 Found 196 hits with Last Name = 'osterkamp' and Initial = 'f'
Found 196 hits with Last Name = 'osterkamp' and Initial = 'f' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50217428

(2S,3R,5S-4-{[2-carboxy-2-(2,4,6-trimethyl-benzoyla...)Show SMILES COc1ccnc(NC[C@@H]2C[C@H](CN2C(=O)OCc2ccccc2)OCC(=O)NC[C@H](NC(=O)c2c(C)cc(C)cc2C)C(O)=O)c1 Show InChI InChI=1S/C34H41N5O8/c1-21-12-22(2)31(23(3)13-21)32(41)38-28(33(42)43)17-37-30(40)20-46-27-14-25(16-36-29-15-26(45-4)10-11-35-29)39(18-27)34(44)47-19-24-8-6-5-7-9-24/h5-13,15,25,27-28H,14,16-20H2,1-4H3,(H,35,36)(H,37,40)(H,38,41)(H,42,43)/t25-,27+,28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50217419

(2S,3R,5S-3-(2-{1-(3,3-dimethyl-butyryl)-5-[(4-meth...)Show SMILES COc1ccnc(NC[C@@H]2C[C@H](CN2C(=O)CC(C)(C)C)OCC(=O)NC[C@H](NC(=O)c2c(C)cc(C)cc2C)C(O)=O)c1 Show InChI InChI=1S/C32H45N5O7/c1-19-10-20(2)29(21(3)11-19)30(40)36-25(31(41)42)16-35-27(38)18-44-24-12-22(37(17-24)28(39)14-32(4,5)6)15-34-26-13-23(43-7)8-9-33-26/h8-11,13,22,24-25H,12,14-18H2,1-7H3,(H,33,34)(H,35,38)(H,36,40)(H,41,42)/t22-,24+,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50217426
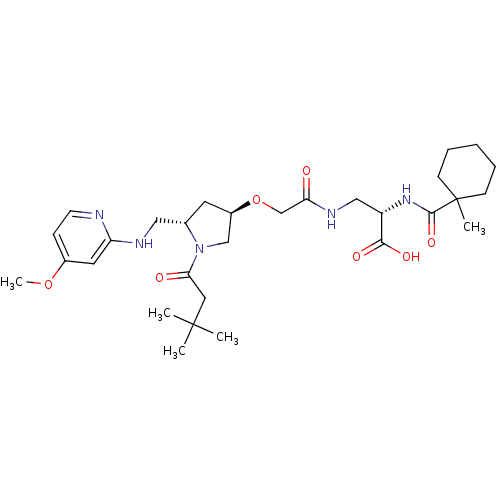
(2S,3R,5S-3-(2-{1-(3,3-dimethyl-butyryl)-5-[(4-meth...)Show SMILES COc1ccnc(NC[C@@H]2C[C@H](CN2C(=O)CC(C)(C)C)OCC(=O)NC[C@H](NC(=O)C2(C)CCCCC2)C(O)=O)c1 Show InChI InChI=1S/C30H47N5O7/c1-29(2,3)15-26(37)35-18-22(13-20(35)16-32-24-14-21(41-5)9-12-31-24)42-19-25(36)33-17-23(27(38)39)34-28(40)30(4)10-7-6-8-11-30/h9,12,14,20,22-23H,6-8,10-11,13,15-19H2,1-5H3,(H,31,32)(H,33,36)(H,34,40)(H,38,39)/t20-,22+,23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50217425
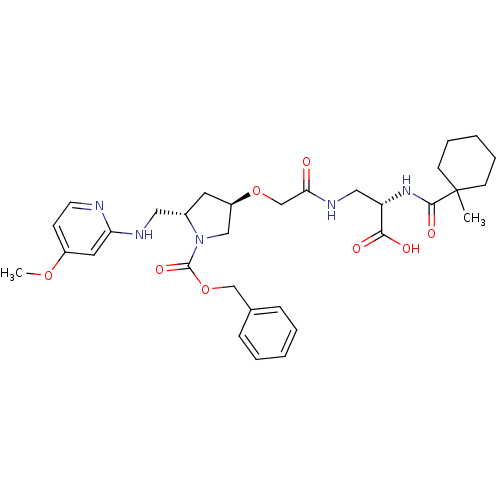
(2S,3R,5S-4-({2-carboxy-2-[(1-methyl-cyclohexanecar...)Show SMILES COc1ccnc(NC[C@@H]2C[C@H](CN2C(=O)OCc2ccccc2)OCC(=O)NC[C@H](NC(=O)C2(C)CCCCC2)C(O)=O)c1 Show InChI InChI=1S/C32H43N5O8/c1-32(12-7-4-8-13-32)30(41)36-26(29(39)40)18-35-28(38)21-44-25-15-23(17-34-27-16-24(43-2)11-14-33-27)37(19-25)31(42)45-20-22-9-5-3-6-10-22/h3,5-6,9-11,14,16,23,25-26H,4,7-8,12-13,15,17-21H2,1-2H3,(H,33,34)(H,35,38)(H,36,41)(H,39,40)/t23-,25+,26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466499
