 Found 22045 hits with Last Name = 'ong' and Initial = 'h'
Found 22045 hits with Last Name = 'ong' and Initial = 'h' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50315177
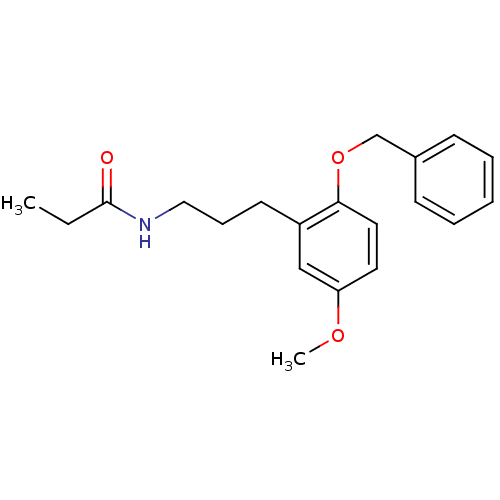
(CHEMBL1091161 | N-(3-(2-(benzyloxy)-5-methoxypheny...)Show InChI InChI=1S/C20H25NO3/c1-3-20(22)21-13-7-10-17-14-18(23-2)11-12-19(17)24-15-16-8-5-4-6-9-16/h4-6,8-9,11-12,14H,3,7,10,13,15H2,1-2H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.000550 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human melatonin MT2 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2582-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.084
BindingDB Entry DOI: 10.7270/Q2S75GG4 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50315171
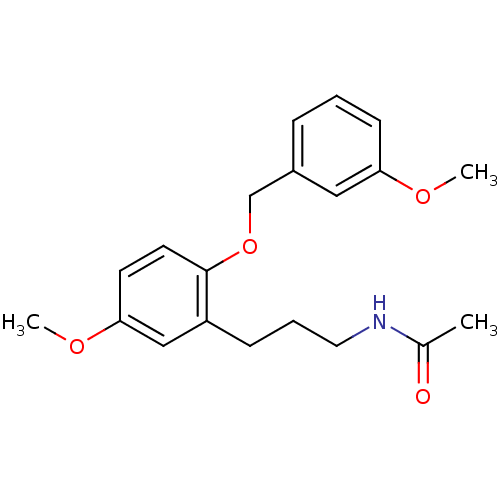
(CHEMBL1092646 | N-(3-(5-methoxy-2-(3-methoxybenzyl...)Show InChI InChI=1S/C20H25NO4/c1-15(22)21-11-5-7-17-13-19(24-3)9-10-20(17)25-14-16-6-4-8-18(12-16)23-2/h4,6,8-10,12-13H,5,7,11,14H2,1-3H3,(H,21,22) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.000690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human melatonin MT2 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2582-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.084
BindingDB Entry DOI: 10.7270/Q2S75GG4 |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50315178
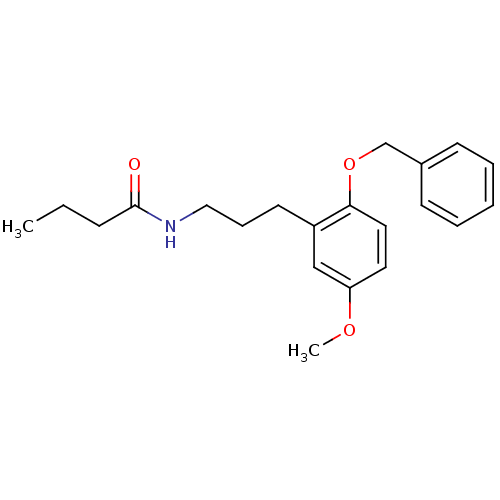
(CHEMBL1088825 | N-(3-(2-(benzyloxy)-5-methoxypheny...)Show InChI InChI=1S/C21H27NO3/c1-3-8-21(23)22-14-7-11-18-15-19(24-2)12-13-20(18)25-16-17-9-5-4-6-10-17/h4-6,9-10,12-13,15H,3,7-8,11,14,16H2,1-2H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00103 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human melatonin MT2 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2582-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.084
BindingDB Entry DOI: 10.7270/Q2S75GG4 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(RAT) | BDBM50097783
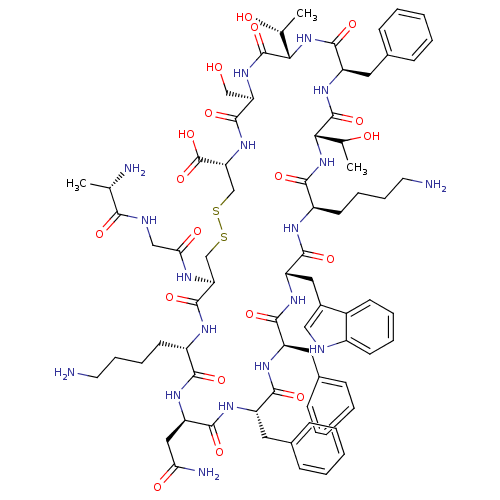
(CHEMBL438401 | D-Trp8 SST-14 | SOMATOSTATIN | SRIF...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@@H](NC(=O)[C@@H](CCCCN)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@@H](Cc2ccccc2)NC(=O)[C@H](Cc2ccccc2)NC(=O)[C@@H](CC(N)=O)NC(=O)[C@H](CCCCN)NC(=O)[C@H](CSSC[C@@H](NC(=O)[C@@H](CO)NC1=O)C(O)=O)NC(=O)CNC(=O)[C@H](C)N)C(C)O Show InChI InChI=1S/C76H104N18O19S2/c1-41(79)64(100)82-37-61(99)83-58-39-114-115-40-59(76(112)113)92-72(108)57(38-95)91-75(111)63(43(3)97)94-71(107)54(33-46-23-11-6-12-24-46)90-74(110)62(42(2)96)93-66(102)51(28-16-18-30-78)84-69(105)55(34-47-36-81-49-26-14-13-25-48(47)49)88-68(104)53(32-45-21-9-5-10-22-45)86-67(103)52(31-44-19-7-4-8-20-44)87-70(106)56(35-60(80)98)89-65(101)50(85-73(58)109)27-15-17-29-77/h4-14,19-26,36,41-43,50-59,62-63,81,95-97H,15-18,27-35,37-40,77-79H2,1-3H3,(H2,80,98)(H,82,100)(H,83,99)(H,84,105)(H,85,109)(H,86,103)(H,87,106)(H,88,104)(H,89,101)(H,90,110)(H,91,111)(H,92,108)(H,93,102)(H,94,107)(H,112,113)/t41-,42?,43+,50-,51+,52-,53+,54+,55-,56+,57+,58-,59+,62-,63-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 44: 385-92 (1993)
BindingDB Entry DOI: 10.7270/Q2X065KZ |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(RAT) | BDBM82253
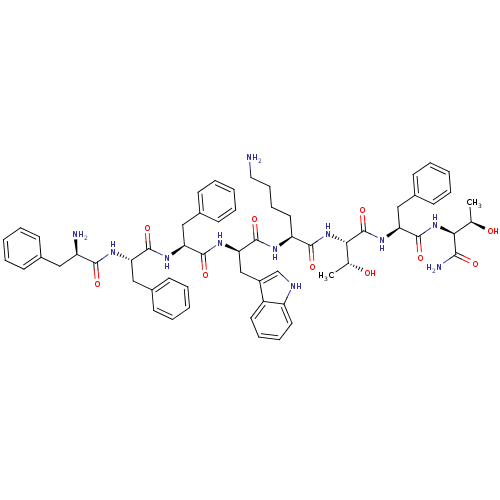
