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00805
BindingDB Entry DOI: 10.7270/Q2XK8HQH |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50368606
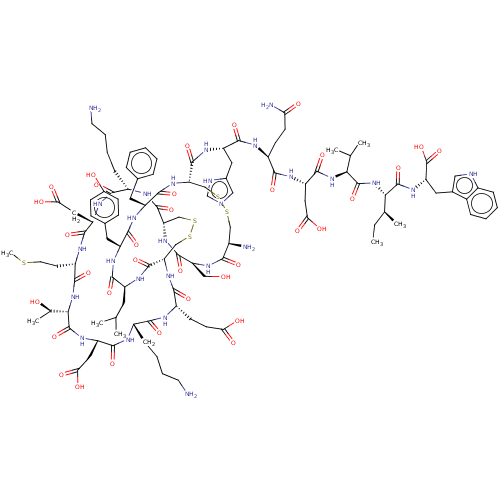
(Sarafotoxin S6B)Show SMILES [H][C@]12CSSC[C@]([H])(NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@]([H])(NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCN)NC1=O)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N2)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| Show InChI InChI=1S/C110H159N27O34S5/c1-9-55(6)88(108(168)130-77(110(170)171)40-59-45-116-64-22-14-13-21-62(59)64)136-107(167)87(54(4)5)135-102(162)76(44-86(148)149)128-93(153)67(29-31-82(114)141)121-99(159)73(41-60-46-115-52-117-60)126-106(166)81-49-174-173-48-63(113)90(150)131-78(47-138)103(163)134-79-50-175-176-51-80(105(165)123-70(37-53(2)3)96(156)124-72(39-58-25-27-61(140)28-26-58)97(157)125-71(98(158)133-81)38-57-19-11-10-12-20-57)132-94(154)68(30-32-83(142)143)120-91(151)65(23-15-17-34-111)118-101(161)75(43-85(146)147)129-109(169)89(56(7)139)137-95(155)69(33-36-172-8)122-100(160)74(42-84(144)145)127-92(152)66(119-104(79)164)24-16-18-35-112/h10-14,19-22,25-28,45-46,52-56,63,65-81,87-89,116,138-140H,9,15-18,23-24,29-44,47-51,111-113H2,1-8H3,(H2,114,141)(H,115,117)(H,118,161)(H,119,164)(H,120,151)(H,121,159)(H,122,160)(H,123,165)(H,124,156)(H,125,157)(H,126,166)(H,127,152)(H,128,153)(H,129,169)(H,130,168)(H,131,150)(H,132,154)(H,133,158)(H,134,163)(H,135,162)(H,136,167)(H,137,155)(H,142,143)(H,144,145)(H,146,147)(H,148,149)(H,170,171)/t55-,56+,63+,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,87-,88-,89-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to Endothelin B receptor in the rat hippocampus |
J Med Chem 35: 1493-508 (1992)
BindingDB Entry DOI: 10.7270/Q27M08KX |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50368606

(Sarafotoxin S6B)Show SMILES [H][C@]12CSSC[C@]([H])(NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@]([H])(NC(=O)[C@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCCCN)NC1=O)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](Cc1ccccc1)C(=O)N[C@@H](CSSC[C@@H](N)C(=O)N[C@@H](CO)C(=O)N2)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CCC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O |r| Show InChI InChI=1S/C110H159N27O34S5/c1-9-55(6)88(108(168)130-77(110(170)171)40-59-45-116-64-22-14-13-21-62(59)64)136-107(167)87(54(4)5)135-102(162)76(44-86(148)149)128-93(153)67(29-31-82(114)141)121-99(159)73(41-60-46-115-52-117-60)126-106(166)81-49-174-173-48-63(113)90(150)131-78(47-138)103(163)134-79-50-175-176-51-80(105(165)123-70(37-53(2)3)96(156)124-72(39-58-25-27-61(140)28-26-58)97(157)125-71(98(158)133-81)38-57-19-11-10-12-20-57)132-94(154)68(30-32-83(142)143)120-91(151)65(23-15-17-34-111)118-101(161)75(43-85(146)147)129-109(169)89(56(7)139)137-95(155)69(33-36-172-8)122-100(160)74(42-84(144)145)127-92(152)66(119-104(79)164)24-16-18-35-112/h10-14,19-22,25-28,45-46,52-56,63,65-81,87-89,116,138-140H,9,15-18,23-24,29-44,47-51,111-113H2,1-8H3,(H2,114,141)(H,115,117)(H,118,161)(H,119,164)(H,120,151)(H,121,159)(H,122,160)(H,123,165)(H,124,156)(H,125,157)(H,126,166)(H,127,152)(H,128,153)(H,129,169)(H,130,168)(H,131,150)(H,132,154)(H,133,158)(H,134,163)(H,135,162)(H,136,167)(H,137,155)(H,142,143)(H,144,145)(H,146,147)(H,148,149)(H,170,171)/t55-,56+,63+,65-,66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,87-,88-,89-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to Endothelin B receptor in the rat cerebellum |
J Med Chem 35: 1493-508 (1992)
BindingDB Entry DOI: 10.7270/Q27M08KX |
More data for this
Ligand-Target Pair | |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50118030
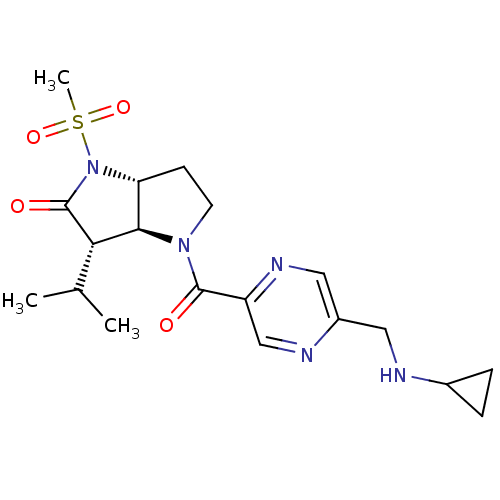
(4-(5-Cyclopropylaminomethyl-pyrazine-2-carbonyl)-3...)Show SMILES CC(C)[C@H]1[C@H]2[C@@H](CCN2C(=O)c2cnc(CNC3CC3)cn2)N(C1=O)S(C)(=O)=O Show InChI InChI=1S/C19H27N5O4S/c1-11(2)16-17-15(24(19(16)26)29(3,27)28)6-7-23(17)18(25)14-10-21-13(9-22-14)8-20-12-4-5-12/h9-12,15-17,20H,4-8H2,1-3H3/t15-,16+,17-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| 0.0140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GSK