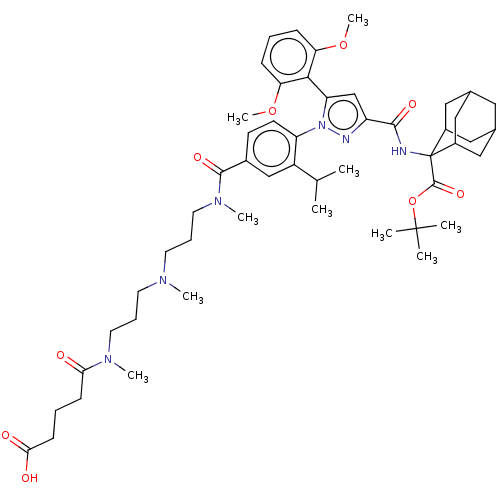
(US10799605, Example 4)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)CCCN(C)C(=O)CCCC(O)=O)C(=O)NC1(C2CC3CC(C2)CC1C3)C(=O)OC(C)(C)C |TLB:48:49:51:54.55.53,THB:49:50:57.58.56:53,49:57:51.50.55:53,56:57:51:54.55.53,56:54:51:49.57.58,59:49:51:54.55.53,(-8.99,4.14,;-7.9,3.05,;-8.3,1.56,;-9.79,1.16,;-10.19,-.33,;-9.1,-1.42,;-7.61,-1.02,;-6.52,-2.11,;-6.92,-3.59,;-7.21,.47,;-5.73,.87,;-5.25,2.33,;-3.71,2.33,;-3.24,.87,;-4.48,-.04,;-4.48,-1.58,;-5.81,-2.35,;-5.81,-3.89,;-4.48,-4.66,;-3.15,-3.89,;-3.15,-2.35,;-1.81,-1.58,;-.48,-2.35,;-1.81,-.04,;-4.48,-6.2,;-5.81,-6.97,;-3.15,-6.97,;-3.15,-8.51,;-1.81,-6.2,;-.48,-6.97,;.85,-6.2,;2.19,-6.97,;2.19,-8.51,;3.52,-6.2,;4.85,-6.97,;6.19,-6.2,;7.52,-6.97,;7.52,-8.51,;8.86,-6.2,;10.19,-6.97,;8.86,-4.66,;7.52,-3.89,;7.52,-2.35,;6.19,-1.58,;6.19,-.04,;4.85,-2.35,;-2.62,3.42,;-3.02,4.91,;-1.13,3.02,;-.05,4.11,;-.69,5.51,;-1.96,6.39,;-1.45,7.84,;-.06,8.51,;1.27,7.86,;.63,6.46,;1.78,6.41,;.46,5.57,;-.8,6.44,;1.29,3.34,;2.62,4.11,;1.29,1.8,;2.62,1.03,;2.62,-.51,;3.96,1.8,;3.96,.26,)| Show InChI InChI=1S/C51H72N6O9/c1-32(2)38-30-35(48(62)56(8)24-14-22-54(6)21-13-23-55(7)44(58)17-12-18-45(59)60)19-20-40(38)57-41(46-42(64-9)15-11-16-43(46)65-10)31-39(53-57)47(61)52-51(49(63)66-50(3,4)5)36-26-33-25-34(28-36)29-37(51)27-33/h11,15-16,19-20,30-34,36-37H,12-14,17-18,21-29H2,1-10H3,(H,52,61)(H,59,60) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
In order to determine the binding affinity of compounds comprising a radiolabel for NTR1, a radioligand binding assay was carried out. A radioligand ... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50423732
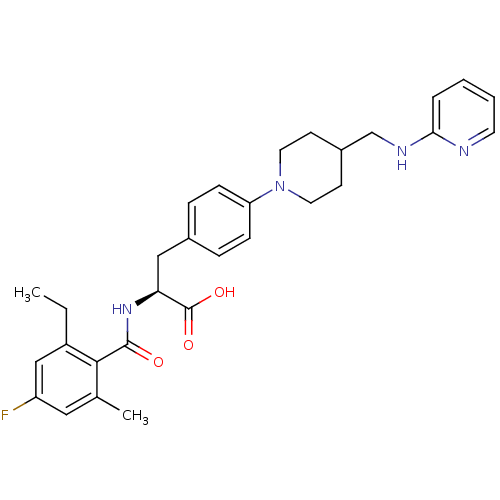
(CHEMBL590389)Show SMILES CCc1cc(F)cc(C)c1C(=O)N[C@@H](Cc1ccc(cc1)N1CCC(CNc2ccccn2)CC1)C(O)=O |r| Show InChI InChI=1S/C30H35FN4O3/c1-3-23-18-24(31)16-20(2)28(23)29(36)34-26(30(37)38)17-21-7-9-25(10-8-21)35-14-11-22(12-15-35)19-33-27-6-4-5-13-32-27/h4-10,13,16,18,22,26H,3,11-12,14-15,17,19H2,1-2H3,(H,32,33)(H,34,36)(H,37,38)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