(BIM 23052 | CAS_133073-82-2)Show SMILES C[C@@H](O)[C@H](NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@@H](NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc1c[nH]c2ccccc12)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](Cc1ccccc1)NC(=O)[C@H](N)Cc1ccccc1)[C@@H](C)O)C(N)=O |r| Show InChI InChI=1S/C61H75N11O10/c1-37(73)52(54(64)75)71-60(81)50(34-42-25-13-6-14-26-42)70-61(82)53(38(2)74)72-56(77)47(29-17-18-30-62)66-59(80)51(35-43-36-65-46-28-16-15-27-44(43)46)69-58(79)49(33-41-23-11-5-12-24-41)68-57(78)48(32-40-21-9-4-10-22-40)67-55(76)45(63)31-39-19-7-3-8-20-39/h3-16,19-28,36-38,45,47-53,65,73-74H,17-18,29-35,62-63H2,1-2H3,(H2,64,75)(H,66,80)(H,67,76)(H,68,78)(H,69,79)(H,70,82)(H,71,81)(H,72,77)/t37-,38-,45-,47+,48+,49+,50+,51-,52+,53+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 44: 385-92 (1993)
BindingDB Entry DOI: 10.7270/Q2X065KZ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50214974
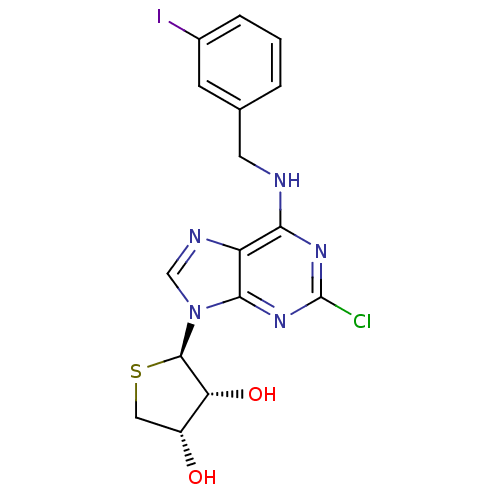
((2R,3R,4S)-2-(2-chloro-6-(3-iodobenzylamino)-9H-pu...)Show SMILES O[C@@H]1CS[C@H]([C@@H]1O)n1cnc2c(NCc3cccc(I)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C16H15ClIN5O2S/c17-16-21-13(19-5-8-2-1-3-9(18)4-8)11-14(22-16)23(7-20-11)15-12(25)10(24)6-26-15/h1-4,7,10,12,15,24-25H,5-6H2,(H,19,21,22)/t10-,12-,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00483 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University
Curated by ChEMBL
| Assay Description
Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARdelta LBD by TR-FRET assay |
J Med Chem 60: 7459-7475 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00805
BindingDB Entry DOI: 10.7270/Q2XK8HQH |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(RAT) | BDBM82463
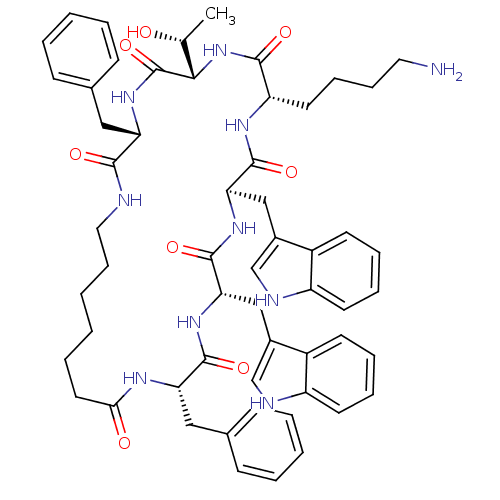
(Cyclo[L-Trp-D-Trp-L-Lys-L-Thr-L-Phe-(7-amino*hepta...)Show SMILES C[C@@H](O)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccccc2)NC(=O)CCCCCCNC(=O)[C@H](Cc2ccccc2)NC1=O Show InChI InChI=1S/C57H70N10O8/c1-36(68)51-57(75)66-46(30-37-18-6-4-7-19-37)52(70)59-29-17-3-2-10-27-50(69)62-47(31-38-20-8-5-9-21-38)54(72)64-49(33-40-35-61-44-25-14-12-23-42(40)44)56(74)65-48(32-39-34-60-43-24-13-11-22-41(39)43)55(73)63-45(53(71)67-51)26-15-16-28-58/h4-9,11-14,18-25,34-36,45-49,51,60-61,68H,2-3,10,15-17,26-33,58H2,1H3,(H,59,70)(H,62,69)(H,63,73)(H,64,72)(H,65,74)(H,66,75)(H,67,71)/t36-,45+,46+,47+,48-,49+,51+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 44: 385-92 (1993)
BindingDB Entry DOI: 10.7270/Q2X065KZ |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50001714
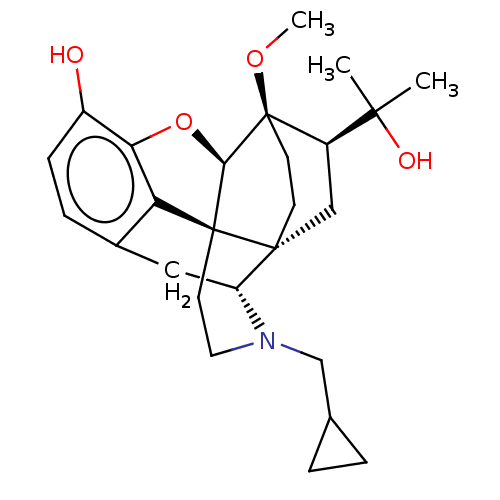
(2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1C(C)(C)O)[C@H]1Cc4ccc(O)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |THB:8:7:22.21:4.3| Show InChI InChI=1S/C26H35NO4/c1-23(2,29)18-13-24-8-9-26(18,30-3)22-25(24)10-11-27(14-15-4-5-15)19(24)12-16-6-7-17(28)21(31-22)20(16)25/h6-7,15,18-19,22,28-29H,4-5,8-14H2,1-3H3/t18-,19-,22-,24-,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50039022
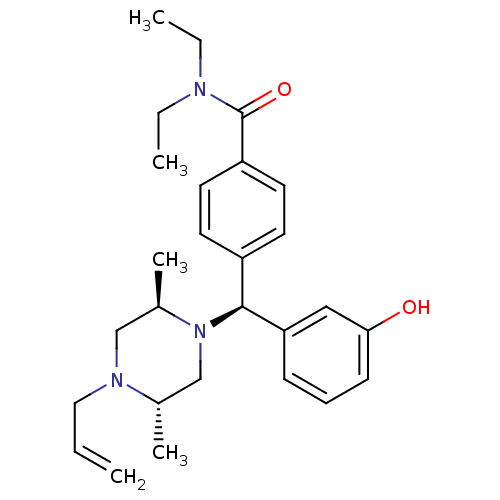
(4-[(S)-((2R,5S)-4-Allyl-2,5-dimethyl-piperazin-1-y...)Show SMILES CCN(CC)C(=O)c1ccc(cc1)[C@H](N1C[C@H](C)N(CC=C)C[C@H]1C)c1cccc(O)c1 Show InChI InChI=1S/C27H37N3O2/c1-6-16-29-18-21(5)30(19-20(29)4)26(24-10-9-11-25(31)17-24)22-12-14-23(15-13-22)27(32)28(7-2)8-3/h6,9-15,17,20-21,26,31H,1,7-8,16,18-19H2,2-5H3/t20-,21+,26-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM82552
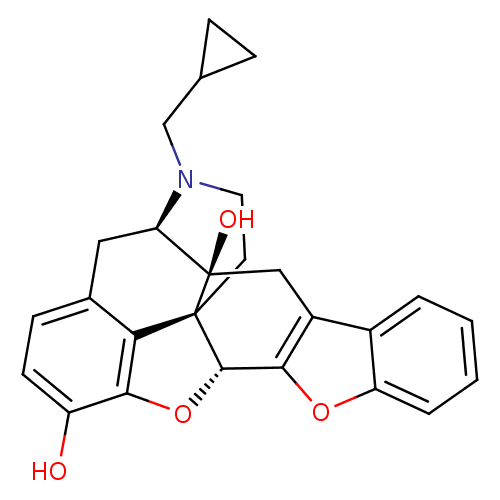
(CAS_111555-58-9 | NTB | naltrindolebenzofuran)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1oc2ccccc2c1C[C@@]35O |r| Show InChI InChI=1S/C26H25NO4/c28-18-8-7-15-11-20-26(29)12-17-16-3-1-2-4-19(16)30-22(17)24-25(26,21(15)23(18)31-24)9-10-27(20)13-14-5-6-14/h1-4,7-8,14,20,24,28-29H,5-6,9-13H2/t20-,24+,25+,26-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50246899