Curated by ChEMBL
| Assay Description
The compound was evaluated for its binding affinity towards human neutrophil elastase (HNE) |
J Med Chem 45: 3878-90 (2002)
BindingDB Entry DOI: 10.7270/Q2HM596S |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50000558

(CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(81)160/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66-,67-,68-,69+,70-,71-,72+,73-,74+,75-,76-,77+,78-,79+,80-,81-,82+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to Endothelin B receptor in the rat cerebellum |
J Med Chem 35: 1493-508 (1992)
BindingDB Entry DOI: 10.7270/Q27M08KX |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50368605
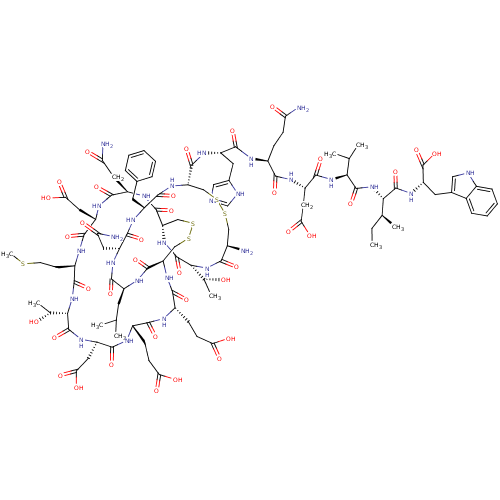
(CHEMBL1790178)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CC(N)=O)NC2=O)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C103H147N27O37S5/c1-10-46(6)80(100(163)123-67(103(166)167)30-50-37-109-54-19-15-14-18-52(50)54)128-99(162)79(45(4)5)127-95(158)66(36-78(144)145)120-85(148)55(20-23-71(105)133)112-90(153)61(31-51-38-108-43-110-51)117-97(160)69-40-170-169-39-53(104)83(146)129-81(47(7)131)102(165)126-70-42-172-171-41-68(96(159)115-59(28-44(2)3)88(151)118-62(32-72(106)134)91(154)116-60(89(152)125-69)29-49-16-12-11-13-17-49)124-86(149)57(22-25-75(138)139)111-84(147)56(21-24-74(136)137)113-94(157)65(35-77(142)143)122-101(164)82(48(8)132)130-87(150)58(26-27-168-9)114-93(156)64(34-76(140)141)121-92(155)63(33-73(107)135)119-98(70)161/h11-19,37-38,43-48,53,55-70,79-82,109,131-132H,10,20-36,39-42,104H2,1-9H3,(H2,105,133)(H2,106,134)(H2,107,135)(H,108,110)(H,111,147)(H,112,153)(H,113,157)(H,114,156)(H,115,159)(H,116,154)(H,117,160)(H,118,151)(H,119,161)(H,120,148)(H,121,155)(H,122,164)(H,123,163)(H,124,149)(H,125,152)(H,126,165)(H,127,158)(H,128,162)(H,129,146)(H,130,150)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H,144,145)(H,166,167)/t46-,47+,48+,53+,55-,56-,57-,58+,59-,60-,61-,62+,63+,64-,65+,66-,67-,68+,69-,70-,79-,80-,81+,82-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to Endothelin B receptor in the rat cerebellum |
J Med Chem 35: 1493-508 (1992)
BindingDB Entry DOI: 10.7270/Q27M08KX |
More data for this
Ligand-Target Pair | |
cGMP-specific 3',5'-cyclic phosphodiesterase
(Rattus norvegicus) | BDBM50163573

((R)-3-(2,3-Dihydro-benzofuran-5-yl)-2-(5-pyridin-2...)Show SMILES Oc1c2CN([C@@H](c2nc2ccccc12)c1ccc2OCCc2c1)c1ncc(cn1)-c1ccccn1 Show InChI InChI=1S/C28H21N5O2/c34-27-20-5-1-2-7-23(20)32-25-21(27)16-33(26(25)18-8-9-24-17(13-18)10-12-35-24)28-30-14-19(15-31-28)22-6-3-4-11-29-22/h1-9,11,13-15,26H,10,12,16H2,(H,32,34)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of phosphodiesterase 5 in rat fetal lung fibroblast (RFL-6) cells |
J Med Chem 48: 2126-33 (2005)
Article DOI: 10.1021/jm0401098
BindingDB Entry DOI: 10.7270/Q2WH2QR5 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50368607
(CHEMBL1790180)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CCc3ccc(O)cc3)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)[C@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C122H170N26O33S4/c1-11-63(7)98(118(176)140-90(122(180)181)50-71-54-127-78-25-17-16-24-76(71)78)146-119(177)99(64(8)12-2)145-113(171)89(53-96(158)159)138-107(165)83(46-61(3)4)133-110(168)87(51-72-55-126-60-128-72)136-114(172)91-57-183-182-56-77(125)102(160)147-100(65(9)149)121(179)143-92-58-184-185-59-93(116(174)144-97(62(5)6)117(175)139-85(49-70-32-39-75(153)40-33-70)108(166)134-84(109(167)142-91)48-69-30-37-74(152)38-31-69)141-106(164)82(42-43-94(154)155)131-103(161)79(26-18-20-44-123)130-111(169)88(52-95(156)157)137-104(162)80(27-19-21-45-124)129-105(163)81(41-34-67-28-35-73(151)36-29-67)132-120(178)101(66(10)150)148-112(170)86(135-115(92)173)47-68-22-14-13-15-23-68/h13-17,22-25,28-33,35-40,54-55,60-66,77,79-93,97-101,127,149-153H,11-12,18-21,26-27,34,41-53,56-59,123-125H2,1-10H3,(H,126,128)(H,129,163)(H,130,169)(H,131,161)(H,132,178)(H,133,168)(H,134,166)(H,135,173)(H,136,172)(H,137,162)(H,138,165)(H,139,175)(H,140,176)(H,141,164)(H,142,167)(H,143,179)(H,144,174)(H,145,171)(H,146,177)(H,147,160)(H,148,170)(H,154,155)(H,156,157)(H,158,159)(H,180,181)/t63-,64-,65+,66-,77+,79-,80-,81+,82-,83-,84-,85+,86+,87-,88+,89-,90-,91-,92-,93+,97-,98-,99-,100+,101-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to Endothelin B receptor in the rat cerebellum |