Bioorg Med Chem Lett 20: 65-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.030
BindingDB Entry DOI: 10.7270/Q2BR8TGJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50217422
(2S,3R,20S-3-(2-{1-(3,3-dimethyl-butyryl)-5-[(4-met...)Show SMILES COc1ccnc(NC[C@@H]2C[C@H](CN2C(=O)CC(C)(C)C)OCC(=O)NC[C@H](NS(=O)(=O)c2c(C)cc(C)cc2C)C(O)=O)c1 Show InChI InChI=1S/C31H45N5O8S/c1-19-10-20(2)29(21(3)11-19)45(41,42)35-25(30(39)40)16-34-27(37)18-44-24-12-22(36(17-24)28(38)14-31(4,5)6)15-33-26-13-23(43-7)8-9-32-26/h8-11,13,22,24-25,35H,12,14-18H2,1-7H3,(H,32,33)(H,34,37)(H,39,40)/t22-,24+,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50423736

(CHEMBL591543)Show SMILES COc1ccnc(NCC2CCN(CC2)c2ccc(CC(NC(=O)c3c(C)cc(C)cc3C)C(O)=O)cc2)c1 Show InChI InChI=1S/C31H38N4O4/c1-20-15-21(2)29(22(3)16-20)30(36)34-27(31(37)38)17-23-5-7-25(8-6-23)35-13-10-24(11-14-35)19-33-28-18-26(39-4)9-12-32-28/h5-9,12,15-16,18,24,27H,10-11,13-14,17,19H2,1-4H3,(H,32,33)(H,34,36)(H,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
Bioorg Med Chem Lett 20: 65-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.030
BindingDB Entry DOI: 10.7270/Q2BR8TGJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50217435

(2S,4R,20S-4-{[2-carboxy-2-(2,4,6-trimethyl-benzene...)Show SMILES COc1ccnc(NC[C@@H]2C[C@H](CN2C(=O)OCc2ccccc2)OCC(=O)NC[C@H](NS(=O)(=O)c2c(C)cc(C)cc2C)C(O)=O)c1 Show InChI InChI=1S/C33H41N5O9S/c1-21-12-22(2)31(23(3)13-21)48(43,44)37-28(32(40)41)17-36-30(39)20-46-27-14-25(16-35-29-15-26(45-4)10-11-34-29)38(18-27)33(42)47-19-24-8-6-5-7-9-24/h5-13,15,25,27-28,37H,14,16-20H2,1-4H3,(H,34,35)(H,36,39)(H,40,41)/t25-,27+,28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50217427

(2S,3R,5S-3-{2-[1-(3,3-dimethyl-butyryl)-5-(pyridin...)Show SMILES CC(C)(C)CC(=O)N1C[C@@H](C[C@H]1CNc1ccccn1)OCC(=O)NC[C@H](NC(=O)C1(C)CCCCC1)C(O)=O Show InChI InChI=1S/C29H45N5O6/c1-28(2,3)15-25(36)34-18-21(14-20(34)16-31-23-10-6-9-13-30-23)40-19-24(35)32-17-22(26(37)38)33-27(39)29(4)11-7-5-8-12-29/h6,9-10,13,20-22H,5,7-8,11-12,14-19H2,1-4H3,(H,30,31)(H,32,35)(H,33,39)(H,37,38)/t20-,21+,22-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50423726