((S)-2-(3-((S)-1-carboxy-5-(4-iodobenzamido)pentyl)...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCCCNC(=O)c1ccc(I)cc1)C(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H24IN3O8/c20-12-6-4-11(5-7-12)16(26)21-10-2-1-3-13(17(27)28)22-19(31)23-14(18(29)30)8-9-15(24)25/h4-7,13-14H,1-3,8-10H2,(H,21,26)(H,24,25)(H,27,28)(H,29,30)(H2,22,23,31)/t13-,14-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yonsei University College of Medicine
Curated by ChEMBL
| Assay Description
Inhibition of PSMA (unknown origin) |
Bioorg Med Chem Lett 28: 572-576 (2018)
Article DOI: 10.1016/j.bmcl.2018.01.047
BindingDB Entry DOI: 10.7270/Q2RN3BGJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM50214981
((2R,3R,4S)-2-(2-chloro-6-(3-chlorobenzylamino)-9H-...)Show SMILES O[C@@H]1CS[C@H]([C@@H]1O)n1cnc2c(NCc3cccc(Cl)c3)nc(Cl)nc12 |r| Show InChI InChI=1S/C16H15Cl2N5O2S/c17-9-3-1-2-8(4-9)5-19-13-11-14(22-16(18)21-13)23(7-20-11)15-12(25)10(24)6-26-15/h1-4,7,10,12,15,24-25H,5-6H2,(H,19,21,22)/t10-,12-,15-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0102 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University
Curated by ChEMBL
| Assay Description
Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARdelta LBD by TR-FRET assay |
J Med Chem 60: 7459-7475 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00805
BindingDB Entry DOI: 10.7270/Q2XK8HQH |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82551

(C18130 | CAS_105618-26-6 | NOR-BNI (HCI)2 | NORBNI)Show SMILES Oc1ccc2C[C@H]3N(CC4CC4)CC[C@@]45[C@@H](Oc1c24)c1[nH]c2[C@@H]4Oc6c7c(C[C@H]8N(CC9CC9)CC[C@@]47[C@@]8(O)Cc2c1C[C@@]35O)ccc6O |r| Show InChI InChI=1S/C40H43N3O6/c44-25-7-5-21-13-27-39(46)15-23-24-16-40(47)28-14-22-6-8-26(45)34-30(22)38(40,10-12-43(28)18-20-3-4-20)36(49-34)32(24)41-31(23)35-37(39,29(21)33(25)48-35)9-11-42(27)17-19-1-2-19/h5-8,19-20,27-28,35-36,41,44-47H,1-4,9-18H2/t27-,28-,35+,36+,37+,38+,39-,40-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50100528
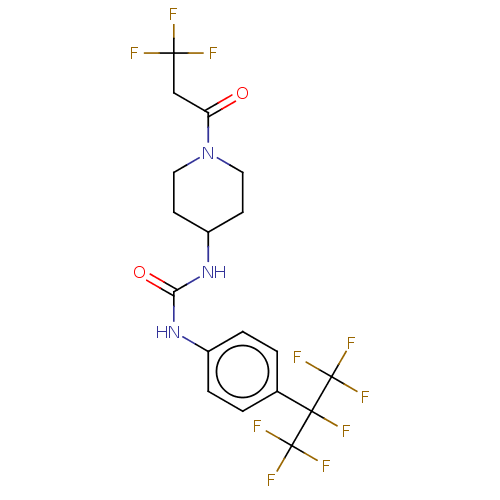
(CHEMBL3327081)Show SMILES FC(F)(F)CC(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C18H17F10N3O2/c19-15(20,21)9-13(32)31-7-5-12(6-8-31)30-14(33)29-11-3-1-10(2-4-11)16(22,17(23,24)25)18(26,27)28/h1-4,12H,5-9H2,(H2,29,30,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| <0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay |
J Med Chem 57: 7016-30 (2014)
Article DOI: 10.1021/jm500694p
BindingDB Entry DOI: 10.7270/Q2FJ2JJQ |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50100535
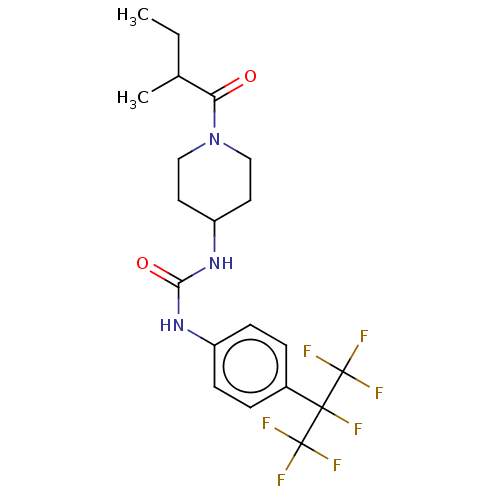
(CHEMBL3327073)Show SMILES CCC(C)C(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C20H24F7N3O2/c1-3-12(2)16(31)30-10-8-15(9-11-30)29-17(32)28-14-6-4-13(5-7-14)18(21,19(22,23)24)20(25,26)27/h4-7,12,15H,3,8-11H2,1-2H3,(H2,28,29,32) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Davis
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant soluble epoxide hydrolase by FRET-based ACPU displacement assay |
J Med Chem 57: 7016-30 (2014)
Article DOI: 10.1021/jm500694p
BindingDB Entry DOI: 10.7270/Q2FJ2JJQ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM21864

((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...)Show SMILES [H][C@@]12Cc3ccc(O)c4OC5c6[nH]c7ccccc7c6CC1(O)C5(CCN2CC1CC1)c34 |THB:27:26:21:31.2.3| Show InChI InChI=1S/C26H26N2O3/c29-19-8-7-15-11-20-26(30)12-17-16-3-1-2-4-18(16)27-22(17)24-25(26,21(15)23(19)31-24)9-10-28(20)13-14-5-6-14/h1-4,7-8,14,20,24,27,29-30H,5-6,9-13H2/t20-,24?,25?,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM83436