J Med Chem 35: 1493-508 (1992)
BindingDB Entry DOI: 10.7270/Q27M08KX |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50561628
(CHEMBL4800615)Show SMILES [H][C@@]12CO[C@]3([H])OCC(CC13)[C@@H]2CC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(N)cc1 |r,TLB:2:1:7.6.4:9,THB:12:11:7.6.4:9,3:4:11.1:9| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV1 protease by fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00670
BindingDB Entry DOI: 10.7270/Q2GF0Z75 |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50368605

(CHEMBL1790178)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CCC(N)=O)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCC(O)=O)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@@H](NC(=O)[C@@H](CCSC)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H](CC(N)=O)NC2=O)[C@@H](C)O)C(=O)N[C@@H](CC(C)C)C(=O)N[C@H](CC(N)=O)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(C)C)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C103H147N27O37S5/c1-10-46(6)80(100(163)123-67(103(166)167)30-50-37-109-54-19-15-14-18-52(50)54)128-99(162)79(45(4)5)127-95(158)66(36-78(144)145)120-85(148)55(20-23-71(105)133)112-90(153)61(31-51-38-108-43-110-51)117-97(160)69-40-170-169-39-53(104)83(146)129-81(47(7)131)102(165)126-70-42-172-171-41-68(96(159)115-59(28-44(2)3)88(151)118-62(32-72(106)134)91(154)116-60(89(152)125-69)29-49-16-12-11-13-17-49)124-86(149)57(22-25-75(138)139)111-84(147)56(21-24-74(136)137)113-94(157)65(35-77(142)143)122-101(164)82(48(8)132)130-87(150)58(26-27-168-9)114-93(156)64(34-76(140)141)121-92(155)63(33-73(107)135)119-98(70)161/h11-19,37-38,43-48,53,55-70,79-82,109,131-132H,10,20-36,39-42,104H2,1-9H3,(H2,105,133)(H2,106,134)(H2,107,135)(H,108,110)(H,111,147)(H,112,153)(H,113,157)(H,114,156)(H,115,159)(H,116,154)(H,117,160)(H,118,151)(H,119,161)(H,120,148)(H,121,155)(H,122,164)(H,123,163)(H,124,149)(H,125,152)(H,126,165)(H,127,158)(H,128,162)(H,129,146)(H,130,150)(H,136,137)(H,138,139)(H,140,141)(H,142,143)(H,144,145)(H,166,167)/t46-,47+,48+,53+,55-,56-,57-,58+,59-,60-,61-,62+,63+,64-,65+,66-,67-,68+,69-,70-,79-,80-,81+,82-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to Endothelin B receptor in the rat hippocampus |
J Med Chem 35: 1493-508 (1992)
BindingDB Entry DOI: 10.7270/Q27M08KX |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 7
(Rattus norvegicus (rat)) | BDBM21392
(3-(2-aminoethyl)-1H-indole-5-carboxamide | 5-CT | ...)Show InChI InChI=1S/C11H13N3O/c12-4-3-8-6-14-10-2-1-7(11(13)15)5-9(8)10/h1-2,5-6,14H,3-4,12H2,(H2,13,15) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| 0.0290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University
Curated by ChEMBL
| Assay Description
Binding affinity against Rat 5-hydroxytryptamine 7 receptor using [3H]-5-HT |
J Med Chem 43: 1339-49 (2001)
BindingDB Entry DOI: 10.7270/Q2RJ4HQ3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50095111
(4-{(4R,5R)-2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phe...)Show SMILES CCOC(=O)N1CCC(CC1)N1CCC(CC1)C1(O[C@H](C)[C@@H](C)O1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C31H40N2O8S/c1-4-37-30(34)33-17-13-25(14-18-33)32-15-11-24(12-16-32)31(40-21(2)22(3)41-31)23-5-7-26(8-6-23)42(35,36)27-9-10-28-29(19-27)39-20-38-28/h5-10,19,21-22,24-25H,4,11-18,20H2,1-3H3/t21-,22-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned acetylcholine receptor M2 in CHO cells. |
Bioorg Med Chem Lett 10: 2727-30 (2000)
BindingDB Entry DOI: 10.7270/Q2V40TGZ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50561630
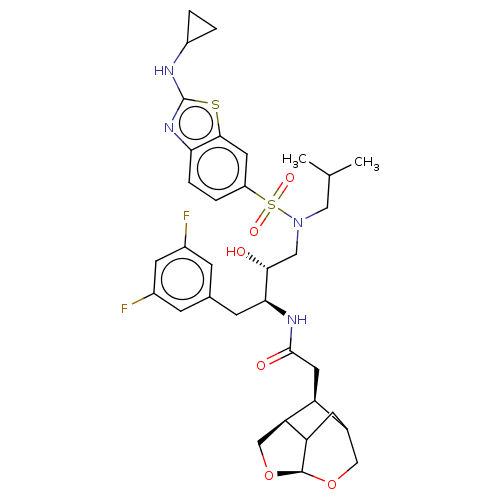