(CHEMBL594161)Show SMILES Cc1ccnc(NCc2cn(nn2)-c2ccc(C[C@H](NC(=O)c3c(C)cc(C)cc3C)C(O)=O)cc2)c1 |r| Show InChI InChI=1S/C28H30N6O3/c1-17-9-10-29-25(13-17)30-15-22-16-34(33-32-22)23-7-5-21(6-8-23)14-24(28(36)37)31-27(35)26-19(3)11-18(2)12-20(26)4/h5-13,16,24H,14-15H2,1-4H3,(H,29,30)(H,31,35)(H,36,37)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to integrin alpha5beta1 receptor by ELISA |
Bioorg Med Chem Lett 20: 380-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.073
BindingDB Entry DOI: 10.7270/Q2M61MKQ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50217432
(2S,3R,5S-3-{2-[1-(3,3-dimethyl-butyryl)-5-(pyridin...)Show SMILES Cc1cc(C)c(C(=O)N[C@@H](CNC(=O)CO[C@@H]2C[C@@H](CNc3ccccn3)N(C2)C(=O)CC(C)(C)C)C(O)=O)c(C)c1 Show InChI InChI=1S/C31H43N5O6/c1-19-11-20(2)28(21(3)12-19)29(39)35-24(30(40)41)16-34-26(37)18-42-23-13-22(15-33-25-9-7-8-10-32-25)36(17-23)27(38)14-31(4,5)6/h7-12,22-24H,13-18H2,1-6H3,(H,32,33)(H,34,37)(H,35,39)(H,40,41)/t22-,23+,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50423717
(CHEMBL594166)Show SMILES Cc1cc(C)c(C(=O)N[C@@H](CNC(=O)CO[C@H]2C[C@H](CNc3ccccn3)N(C2)C(=O)CC(C)(C)C)C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C31H43N5O6/c1-19-11-20(2)28(21(3)12-19)29(39)35-24(30(40)41)16-34-26(37)18-42-23-13-22(15-33-25-9-7-8-10-32-25)36(17-23)27(38)14-31(4,5)6/h7-12,22-24H,13-18H2,1-6H3,(H,32,33)(H,34,37)(H,35,39)(H,40,41)/t22-,23+,24+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to integrin alpha5beta1 receptor by ELISA |
Bioorg Med Chem Lett 20: 380-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.073
BindingDB Entry DOI: 10.7270/Q2M61MKQ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50423737
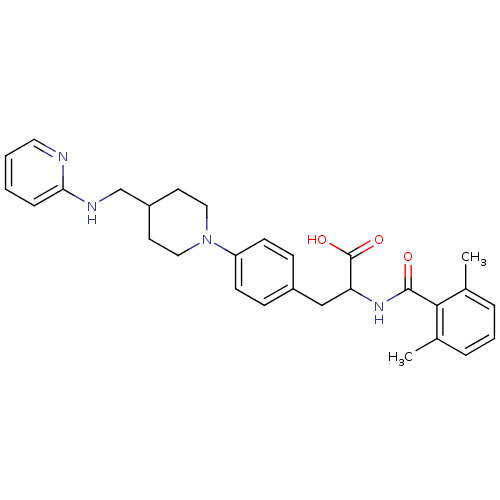
(CHEMBL591544)Show SMILES Cc1cccc(C)c1C(=O)NC(Cc1ccc(cc1)N1CCC(CNc2ccccn2)CC1)C(O)=O Show InChI InChI=1S/C29H34N4O3/c1-20-6-5-7-21(2)27(20)28(34)32-25(29(35)36)18-22-9-11-24(12-10-22)33-16-13-23(14-17-33)19-31-26-8-3-4-15-30-26/h3-12,15,23,25H,13-14,16-19H2,1-2H3,(H,30,31)(H,32,34)(H,35,36) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