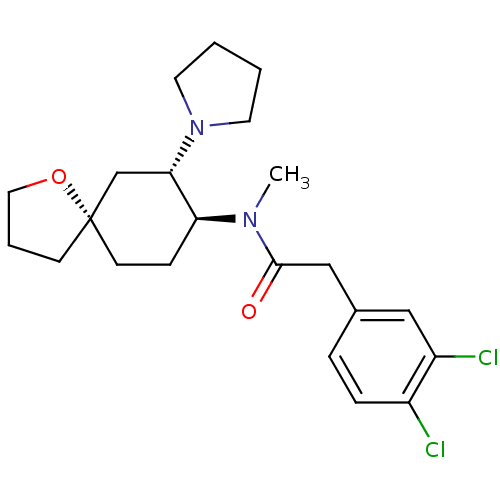
(2-(3,4-dichlorophenyl)-N-methyl-N-[(5R,7S,8S)-7-(1...)Show SMILES CN([C@H]1CC[C@@]2(CCCO2)C[C@@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C22H30Cl2N2O2/c1-25(21(27)14-16-5-6-17(23)18(24)13-16)19-7-9-22(8-4-12-28-22)15-20(19)26-10-2-3-11-26/h5-6,13,19-20H,2-4,7-12,14-15H2,1H3/t19-,20-,22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442922
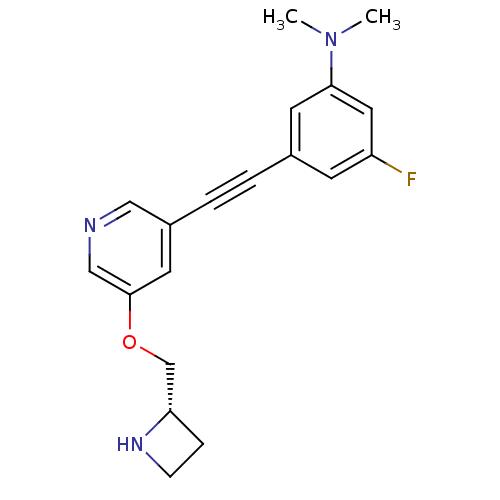
(CHEMBL3086984)Show SMILES CN(C)c1cc(F)cc(c1)C#Cc1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C19H20FN3O/c1-23(2)18-8-14(7-16(20)10-18)3-4-15-9-19(12-21-11-15)24-13-17-5-6-22-17/h7-12,17,22H,5-6,13H2,1-2H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442927

(CHEMBL3086994)Show InChI InChI=1S/C17H15FN2O/c18-15-3-1-2-13(8-15)4-5-14-9-17(11-19-10-14)21-12-16-6-7-20-16/h1-3,8-11,16,20H,6-7,12H2/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50315183
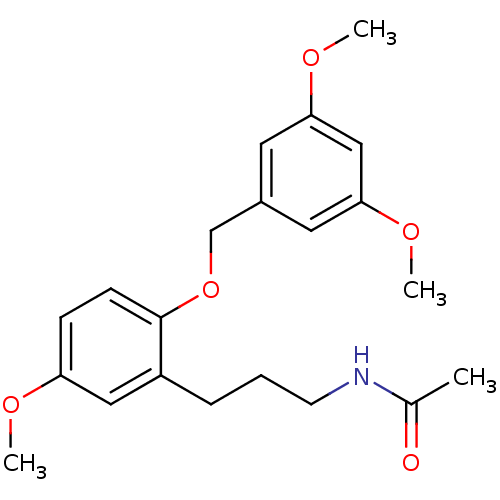
(CHEMBL1091998 | N-(3-(2-(3,5-dimethoxybenzyloxy)-5...)Show InChI InChI=1S/C21H27NO5/c1-15(23)22-9-5-6-17-12-18(24-2)7-8-21(17)27-14-16-10-19(25-3)13-20(11-16)26-4/h7-8,10-13H,5-6,9,14H2,1-4H3,(H,22,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0326 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human melatonin MT2 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2582-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.084
BindingDB Entry DOI: 10.7270/Q2S75GG4 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442921

(CHEMBL3086985)Show SMILES Cc1cc(F)cc(c1)C#Cc1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C18H17FN2O/c1-13-6-14(8-16(19)7-13)2-3-15-9-18(11-20-10-15)22-12-17-4-5-21-17/h6-11,17,21H,4-5,12H2,1H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442924

(CHEMBL3086982)Show InChI InChI=1S/C18H18N2O/c1-14-3-2-4-15(9-14)5-6-16-10-18(12-19-11-16)21-13-17-7-8-20-17/h2-4,9-12,17,20H,7-8,13H2,1H3/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50315176
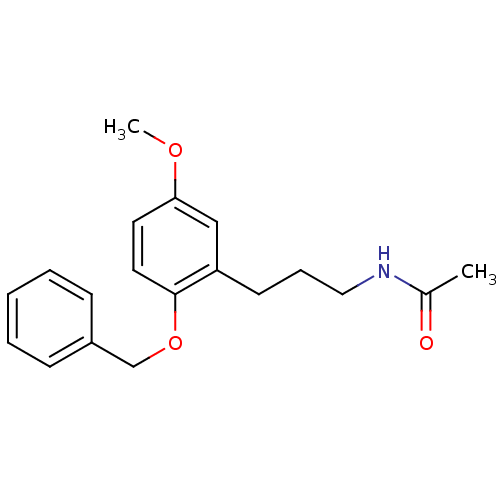
(CHEMBL1091160 | N-(3-(2-(benzyloxy)-5-methoxypheny...)Show InChI InChI=1S/C19H23NO3/c1-15(21)20-12-6-9-17-13-18(22-2)10-11-19(17)23-14-16-7-4-3-5-8-16/h3-5,7-8,10-11,13H,6,9,12,14H2,1-2H3,(H,20,21) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0471 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human melatonin MT2 receptor expressed in CHO cells after 60 mins by scintillation counting |
Bioorg Med Chem Lett 20: 2582-5 (2010)
Article DOI: 10.1016/j.bmcl.2010.02.084
BindingDB Entry DOI: 10.7270/Q2S75GG4 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor delta
(Homo sapiens (Human)) | BDBM28661
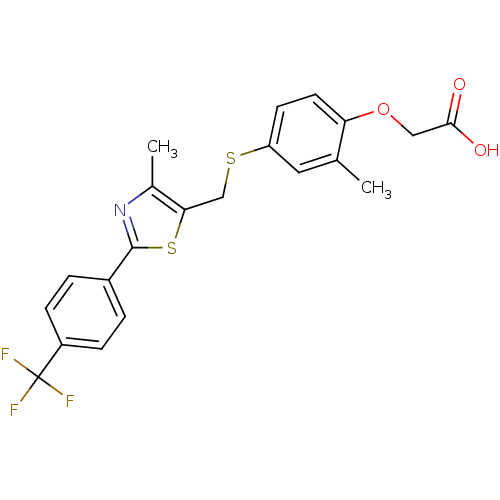
(2-{2-methyl-4-[({4-methyl-2-[4-(trifluoromethyl)ph...)Show SMILES Cc1nc(sc1CSc1ccc(OCC(O)=O)c(C)c1)-c1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C21H18F3NO3S2/c1-12-9-16(7-8-17(12)28-10-19(26)27)29-11-18-13(2)25-20(30-18)14-3-5-15(6-4-14)21(22,23)24/h3-9H,10-11H2,1-2H3,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University
Curated by ChEMBL
| Assay Description
Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARdelta LBD by TR-FRET assay |
J Med Chem 60: 7459-7475 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00805
BindingDB Entry DOI: 10.7270/Q2XK8HQH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442930
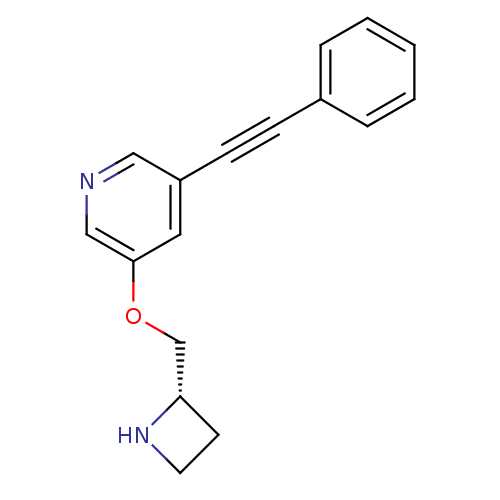
(CHEMBL3086991)Show InChI InChI=1S/C17H16N2O/c1-2-4-14(5-3-1)6-7-15-10-17(12-18-11-15)20-13-16-8-9-19-16/h1-5,10-12,16,19H,8-9,13H2/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50085048