(CHEMBL4743665)Show SMILES [H][C@]12OC[C@@]3([H])[C@@H](CC(=O)N[C@@H](Cc4cc(F)cc(F)c4)[C@H](O)CN(CC(C)C)S(=O)(=O)c4ccc5nc(NC6CC6)sc5c4)C(CC13)CO2 |r,TLB:7:6:48.49.1:46,2:1:6.4:46,THB:3:4:48.49.1:46| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV1 protease by fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00670
BindingDB Entry DOI: 10.7270/Q2GF0Z75 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50095097
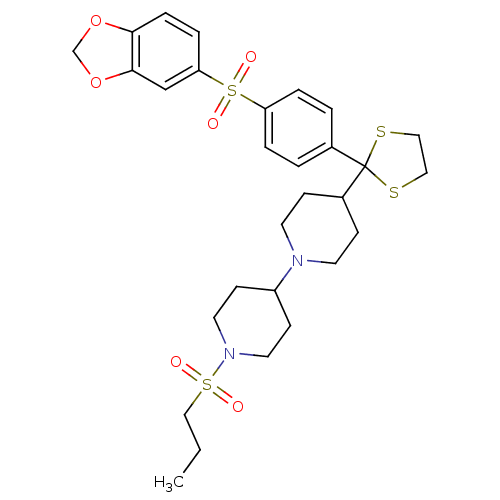
(4-{2-[4-(Benzo[1,3]dioxole-5-sulfonyl)-phenyl]-[1,...)Show SMILES CCCS(=O)(=O)N1CCC(CC1)N1CCC(CC1)C1(SCCS1)c1ccc(cc1)S(=O)(=O)c1ccc2OCOc2c1 Show InChI InChI=1S/C29H38N2O6S4/c1-2-19-40(32,33)31-15-11-24(12-16-31)30-13-9-23(10-14-30)29(38-17-18-39-29)22-3-5-25(6-4-22)41(34,35)26-7-8-27-28(20-26)37-21-36-27/h3-8,20,23-24H,2,9-19,21H2,1H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned acetylcholine receptor M2 in CHO cells. |
Bioorg Med Chem Lett 10: 2727-30 (2000)
BindingDB Entry DOI: 10.7270/Q2V40TGZ |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50561627

(CHEMBL4744116)Show SMILES [H][C@@]12CO[C@]3([H])OCC(CC13)[C@@H]2CC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN(CC(C)C)S(=O)(=O)c1ccc(OC)cc1 |r,TLB:2:1:7.6.4:9,THB:12:11:7.6.4:9,3:4:11.1:9| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV1 protease by fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00670
BindingDB Entry DOI: 10.7270/Q2GF0Z75 |
More data for this
Ligand-Target Pair | |
Endothelin-1 receptor
(RAT) | BDBM50000558

(CHEMBL437472 | ET-1 | Endothelin -1 | Endothelin 1...)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H](CO)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCSC)NC(=O)[C@@H](CC(C)C)NC(=O)[C@H](CO)NC(=O)[C@@H](CO)NC2=O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccccc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C109H159N25O32S5/c1-12-56(9)87(107(163)125-76(109(165)166)39-60-43-113-65-24-18-17-23-63(60)65)134-108(164)88(57(10)13-2)133-99(155)75(42-85(143)144)123-94(150)70(36-54(5)6)118-97(153)73(40-61-44-112-52-114-61)121-103(159)80-49-169-168-48-64(111)89(145)126-77(45-135)102(158)131-81-50-170-171-51-82(105(161)132-86(55(7)8)106(162)124-72(38-59-26-28-62(138)29-27-59)95(151)120-71(96(152)130-80)37-58-21-15-14-16-22-58)129-91(147)67(30-31-83(139)140)116-90(146)66(25-19-20-33-110)115-98(154)74(41-84(141)142)122-92(148)68(32-34-167-11)117-93(149)69(35-53(3)4)119-100(156)78(46-136)127-101(157)79(47-137)128-104(81)160/h14-18,21-24,26-29,43-44,52-57,64,66-82,86-88,113,135-138H,12-13,19-20,25,30-42,45-51,110-111H2,1-11H3,(H,112,114)(H,115,154)(H,116,146)(H,117,149)(H,118,153)(H,119,156)(H,120,151)(H,121,159)(H,122,148)(H,123,150)(H,124,162)(H,125,163)(H,126,145)(H,127,157)(H,128,160)(H,129,147)(H,130,152)(H,131,158)(H,132,161)(H,133,155)(H,134,164)(H,139,140)(H,141,142)(H,143,144)(H,165,166)/t56-,57-,64+,66-,67-,68-,69+,70-,71-,72+,73-,74+,75-,76-,77+,78-,79+,80-,81-,82+,86-,87-,88-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to Endothelin A receptor in the rat atrium |
J Med Chem 35: 1493-508 (1992)
BindingDB Entry DOI: 10.7270/Q27M08KX |
More data for this
Ligand-Target Pair | |
Endothelin receptor type B
(RAT) | BDBM50368607

(CHEMBL1790180)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@@H](N)C(=O)N[C@H]([C@@H](C)O)C(=O)N[C@H]2CSSC[C@@H](NC(=O)[C@H](CCC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CC(O)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@@H](CCc3ccc(O)cc3)NC(=O)[C@@H](NC(=O)[C@@H](Cc3ccccc3)NC2=O)[C@H](C)O)C(=O)N[C@@H](C(C)C)C(=O)N[C@H](Cc2ccc(O)cc2)C(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N1)C(=O)N[C@@H]([C@@H](C)CC)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O Show InChI