Bioorg Med Chem Lett 20: 65-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.030
BindingDB Entry DOI: 10.7270/Q2BR8TGJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50423728
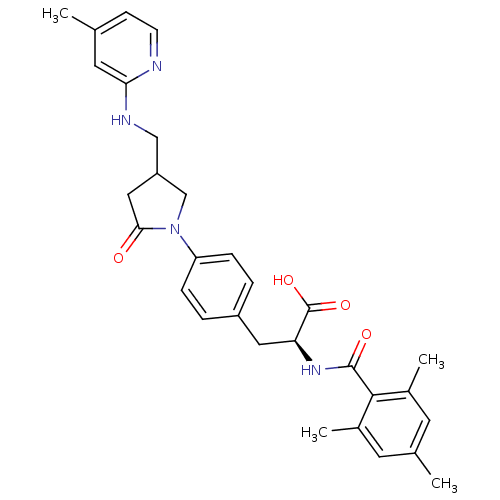
(CHEMBL593938)Show SMILES Cc1ccnc(NCC2CN(C(=O)C2)c2ccc(C[C@H](NC(=O)c3c(C)cc(C)cc3C)C(O)=O)cc2)c1 |r| Show InChI InChI=1S/C30H34N4O4/c1-18-9-10-31-26(13-18)32-16-23-15-27(35)34(17-23)24-7-5-22(6-8-24)14-25(30(37)38)33-29(36)28-20(3)11-19(2)12-21(28)4/h5-13,23,25H,14-17H2,1-4H3,(H,31,32)(H,33,36)(H,37,38)/t23?,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to integrin alpha5beta1 receptor by ELISA |
Bioorg Med Chem Lett 20: 380-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.073
BindingDB Entry DOI: 10.7270/Q2M61MKQ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50217423

(2S,4R,20S-4-({2-carboxy-2-[(1-methyl-cyclohexaneca...)Show SMILES CC1(CCCCC1)C(=O)N[C@@H](CNC(=O)CO[C@@H]1C[C@@H](CNc2ccccn2)N(C1)C(=O)OCc1ccccc1)C(O)=O Show InChI InChI=1S/C31H41N5O7/c1-31(13-7-3-8-14-31)29(40)35-25(28(38)39)18-34-27(37)21-42-24-16-23(17-33-26-12-6-9-15-32-26)36(19-24)30(41)43-20-22-10-4-2-5-11-22/h2,4-6,9-12,15,23-25H,3,7-8,13-14,16-21H2,1H3,(H,32,33)(H,34,37)(H,35,40)(H,38,39)/t23-,24+,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50423731
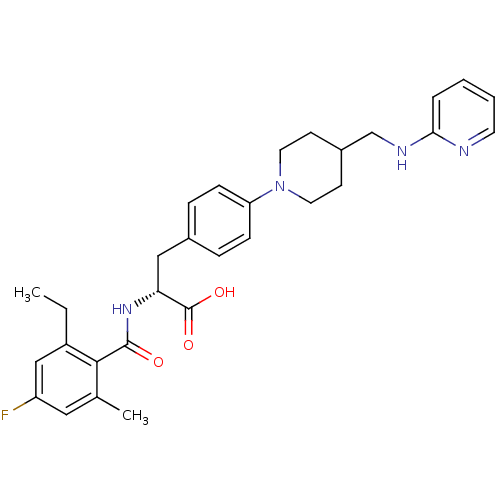
(CHEMBL590622)Show SMILES CCc1cc(F)cc(C)c1C(=O)N[C@H](Cc1ccc(cc1)N1CCC(CNc2ccccn2)CC1)C(O)=O |r| Show InChI InChI=1S/C30H35FN4O3/c1-3-23-18-24(31)16-20(2)28(23)29(36)34-26(30(37)38)17-21-7-9-25(10-8-21)35-14-11-22(12-15-35)19-33-27-6-4-5-13-32-27/h4-10,13,16,18,22,26H,3,11-12,14-15,17,19H2,1-2H3,(H,32,33)(H,34,36)(H,37,38)/t26-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
Bioorg Med Chem Lett 20: 65-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.030
BindingDB Entry DOI: 10.7270/Q2BR8TGJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50423735
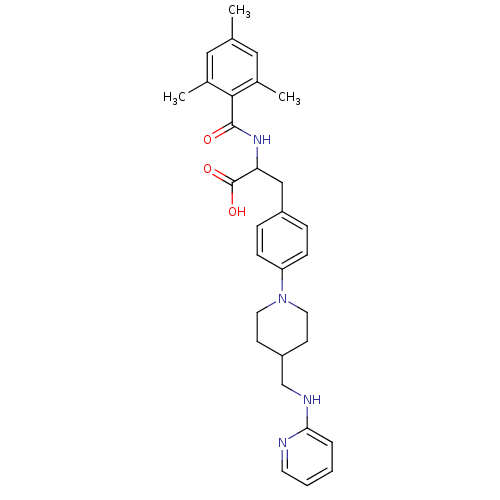