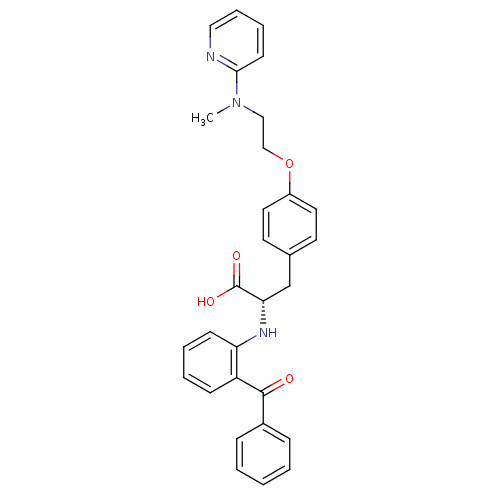
((S)-2-(2-Benzoyl-phenylamino)-3-{4-[2-(methyl-pyri...)Show SMILES CN(CCOc1ccc(C[C@H](Nc2ccccc2C(=O)c2ccccc2)C(O)=O)cc1)c1ccccn1 Show InChI InChI=1S/C30H29N3O4/c1-33(28-13-7-8-18-31-28)19-20-37-24-16-14-22(15-17-24)21-27(30(35)36)32-26-12-6-5-11-25(26)29(34)23-9-3-2-4-10-23/h2-18,27,32H,19-21H2,1H3,(H,35,36)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0497 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University
Curated by ChEMBL
| Assay Description
Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARgamma LBD by TR-FRET assay |
J Med Chem 60: 7459-7475 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00805
BindingDB Entry DOI: 10.7270/Q2XK8HQH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM82555

(CAS_4424 | NSC_4424 | Naloxonazine)Show SMILES Oc1ccc2CC3N(CC=C)CCC45C(Oc1c24)C(CCC35O)=NN=C1CCC2(O)C3Cc4ccc(O)c5OC1C2(CCN3CC=C)c45 |w:24.28,18.21,TLB:27:28:43.42.41:31.32.47,22:21:5.4.17:12.11.7,20:21:5.4.17:12.11.7,THB:29:28:43.42.41:31.32.47| Show InChI InChI=1S/C38H42N4O6/c1-3-15-41-17-13-35-29-21-5-7-25(43)31(29)47-33(35)23(9-11-37(35,45)27(41)19-21)39-40-24-10-12-38(46)28-20-22-6-8-26(44)32-30(22)36(38,34(24)48-32)14-18-42(28)16-4-2/h3-8,27-28,33-34,43-46H,1-2,9-20H2 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442926
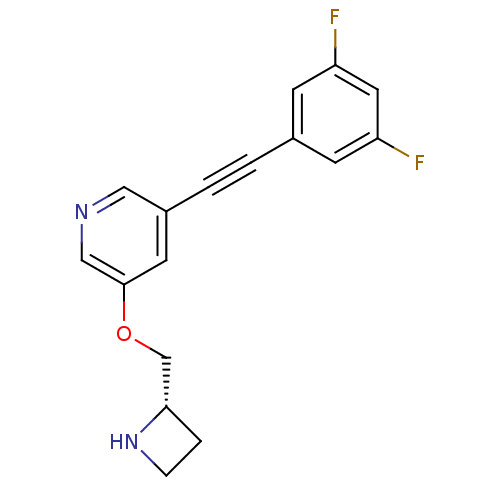
(CHEMBL3086995)Show SMILES Fc1cc(F)cc(c1)C#Cc1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C17H14F2N2O/c18-14-5-12(6-15(19)8-14)1-2-13-7-17(10-20-9-13)22-11-16-3-4-21-16/h5-10,16,21H,3-4,11H2/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor alpha
(Homo sapiens (Human)) | BDBM50099491
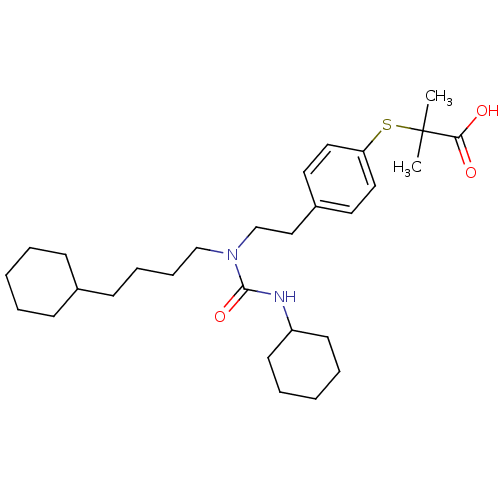
(2-(4-(2-(3-cyclohexyl-1-(4-cyclohexylbutyl)ureido)...)Show SMILES CC(C)(Sc1ccc(CCN(CCCCC2CCCCC2)C(=O)NC2CCCCC2)cc1)C(O)=O Show InChI InChI=1S/C29H46N2O3S/c1-29(2,27(32)33)35-26-18-16-24(17-19-26)20-22-31(28(34)30-25-14-7-4-8-15-25)21-10-9-13-23-11-5-3-6-12-23/h16-19,23,25H,3-15,20-22H2,1-2H3,(H,30,34)(H,32,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0541 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sahmyook University
Curated by ChEMBL
| Assay Description
Displacement of Fluormone-Pan-PPAR Green from human GST-tagged PPARalpha LBD by TR-FRET assay |
J Med Chem 60: 7459-7475 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00805
BindingDB Entry DOI: 10.7270/Q2XK8HQH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50382474

(CHEMBL2024096 | US9303017, Sazetidine-A)Show InChI InChI=1S/C15H20N2O2/c18-8-4-2-1-3-5-13-9-15(11-16-10-13)19-12-14-6-7-17-14/h9-11,14,17-18H,1-2,4,6-8,12H2/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50001714

(2-[3-cyclopropylmethyl-11-hydroxy-15-methoxy-(1S,2...)Show SMILES CO[C@]12CC[C@@]3(C[C@@H]1C(C)(C)O)[C@H]1Cc4ccc(O)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |THB:8:7:22.21:4.3| Show InChI InChI=1S/C26H35NO4/c1-23(2,29)18-13-24-8-9-26(18,30-3)22-25(24)10-11-27(14-15-4-5-15)19(24)12-16-6-7-17(28)21(31-22)20(16)25/h6-7,15,18-19,22,28-29H,4-5,8-14H2,1-3H3/t18-,19-,22-,24-,25+,26-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442928
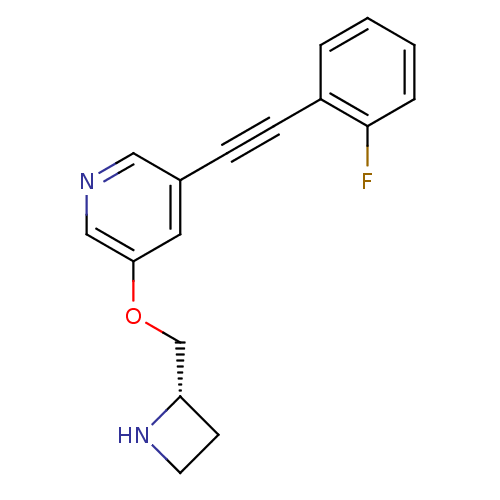
(CHEMBL3086993)Show InChI InChI=1S/C17H15FN2O/c18-17-4-2-1-3-14(17)6-5-13-9-16(11-19-10-13)21-12-15-7-8-20-15/h1-4,9-11,15,20H,7-8,12H2/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0720 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Melatonin receptor type 1B
(Homo sapiens (Human)) | BDBM50426656