InChI=1S/C122H170N26O33S4/c1-11-63(7)98(118(176)140-90(122(180)181)50-71-54-127-78-25-17-16-24-76(71)78)146-119(177)99(64(8)12-2)145-113(171)89(53-96(158)159)138-107(165)83(46-61(3)4)133-110(168)87(51-72-55-126-60-128-72)136-114(172)91-57-183-182-56-77(125)102(160)147-100(65(9)149)121(179)143-92-58-184-185-59-93(116(174)144-97(62(5)6)117(175)139-85(49-70-32-39-75(153)40-33-70)108(166)134-84(109(167)142-91)48-69-30-37-74(152)38-31-69)141-106(164)82(42-43-94(154)155)131-103(161)79(26-18-20-44-123)130-111(169)88(52-95(156)157)137-104(162)80(27-19-21-45-124)129-105(163)81(41-34-67-28-35-73(151)36-29-67)132-120(178)101(66(10)150)148-112(170)86(135-115(92)173)47-68-22-14-13-15-23-68/h13-17,22-25,28-33,35-40,54-55,60-66,77,79-93,97-101,127,149-153H,11-12,18-21,26-27,34,41-53,56-59,123-125H2,1-10H3,(H,126,128)(H,129,163)(H,130,169)(H,131,161)(H,132,178)(H,133,168)(H,134,166)(H,135,173)(H,136,172)(H,137,162)(H,138,165)(H,139,175)(H,140,176)(H,141,164)(H,142,167)(H,143,179)(H,144,174)(H,145,171)(H,146,177)(H,147,160)(H,148,170)(H,154,155)(H,156,157)(H,158,159)(H,180,181)/t63-,64-,65+,66-,77+,79-,80-,81+,82-,83-,84-,85+,86+,87-,88+,89-,90-,91-,92-,93+,97-,98-,99-,100+,101-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Warner-Lambert Company
Curated by ChEMBL
| Assay Description
Compound was evaluated for the binding affinity to Endothelin B receptor in the rat hippocampus |
J Med Chem 35: 1493-508 (1992)
BindingDB Entry DOI: 10.7270/Q27M08KX |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311157
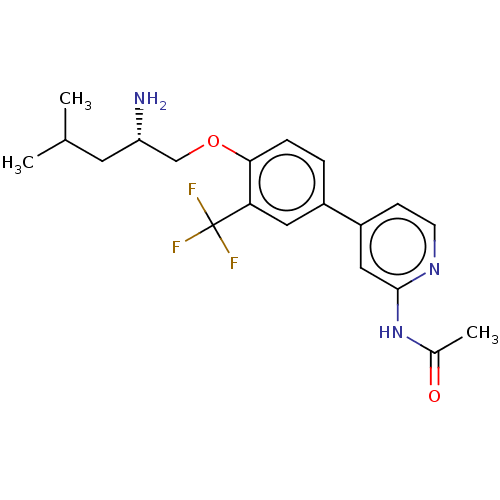
((S)-N-(4-(4-((2-amino-4-methylpentyl)oxy)-3-(trifl...)Show SMILES CC(C)C[C@H](N)COc1ccc(cc1C(F)(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C20H24F3N3O2/c1-12(2)8-16(24)11-28-18-5-4-14(9-17(18)20(21,22)23)15-6-7-25-19(10-15)26-13(3)27/h4-7,9-10,12,16H,8,11,24H2,1-3H3,(H,25,26,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311180
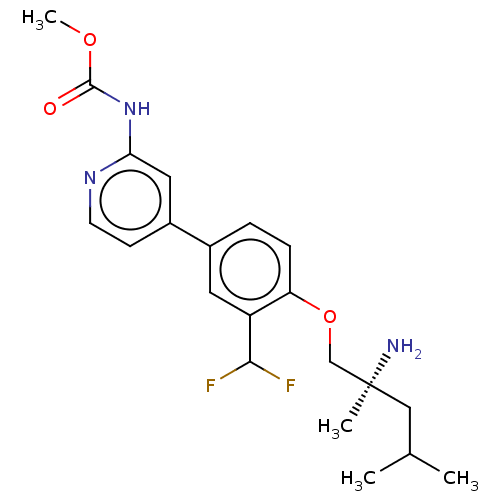
((S)-methyl (4-(4-((2-amino-2,4-dimethylpentyl)oxy)...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(c1)C(F)F |r| Show InChI InChI=1S/C21H27F2N3O3/c1-13(2)11-21(3,24)12-29-17-6-5-14(9-16(17)19(22)23)15-7-8-25-18(10-15)26-20(27)28-4/h5-10,13,19H,11-12,24H2,1-4H3,(H,25,26,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311170
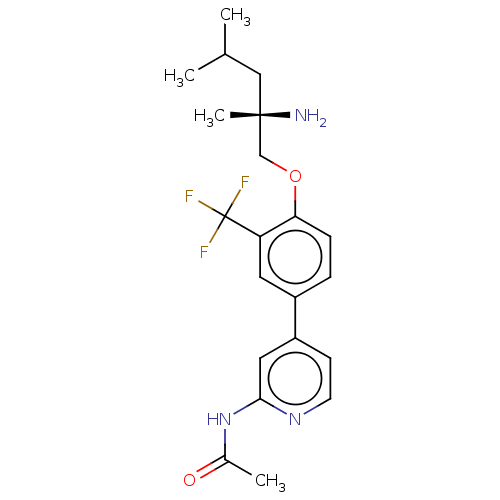
((S)-N-(4-(4-((2-amino-2,4-dimethylpentyl)oxy)-3-(t...)Show SMILES CC(C)C[C@](C)(N)COc1ccc(cc1C(F)(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C21H26F3N3O2/c1-13(2)11-20(4,25)12-29-18-6-5-15(9-17(18)21(22,23)24)16-7-8-26-19(10-16)27-14(3)28/h5-10,13H,11-12,25H2,1-4H3,(H,26,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311155
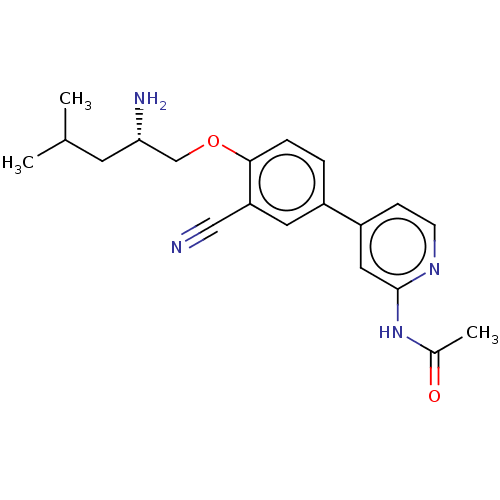
((S)-N-(4-(4-((2-amino-4-methylpentyl)oxy)-3-cyanop...)Show SMILES CC(C)C[C@H](N)COc1ccc(cc1C#N)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C20H24N4O2/c1-13(2)8-18(22)12-26-19-5-4-15(9-17(19)11-21)16-6-7-23-20(10-16)24-14(3)25/h4-7,9-10,13,18H,8,12,22H2,1-3H3,(H,23,24,25)/t18-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311182

((S)-methyl (4-(4-((2-amino-2,4-dimethylpentyl)oxy)...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(OC(F)(F)F)c1 |r| Show InChI InChI=1S/C21H26F3N3O4/c1-13(2)11-20(3,25)12-30-16-6-5-14(9-17(16)31-21(22,23)24)15-7-8-26-18(10-15)27-19(28)29-4/h5-10,13H,11-12,25H2,1-4H3,(H,26,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311158
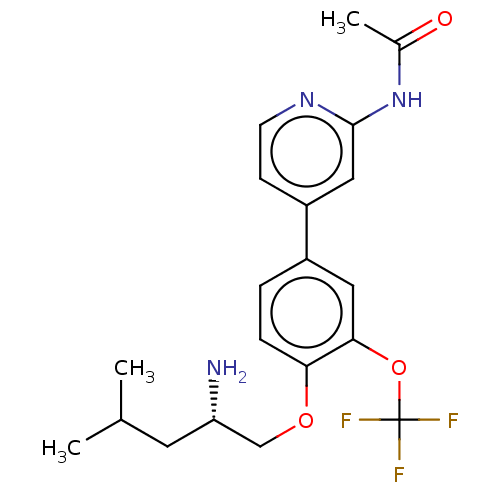
((S)-N-(4-(4-((2-amino-4-methylpentyl)oxy)-3-(trifl...)Show SMILES CC(C)C[C@H](N)COc1ccc(cc1OC(F)(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C20H24F3N3O3/c1-12(2)8-16(24)11-28-17-5-4-14(9-18(17)29-20(21,22)23)15-6-7-25-19(10-15)26-13(3)27/h4-7,9-10,12,16H,8,11,24H2,1-3H3,(H,25,26,27)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM28661
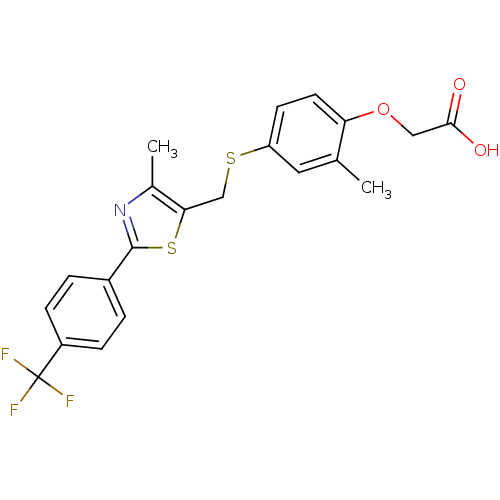
(2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...)Show SMILES Cc1nc(sc1CSc1ccc(OCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO3S2/c1-12-9-16(7-8-17(12)28-10-19(26)27)29-11-18-13(2)25-20(30-18)14-3-5-15(6-4-14)21(22,23)24/h3-9H,10-11H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University
Curated by ChEMBL
| Assay Description
Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARdelta LBD by TR-FRET assay |
J Med Chem 60: 7459-7475 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00805
BindingDB Entry DOI: 10.7270/Q2XK8HQH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50250450