(CHEMBL2326200)Show InChI InChI=1S/C22H25NO3/c1-4-22(24)23-14-6-8-19-16-21(26-3)13-12-18(19)11-10-17-7-5-9-20(15-17)25-2/h5,7,9,12-13,15-16H,4,6,8,14H2,1-3H3,(H,23,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0730 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hong Kong University of Science and Technology
Curated by ChEMBL
| Assay Description
Displacement of [3H]melatonin from human MT2 receptor expressed in CHO cells after 60 mins by microbeta scintillation method |
Bioorg Med Chem 21: 547-52 (2012)
Article DOI: 10.1016/j.bmc.2012.10.060
BindingDB Entry DOI: 10.7270/Q29W0GTX |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442920

(CHEMBL3086986)Show SMILES COc1cc(F)cc(c1)C#Cc1cncc(OC[C@@H]2CCN2)c1 |r| Show InChI InChI=1S/C18H17FN2O2/c1-22-17-7-13(6-15(19)9-17)2-3-14-8-18(11-20-10-14)23-12-16-4-5-21-16/h6-11,16,21H,4-5,12H2,1H3/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM82427
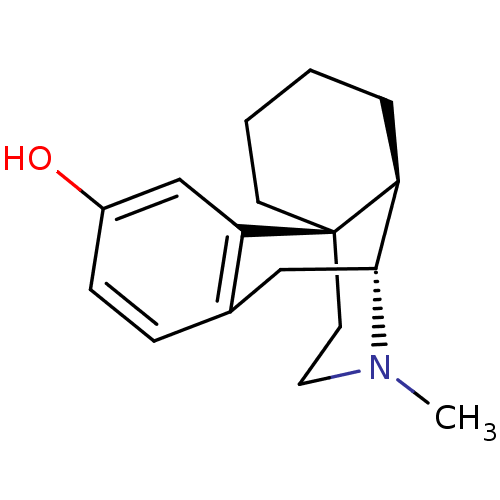
(CAS_5985-38-6 | LEVORPHANOL-tartarate)Show SMILES CN1CC[C@]23CCCC[C@H]2[C@H]1Cc1ccc(O)cc31 |r| Show InChI InChI=1S/C17H23NO/c1-18-9-8-17-7-3-2-4-14(17)16(18)10-12-5-6-13(19)11-15(12)17/h5-6,11,14,16,19H,2-4,7-10H2,1H3/t14-,16+,17+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
PC cid
PC sid
UniChem
Similars
| DrugBank
Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM82554

(Beta C N A | CAS_115070 | NSC_115070)Show SMILES Oc1ccc2CC3N(CC4CC4)CCC45C(Oc1c24)C(CCC35O)N(CCCl)CCCl |TLB:21:22:7.12.13:5.4.18,THB:23:22:7.12.13:5.4.18| Show InChI InChI=1S/C24H32Cl2N2O3/c25-8-11-27(12-9-26)17-5-6-24(30)19-13-16-3-4-18(29)21-20(16)23(24,22(17)31-21)7-10-28(19)14-15-1-2-15/h3-4,15,17,19,22,29-30H,1-2,5-14H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50013388
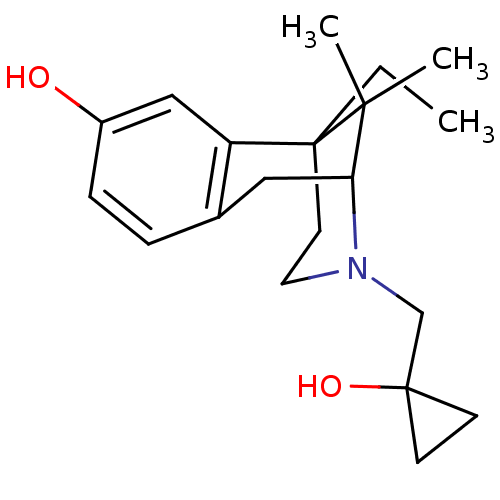
(6-Ethyl-3-(1-hydroxy-cyclopropylmethyl)-11,11-dime...)Show SMILES CCC12CCN(CC3(O)CC3)C(Cc3ccc(O)cc13)C2(C)C |TLB:6:5:20:19.13.12| Show InChI InChI=1S/C20H29NO2/c1-4-20-9-10-21(13-19(23)7-8-19)17(18(20,2)3)11-14-5-6-15(22)12-16(14)20/h5-6,12,17,22-23H,4,7-11,13H2,1-3H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50263483
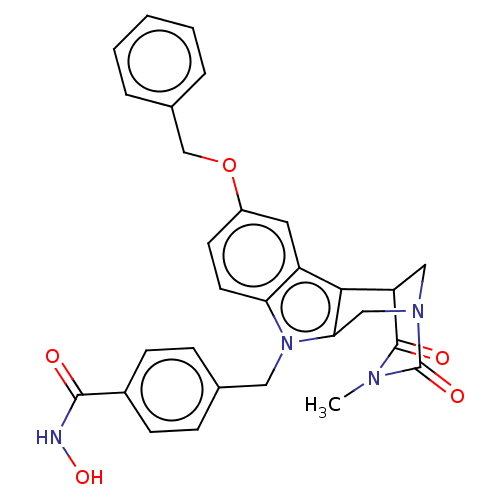
(CHEMBL4082520)Show SMILES CN1C(=O)C2CN(Cc3c2c2cc(OCc4ccccc4)ccc2n3Cc2ccc(cc2)C(=O)NO)C1=O Show InChI InChI=1S/C29H26N4O5/c1-31-28(35)23-15-32(29(31)36)16-25-26(23)22-13-21(38-17-19-5-3-2-4-6-19)11-12-24(22)33(25)14-18-7-9-20(10-8-18)27(34)30-37/h2-13,23,37H,14-17H2,1H3,(H,30,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmacy, Faculty of Chemistry and Pharmacy , University of Regensburg , 93040 Regensburg , Germany.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal GST-tagged HDAC6 expressed in baculovirus-infected Sf9 insect cells using RHKK(Ac)AMC as subst... |
J Med Chem 61: 3454-3477 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01593
BindingDB Entry DOI: 10.7270/Q24B33S0 |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(RAT) | BDBM82462
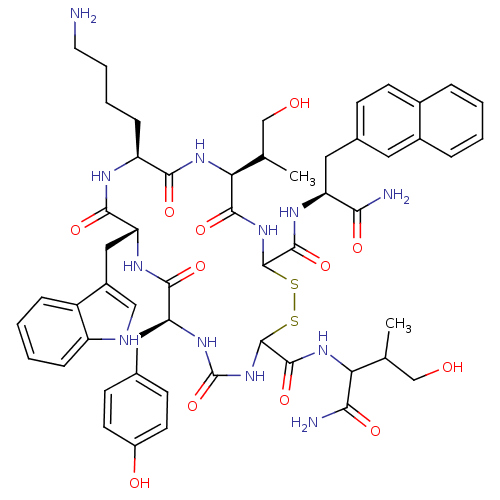
(BIM 23059 | D-Nal-c[Cys-Tyr-D-Trp-Lys-Thr-Cys]-Thr...)Show SMILES CC(CO)C(NC(=O)C1NC(=O)N[C@@H](Cc2ccc(O)cc2)C(=O)N[C@H](Cc2c[nH]c3ccccc23)C(=O)N[C@@H](CCCCN)C(=O)N[C@@H](C(C)CO)C(=O)NC(SS1)C(=O)N[C@@H](Cc1ccc2ccccc2c1)C(N)=O)C(N)=O |r| Show InChI InChI=1S/C54H68N12O12S2/c1-28(26-67)42(45(57)71)63-51(77)53-66-54(78)62-40(22-30-15-18-35(69)19-16-30)47(73)61-41(24-34-25-58-37-12-6-5-11-36(34)37)48(74)59-38(13-7-8-20-55)46(72)64-43(29(2)27-68)49(75)65-52(79-80-53)50(76)60-39(44(56)70)23-31-14-17-32-9-3-4-10-33(32)21-31/h3-6,9-12,14-19,21,25,28-29,38-43,52-53,58,67-69H,7-8,13,20,22-24,26-27,55H2,1-2H3,(H2,56,70)(H2,57,71)(H,59,74)(H,60,76)(H,61,73)(H,63,77)(H,64,72)(H,65,75)(H2,62,66,78)/t28?,29?,38-,39-,40-,41+,42?,43-,52?,53?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 44: 385-92 (1993)
BindingDB Entry DOI: 10.7270/Q2X065KZ |
More data for this
Ligand-Target Pair | |
Nicotinic acetylcholine receptor
(RAT) | BDBM50049757

(()-2-(6-Chloro-pyridin-3-yl)-7-aza-bicyclo[2.2.1]h...)Show InChI InChI=1S/C11H13ClN2/c12-11-4-1-7(6-13-11)9-5-8-2-3-10(9)14-8/h1,4,6,8-10,14H,2-3,5H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Kentucky
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 302: 687-95 (2002)
Article DOI: 10.1124/jpet.302.2.687
BindingDB Entry DOI: 10.7270/Q21R6P3D |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442929
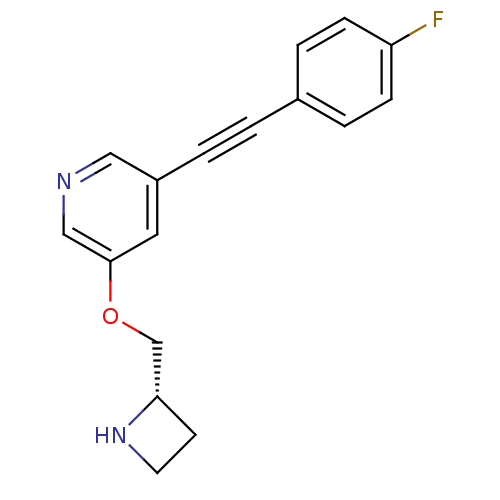
(CHEMBL3086992)Show InChI InChI=1S/C17H15FN2O/c18-15-5-3-13(4-6-15)1-2-14-9-17(11-19-10-14)21-12-16-7-8-20-16/h3-6,9-11,16,20H,7-8,12H2/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(RAT) | BDBM84630
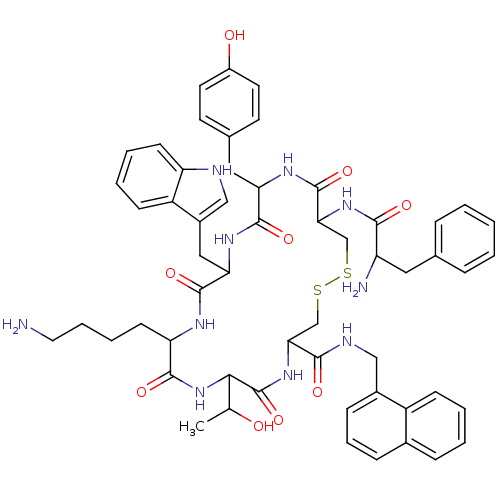
(BIM 23060)Show SMILES CC(O)C1NC(=O)C(CCCCN)NC(=O)C(Cc2c[nH]c3ccccc23)NC(=O)C(Cc2ccc(O)cc2)NC(=O)C(CSSCC(NC1=O)C(=O)NCc1cccc2ccccc12)NC(=O)C(N)Cc1ccccc1 Show InChI InChI=1S/C56H66N10O9S2/c1-33(67)49-56(75)65-47(51(70)60-29-37-16-11-15-36-14-5-6-17-40(36)37)31-76-77-32-48(64-50(69)42(58)26-34-12-3-2-4-13-34)55(74)62-45(27-35-21-23-39(68)24-22-35)53(72)63-46(28-38-30-59-43-19-8-7-18-41(38)43)54(73)61-44(52(71)66-49)20-9-10-25-57/h2-8,11-19,21-24,30,33,42,44-49,59,67-68H,9-10,20,25-29,31-32,57-58H2,1H3,(H,60,70)(H,61,73)(H,62,74)(H,63,72)(H,64,69)(H,65,75)(H,66,71) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 44: 385-92 (1993)
BindingDB Entry DOI: 10.7270/Q2X065KZ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442923

(CHEMBL3086983)Show InChI InChI=1S/C17H15ClN2O/c18-15-3-1-2-13(8-15)4-5-14-9-17(11-19-10-14)21-12-16-6-7-20-16/h1-3,8-11,16,20H,6-7,12H2/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0930 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Somatostatin receptor type 4
(RAT) | BDBM82470
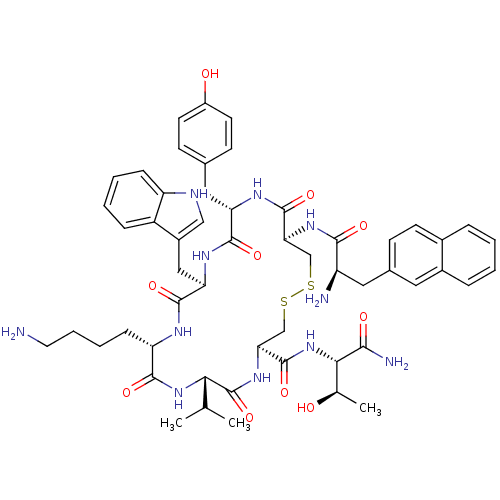
(3-(2-Naphtyl)-D-Ala-L-Cys(1)-L-Tyr-D-Trp-L-Lys-L-V...)Show SMILES CC(C)[C@@H]1NC(=O)[C@H](CCCCN)NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](Cc2ccc(O)cc2)NC(=O)[C@H](CSSC[C@H](NC1=O)C(=O)N[C@@H]([C@@H](C)O)C(N)=O)NC(=O)[C@H](N)Cc1ccc2ccccc2c1 Show InChI InChI=1S/C54H69N11O10S2/c1-29(2)45-54(75)63-44(53(74)65-46(30(3)66)47(57)68)28-77-76-27-43(62-48(69)38(56)23-32-15-18-33-10-4-5-11-34(33)22-32)52(73)60-41(24-31-16-19-36(67)20-17-31)50(71)61-42(25-35-26-58-39-13-7-6-12-37(35)39)51(72)59-40(49(70)64-45)14-8-9-21-55/h4-7,10-13,15-20,22,26,29-30,38,40-46,58,66-67H,8-9,14,21,23-25,27-28,55-56H2,1-3H3,(H2,57,68)(H,59,72)(H,60,73)(H,61,71)(H,62,69)(H,63,75)(H,64,70)(H,65,74)/t30-,38-,40+,41+,42-,43+,44+,45+,46+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 44: 385-92 (1993)
BindingDB Entry DOI: 10.7270/Q2X065KZ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50046377
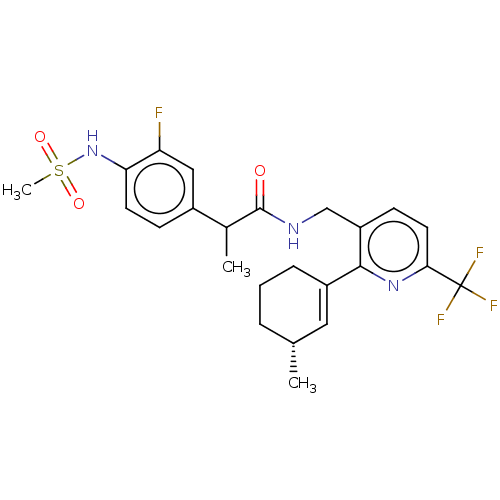
(CHEMBL3314406)Show SMILES CC(C(=O)NCc1ccc(nc1C1=C[C@H](C)CCC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r,t:13| Show InChI InChI=1S/C24H27F4N3O3S/c1-14-5-4-6-17(11-14)22-18(8-10-21(30-22)24(26,27)28)13-29-23(32)15(2)16-7-9-20(19(25)12-16)31-35(3,33)34/h7-12,14-15,31H,4-6,13H2,1-3H3,(H,29,32)/t14-,15?/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced increase in intracellular Ca2+ level by FLIPR a... |