(CHEMBL4103328)Show SMILES [H][C@@]12CCC[C@@]3(OCc4ccccc4)[C@@]4([H])Cc5ccc(O)c(O1)c5[C@@]23CCN4C |r,TLB:29:28:5:24.17.16| Show InChI InChI=1S/C24H27NO3/c1-25-13-12-23-20-8-5-11-24(23,27-15-16-6-3-2-4-7-16)19(25)14-17-9-10-18(26)22(28-20)21(17)23/h2-4,6-7,9-10,19-20,26H,5,8,11-15H2,1H3/t19-,20+,23-,24-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck
Curated by ChEMBL
| Assay Description
Displacement of [3H]-DAMGO from recombinant human MOR expressed in CHO cell membranes after 60 mins by liquid scintillation counting |
J Med Chem 60: 9407-9412 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01363
BindingDB Entry DOI: 10.7270/Q2PZ5C72 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085048
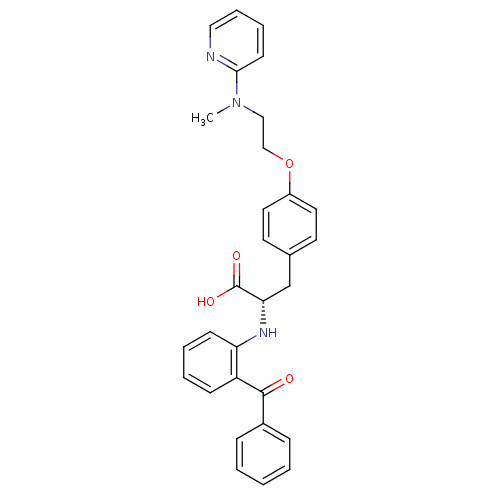
((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(methyl-pyri...)Show SMILES CN(CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)c1ccccn1 Show InChI InChI=1S/C30H29N3O4/c1-33(28-13-7-8-18-31-28)19-20-37-24-16-14-22(15-17-24)21-27(30(35)36)32-26-12-6-5-11-25(26)29(34)23-9-3-2-4-10-23/h2-18,27,32H,19-21H2,1H3,(H,35,36)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0497 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University
Curated by ChEMBL
| Assay Description
Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARgamma LBD by TR-FRET assay |
J Med Chem 60: 7459-7475 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00805
BindingDB Entry DOI: 10.7270/Q2XK8HQH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Genome polyprotein
(Hepatitis C virus) | BDBM123410
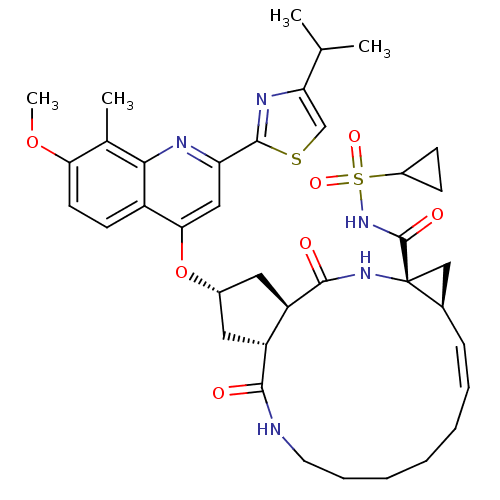
(US8741926, 82 | US8754106, 82 | US8754106, 91)Show SMILES COc1ccc2c(O[C@H]3C[C@@H]4[C@@H](C3)C(=O)N[C@@]3(C[C@H]3\C=C/CCCCCNC4=O)C(=O)NS(=O)(=O)C3CC3)cc(nc2c1C)-c1nc(cs1)C(C)C |r,c:21| Show InChI InChI=1S/C38H47N5O7S2/c1-21(2)30-20-51-36(41-30)29-18-32(26-13-14-31(49-4)22(3)33(26)40-29)50-24-16-27-28(17-24)35(45)42-38(37(46)43-52(47,48)25-11-12-25)19-23(38)10-8-6-5-7-9-15-39-34(27)44/h8,10,13-14,18,20-21,23-25,27-28H,5-7,9,11-12,15-17,19H2,1-4H3,(H,39,44)(H,42,45)(H,43,46)/b10-8-/t23-,24+,27-,28-,38-/m1/s1 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB
US Patent
| Assay Description
The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... |
US Patent US8754106 (2014)
BindingDB Entry DOI: 10.7270/Q2V40SWX |
More data for this
Ligand-Target Pair | |
Genome polyprotein
(Hepatitis C virus) | BDBM123407
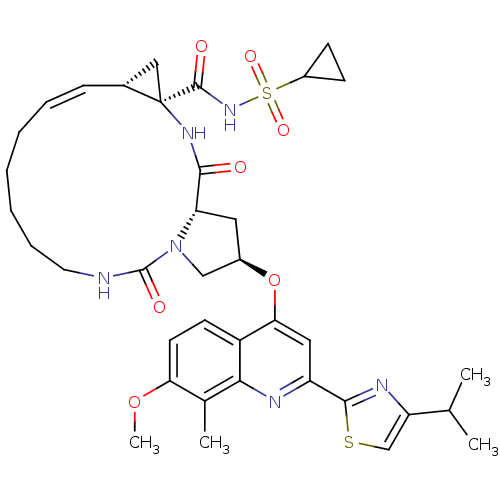
(US8741926, 91)Show SMILES COc1ccc2c(O[C@@H]3C[C@@H]4N(C3)C(=O)NCCCCC\C=C/[C@@H]3C[C@]3(NC4=O)C(=O)NS(=O)(=O)C3CC3)cc(nc2c1C)-c1nc(cs1)C(C)C |r,c:22| Show InChI InChI=1S/C37H46N6O7S2/c1-21(2)28-20-51-34(40-28)27-17-31(26-13-14-30(49-4)22(3)32(26)39-27)50-24-16-29-33(44)41-37(35(45)42-52(47,48)25-11-12-25)18-23(37)10-8-6-5-7-9-15-38-36(46)43(29)19-24/h8,10,13-14,17,20-21,23-25,29H,5-7,9,11-12,15-16,18-19H2,1-4H3,(H,38,46)(H,41,44)(H,42,45)/b10-8-/t23-,24-,29+,37-/m1/s1 | PDB
MMDB
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.0500 | -59.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB
US Patent
| Assay Description
The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... |
US Patent US8741926 (2014)
BindingDB Entry DOI: 10.7270/Q2Z31XBC |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311179
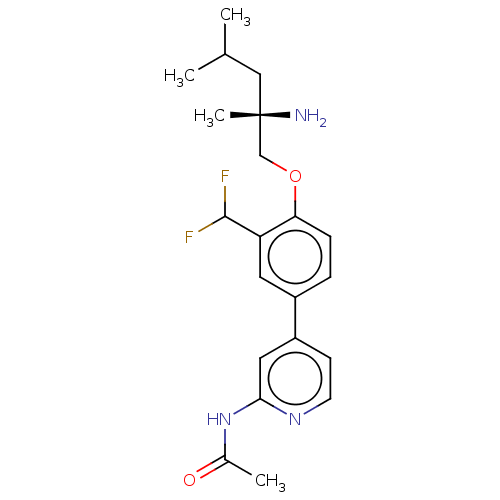
((S)-N-(4-(4-((2-amino-2,4-dimethylpentyl)oxy)-3-(d...)Show SMILES CC(C)C[C@](C)(N)COc1ccc(cc1C(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C21H27F2N3O2/c1-13(2)11-21(4,24)12-28-18-6-5-15(9-17(18)20(22)23)16-7-8-25-19(10-16)26-14(3)27/h5-10,13,20H,11-12,24H2,1-4H3,(H,25,26,27)/t21-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311268
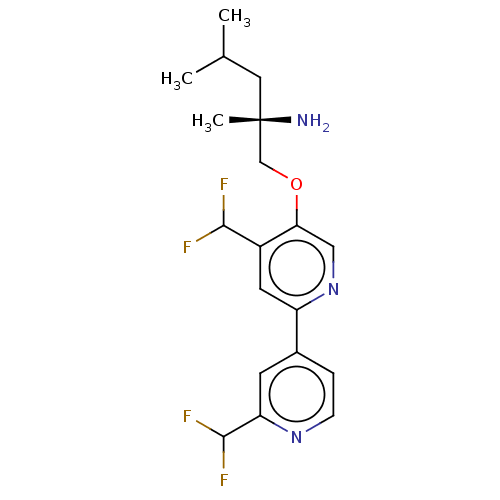
((S)-1-((2′,4-bis(difluoromethyl)-[2,4′...)Show SMILES CC(C)C[C@](C)(N)COc1cnc(cc1C(F)F)-c1ccnc(c1)C(F)F |r| Show InChI InChI=1S/C19H23F4N3O/c1-11(2)8-19(3,24)10-27-16-9-26-14(7-13(16)17(20)21)12-4-5-25-15(6-12)18(22)23/h4-7,9,11,17-18H,8,10,24H2,1-3H3/t19-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311176
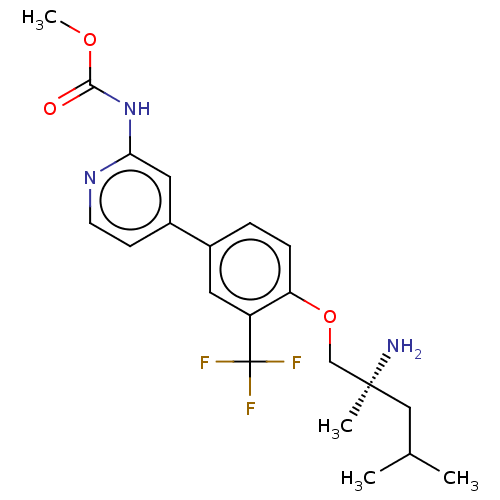