Bioorg Med Chem Lett 24: 4039-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.074
BindingDB Entry DOI: 10.7270/Q2B859RZ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50046396
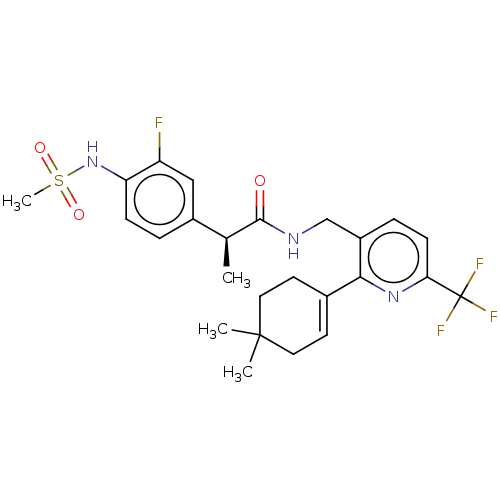
(CHEMBL3314411)Show SMILES C[C@H](C(=O)NCc1ccc(nc1C1=CCC(C)(C)CC1)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r,t:13| Show InChI InChI=1S/C25H29F4N3O3S/c1-15(17-5-7-20(19(26)13-17)32-36(4,34)35)23(33)30-14-18-6-8-21(25(27,28)29)31-22(18)16-9-11-24(2,3)12-10-16/h5-9,13,15,32H,10-12,14H2,1-4H3,(H,30,33)/t15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced increase in intracellular Ca2+ level by FLIPR a... |
Bioorg Med Chem Lett 24: 4039-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.074
BindingDB Entry DOI: 10.7270/Q2B859RZ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50046394
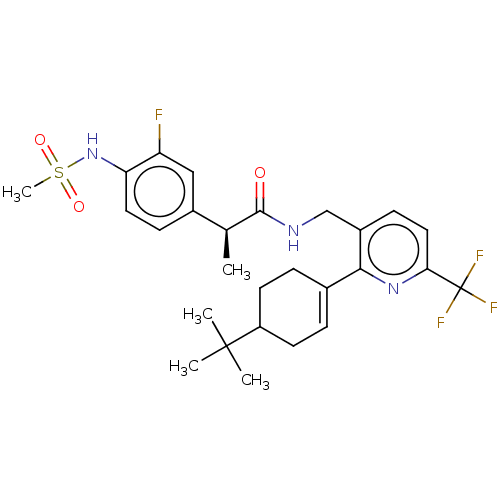
(CHEMBL3314409)Show SMILES C[C@H](C(=O)NCc1ccc(nc1C1=CCC(CC1)C(C)(C)C)C(F)(F)F)c1ccc(NS(C)(=O)=O)c(F)c1 |r,t:13| Show InChI InChI=1S/C27H33F4N3O3S/c1-16(18-8-12-22(21(28)14-18)34-38(5,36)37)25(35)32-15-19-9-13-23(27(29,30)31)33-24(19)17-6-10-20(11-7-17)26(2,3)4/h6,8-9,12-14,16,20,34H,7,10-11,15H2,1-5H3,(H,32,35)/t16-,20?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced increase in intracellular Ca2+ level by FLIPR a... |
Bioorg Med Chem Lett 24: 4039-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.074
BindingDB Entry DOI: 10.7270/Q2B859RZ |
More data for this
Ligand-Target Pair | |
Transient receptor potential cation channel subfamily V member 1
(Homo sapiens (Human)) | BDBM50046379
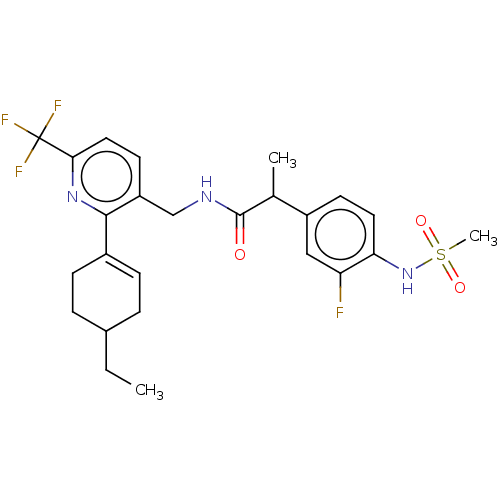
(CHEMBL3314407)Show SMILES CCC1CCC(=CC1)c1nc(ccc1CNC(=O)C(C)c1ccc(NS(C)(=O)=O)c(F)c1)C(F)(F)F |c:5| Show InChI InChI=1S/C25H29F4N3O3S/c1-4-16-5-7-17(8-6-16)23-19(10-12-22(31-23)25(27,28)29)14-30-24(33)15(2)18-9-11-21(20(26)13-18)32-36(3,34)35/h7,9-13,15-16,32H,4-6,8,14H2,1-3H3,(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Seoul National University
Curated by ChEMBL
| Assay Description
Antagonist activity at human TRPV1 expressed in CHO cells assessed as inhibition of capsaicin-induced increase in intracellular Ca2+ level by FLIPR a... |
Bioorg Med Chem Lett 24: 4039-43 (2014)
Article DOI: 10.1016/j.bmcl.2014.05.074
BindingDB Entry DOI: 10.7270/Q2B859RZ |
More data for this
Ligand-Target Pair | |
Neuronal acetylcholine receptor subunit alpha-4/beta-2
(Homo sapiens (Human)) | BDBM50442932
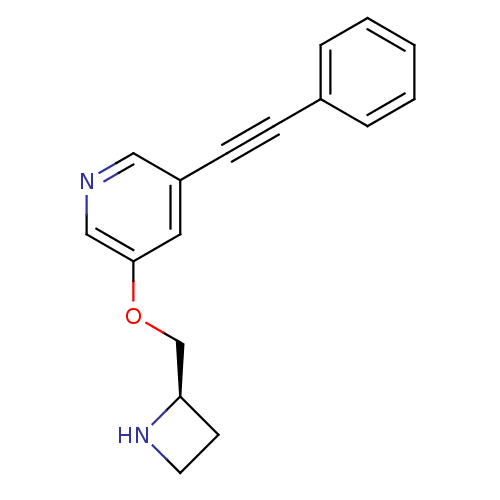
(CHEMBL3086989)Show InChI InChI=1S/C17H16N2O/c1-2-4-14(5-3-1)6-7-15-10-17(12-18-11-15)20-13-16-8-9-19-16/h1-5,10-12,16,19H,8-9,13H2/t16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Georgetown University
Curated by ChEMBL
| Assay Description
Displacement of [3H]epibatidine from human alpha4beta2 nAChR after 4 hrs by cell-based assay |
J Med Chem 56: 8404-21 (2013)
Article DOI: 10.1021/jm4008455
BindingDB Entry DOI: 10.7270/Q2JW8GBV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50169774
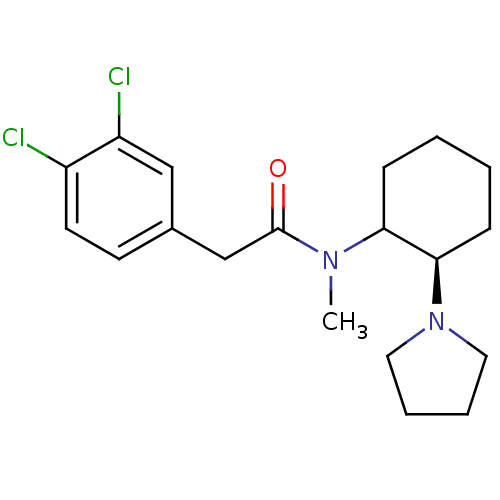
(2-(3,4-Dichloro-phenyl)-N-methyl-N-(2-pyrrolidin-1...)Show SMILES CN(C1CCCC[C@H]1N1CCCC1)C(=O)Cc1ccc(Cl)c(Cl)c1 Show InChI InChI=1S/C19H26Cl2N2O/c1-22(19(24)13-14-8-9-15(20)16(21)12-14)17-6-2-3-7-18(17)23-10-4-5-11-23/h8-9,12,17-18H,2-7,10-11,13H2,1H3/t17?,18-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pennsylvania
Curated by PDSP Ki Database
| |
Mol Pharmacol 45: 330-4 (1994)
Article DOI: 10.1016/j.bioorg.2015.02.008
BindingDB Entry DOI: 10.7270/Q21Z42X1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data