((S)-methyl (4-(4-((2-amino-2,4-dimethylpentyl)oxy)...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(c1)C(F)(F)F |r| Show InChI InChI=1S/C21H26F3N3O3/c1-13(2)11-20(3,25)12-30-17-6-5-14(9-16(17)21(22,23)24)15-7-8-26-18(10-15)27-19(28)29-4/h5-10,13H,11-12,25H2,1-4H3,(H,26,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311189
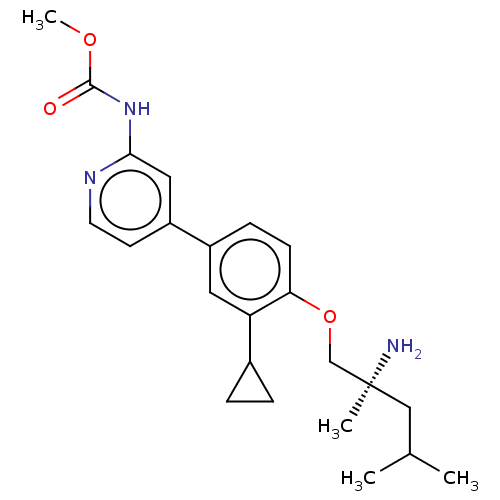
((S)-methyl (4-(4-((2-amino-2,4-dimethylpentyl)oxy)...)Show SMILES COC(=O)Nc1cc(ccn1)-c1ccc(OC[C@@](C)(N)CC(C)C)c(c1)C1CC1 |r| Show InChI InChI=1S/C23H31N3O3/c1-15(2)13-23(3,24)14-29-20-8-7-17(11-19(20)16-5-6-16)18-9-10-25-21(12-18)26-22(27)28-4/h7-12,15-16H,5-6,13-14,24H2,1-4H3,(H,25,26,27)/t23-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50099491
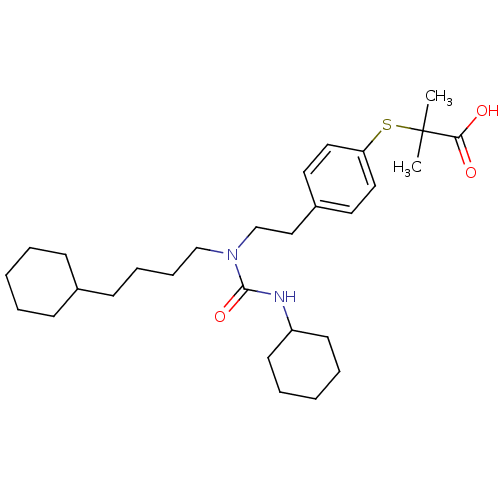
(2-(4-(2-(3-cyclohexyl-1-(4-cyclohexylbutyl)ureido)...)Show SMILES CC(C)(Sc1ccc(CCN(CCCCC2CCCCC2)C(=O)NC2CCCCC2)cc1)C(O)=O Show InChI InChI=1S/C29H46N2O3S/c1-29(2,27(32)33)35-26-18-16-24(17-19-26)20-22-31(28(34)30-25-14-7-4-8-15-25)21-10-9-13-23-11-5-3-6-12-23/h16-19,23,25H,3-15,20-22H2,1-2H3,(H,30,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0541 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University
Curated by ChEMBL
| Assay Description
Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARalpha LBD by TR-FRET assay |
J Med Chem 60: 7459-7475 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00805
BindingDB Entry DOI: 10.7270/Q2XK8HQH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neutrophil elastase
(Homo sapiens (Human)) | BDBM50061031
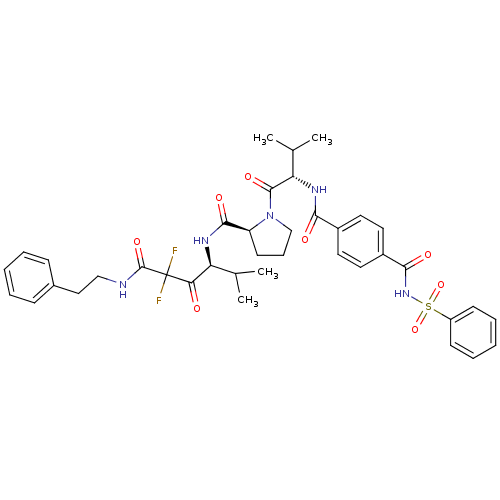
((S)-1-[(S)-2-(4-Benzenesulfonylaminocarbonyl-benzo...)Show SMILES CC(C)[C@H](NC(=O)c1ccc(cc1)C(=O)NS(=O)(=O)c1ccccc1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](C(C)C)C(=O)C(F)(F)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C39H45F2N5O8S/c1-24(2)31(33(47)39(40,41)38(52)42-22-21-26-12-7-5-8-13-26)43-36(50)30-16-11-23-46(30)37(51)32(25(3)4)44-34(48)27-17-19-28(20-18-27)35(49)45-55(53,54)29-14-9-6-10-15-29/h5-10,12-15,17-20,24-25,30-32H,11,16,21-23H2,1-4H3,(H,42,52)(H,43,50)(H,44,48)(H,45,49)/t30-,31-,32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
ZENECA Pharmaceuticals
Curated by ChEMBL
| Assay Description
Binding affinity for human leukocyte elastase |
J Med Chem 40: 3173-81 (1997)
Article DOI: 10.1021/jm970250z
BindingDB Entry DOI: 10.7270/Q2GT5M85 |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50111036
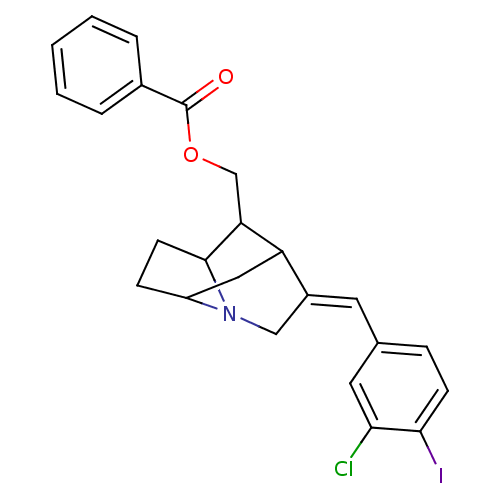
(Benzoic acid 9-(3-chloro-4-iodo-benzylidene)-7-aza...)Show SMILES Clc1cc(\C=C2/CN3C4CCC3C(COC(=O)c3ccccc3)C2C4)ccc1I |TLB:13:12:5.6:8.24,THB:10:11:5.6:8.24| Show InChI InChI=1S/C24H23ClINO2/c25-21-11-15(6-8-22(21)26)10-17-13-27-18-7-9-23(27)20(19(17)12-18)14-29-24(28)16-4-2-1-3-5-16/h1-6,8,10-11,18-20,23H,7,9,12-14H2/b17-10+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of high affinity re-uptake of [3H]5-HT (serotonin) into nerve ending synaptosomes |
J Med Chem 45: 1930-41 (2002)
BindingDB Entry DOI: 10.7270/Q2M32WHG |
More data for this
Ligand-Target Pair | |
Sodium-dependent serotonin transporter
(Rattus norvegicus (rat)) | BDBM50111036

(Benzoic acid 9-(3-chloro-4-iodo-benzylidene)-7-aza...)Show SMILES Clc1cc(\C=C2/CN3C4CCC3C(COC(=O)c3ccccc3)C2C4)ccc1I |TLB:13:12:5.6:8.24,THB:10:11:5.6:8.24| Show InChI InChI=1S/C24H23ClINO2/c25-21-11-15(6-8-22(21)26)10-17-13-27-18-7-9-23(27)20(19(17)12-18)14-29-24(28)16-4-2-1-3-5-16/h1-6,8,10-11,18-20,23H,7,9,12-14H2/b17-10+ | PDB
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University Medical Center
Curated by ChEMBL
| Assay Description
Inhibition of [3H]5-HT reuptake at serotonin transporter in rat mid brain |
Bioorg Med Chem Lett 12: 993-5 (2002)
BindingDB Entry DOI: 10.7270/Q20K294R |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311160
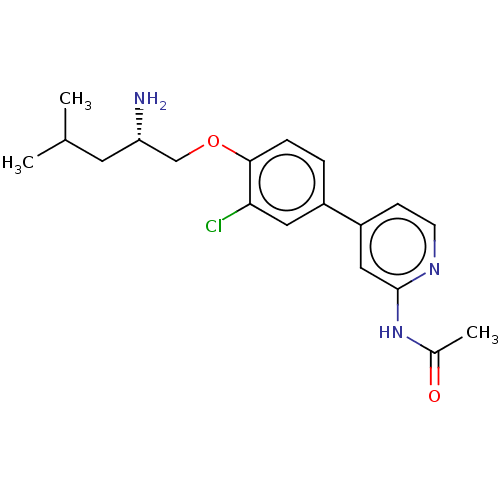
((S)-N-(4-(4-((2-amino-4-methylpentyl)oxy)-3-chloro...)Show SMILES CC(C)C[C@H](N)COc1ccc(cc1Cl)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C19H24ClN3O2/c1-12(2)8-16(21)11-25-18-5-4-14(9-17(18)20)15-6-7-22-19(10-15)23-13(3)24/h4-7,9-10,12,16H,8,11,21H2,1-3H3,(H,22,23,24)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
5-hydroxytryptamine receptor 3A
(Homo sapiens (Human)) | BDBM50334454

(CHEMBL1643895 | Ramosetron | US9045501, Ramosetron)Show SMILES Cn1cc(C(=O)[C@@H]2CCc3nc[nH]c3C2)c2ccccc12 |r| Show InChI InChI=1S/C17H17N3O/c1-20-9-13(12-4-2-3-5-16(12)20)17(21)11-6-7-14-15(8-11)19-10-18-14/h2-5,9-11H,6-8H2,1H3,(H,18,19)/t11-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AMRI
Curated by ChEMBL
| Assay Description
Binding affinity to human 5HT3A receptor |
Bioorg Med Chem Lett 21: 58-61 (2010)
Article DOI: 10.1016/j.bmcl.2010.11.080
BindingDB Entry DOI: 10.7270/Q2474B4Q |
More data for this
Ligand-Target Pair | |
Histamine H3 receptor
(Homo sapiens (Human)) | BDBM50159110
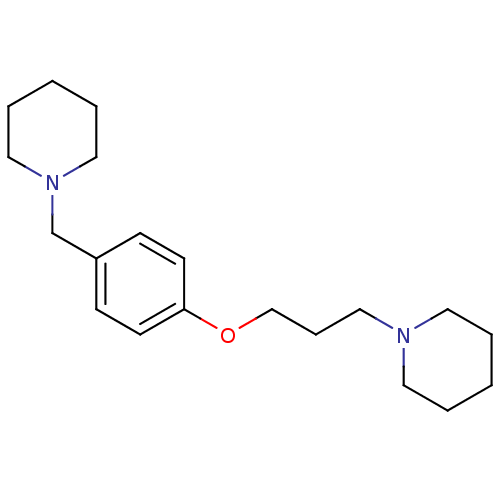
(1-(3-(4-(piperidin-1-ylmethyl)phenoxy)propyl)piper...)Show InChI InChI=1S/C20H32N2O/c1-3-12-21(13-4-1)16-7-17-23-20-10-8-19(9-11-20)18-22-14-5-2-6-15-22/h8-11H,1-7,12-18H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0603 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity in Nluc-hH3R assessed in HEK293 cells by NanoBRET binding assay |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01089
BindingDB Entry DOI: 10.7270/Q2TB1BTS |
More data for this
Ligand-Target Pair | |
Gag-Pol polyprotein [489-587]
(Human immunodeficiency virus type 1) | BDBM50561629
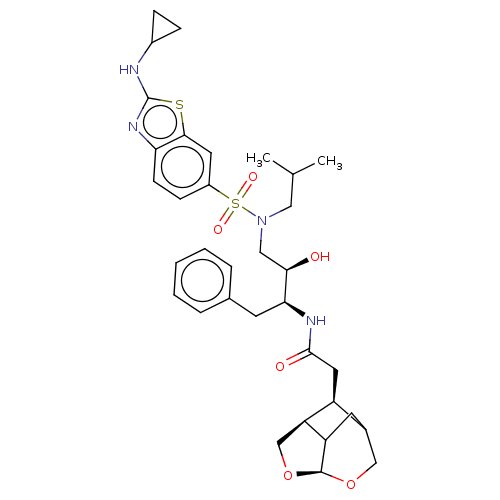
(CHEMBL4740468)Show SMILES [H][C@]12OC[C@@]3([H])[C@@H](CC(=O)N[C@@H](Cc4ccccc4)[C@H](O)CN(CC(C)C)S(=O)(=O)c4ccc5nc(NC6CC6)sc5c4)C(CC13)CO2 |r,TLB:2:1:6.4:44,7:6:46.47.1:44,THB:3:4:46.47.1:44| | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0660 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of HIV1 protease by fluorescence based assay |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.9b00670
BindingDB Entry DOI: 10.7270/Q2GF0Z75 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(BOVINE) | BDBM21173
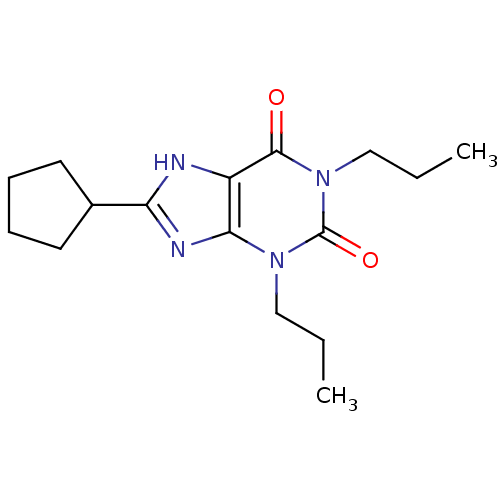
(1,3-dipropyl-8-cyclopentylxanthine | 8-cyclopentyl...)Show InChI InChI=1S/C16H24N4O2/c1-3-9-19-14-12(15(21)20(10-4-2)16(19)22)17-13(18-14)11-7-5-6-8-11/h11H,3-10H2,1-2H3,(H,17,18) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universität Heidelberg
Curated by PDSP Ki Database
| |
Naunyn Schmiedebergs Arch Pharmacol 336: 204-10 (1987)
Article DOI: 10.1007/bf00165806
BindingDB Entry DOI: 10.7270/Q26H4FXN |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50092959
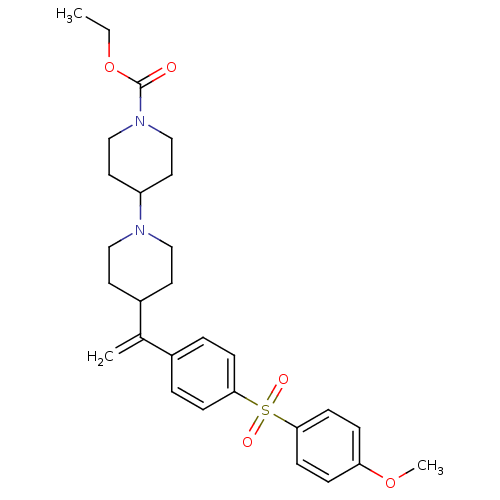
(4-{1-[4-(4-Methoxy-benzenesulfonyl)-phenyl]-vinyl}...)Show SMILES CCOC(=O)N1CCC(CC1)N1CCC(CC1)C(=C)c1ccc(cc1)S(=O)(=O)c1ccc(OC)cc1 Show InChI InChI=1S/C28H36N2O5S/c1-4-35-28(31)30-19-15-24(16-20-30)29-17-13-23(14-18-29)21(2)22-5-9-26(10-6-22)36(32,33)27-11-7-25(34-3)8-12-27/h5-12,23-24H,2,4,13-20H2,1,3H3 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human cloned acetylcholine receptor M2 in CHO cells. |
Bioorg Med Chem Lett 10: 2727-30 (2000)
BindingDB Entry DOI: 10.7270/Q2V40TGZ |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311181
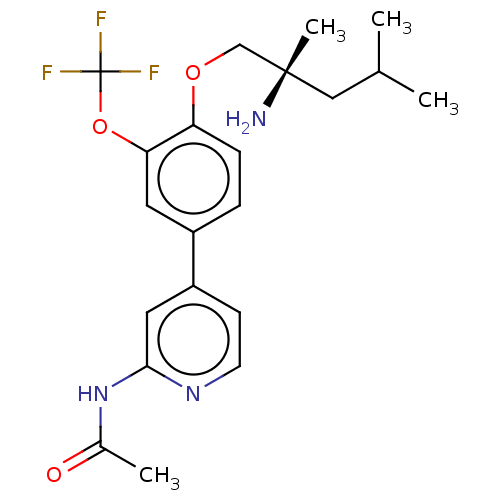
((S)-N-(4-(4-((2-amino-2,4-dimethylpentyl)oxy)-3-(t...)Show SMILES CC(C)C[C@](C)(N)COc1ccc(cc1OC(F)(F)F)-c1ccnc(NC(C)=O)c1 |r| Show InChI InChI=1S/C21H26F3N3O3/c1-13(2)11-20(4,25)12-29-17-6-5-15(9-18(17)30-21(22,23)24)16-7-8-26-19(10-16)27-14(3)28/h5-10,13H,11-12,25H2,1-4H3,(H,26,27,28)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |
AP2-associated protein kinase 1
(Mus musculus) | BDBM311264
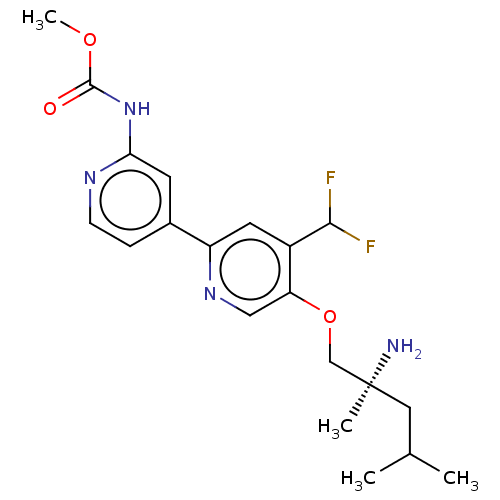
((S)-methyl (5-((2-amino-2,4-dimethylpentyl)oxy)-4-...)Show SMILES COC(=O)Nc1cc(ccn1)-c1cc(C(F)F)c(OC[C@@](C)(N)CC(C)C)cn1 |r| Show InChI InChI=1S/C20H26F2N4O3/c1-12(2)9-20(3,23)11-29-16-10-25-15(8-14(16)18(21)22)13-5-6-24-17(7-13)26-19(27)28-4/h5-8,10,12,18H,9,11,23H2,1-4H3,(H,24,26,27)/t20-/m0/s1 | PDB
MMDB
Reactome pathway
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02132
BindingDB Entry DOI: 10.7270/Q2TH8RSP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data