(CHEMBL592769)Show SMILES Cc1cc(C)c(C(=O)NC(Cc2ccc(cc2)N2CCC(CNc3ccccn3)CC2)C(O)=O)c(C)c1 Show InChI InChI=1S/C30H36N4O3/c1-20-16-21(2)28(22(3)17-20)29(35)33-26(30(36)37)18-23-7-9-25(10-8-23)34-14-11-24(12-15-34)19-32-27-6-4-5-13-31-27/h4-10,13,16-17,24,26H,11-12,14-15,18-19H2,1-3H3,(H,31,32)(H,33,35)(H,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
Bioorg Med Chem Lett 20: 65-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.030
BindingDB Entry DOI: 10.7270/Q2BR8TGJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50423738
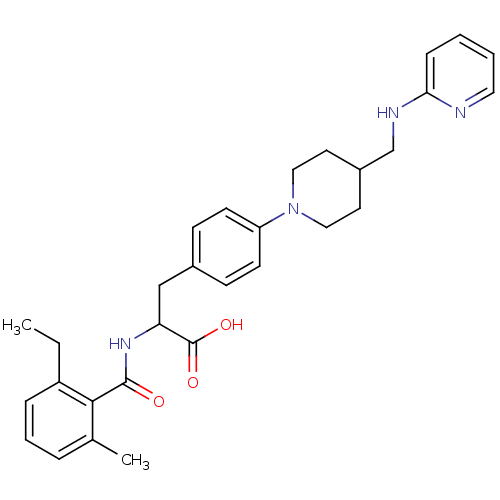
(CHEMBL591545)Show SMILES CCc1cccc(C)c1C(=O)NC(Cc1ccc(cc1)N1CCC(CNc2ccccn2)CC1)C(O)=O Show InChI InChI=1S/C30H36N4O3/c1-3-24-8-6-7-21(2)28(24)29(35)33-26(30(36)37)19-22-10-12-25(13-11-22)34-17-14-23(15-18-34)20-32-27-9-4-5-16-31-27/h4-13,16,23,26H,3,14-15,17-20H2,1-2H3,(H,31,32)(H,33,35)(H,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
Bioorg Med Chem Lett 20: 65-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.030
BindingDB Entry DOI: 10.7270/Q2BR8TGJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50423721
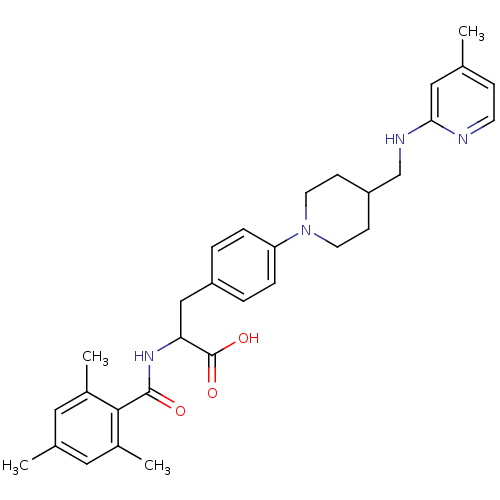
(CHEMBL595105)Show SMILES Cc1ccnc(NCC2CCN(CC2)c2ccc(CC(NC(=O)c3c(C)cc(C)cc3C)C(O)=O)cc2)c1 Show InChI InChI=1S/C31H38N4O3/c1-20-9-12-32-28(17-20)33-19-25-10-13-35(14-11-25)26-7-5-24(6-8-26)18-27(31(37)38)34-30(36)29-22(3)15-21(2)16-23(29)4/h5-9,12,15-17,25,27H,10-11,13-14,18-19H2,1-4H3,(H,32,33)(H,34,36)(H,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
Bioorg Med Chem Lett 20: 65-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.030
BindingDB Entry DOI: 10.7270/Q2BR8TGJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50423721

(CHEMBL595105)Show SMILES Cc1ccnc(NCC2CCN(CC2)c2ccc(CC(NC(=O)c3c(C)cc(C)cc3C)C(O)=O)cc2)c1 Show InChI InChI=1S/C31H38N4O3/c1-20-9-12-32-28(17-20)33-19-25-10-13-35(14-11-25)26-7-5-24(6-8-26)18-27(31(37)38)34-30(36)29-22(3)15-21(2)16-23(29)4/h5-9,12,15-17,25,27H,10-11,13-14,18-19H2,1-4H3,(H,32,33)(H,34,36)(H,37,38) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to integrin alpha5beta1 receptor by ELISA |
Bioorg Med Chem Lett 20: 380-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.073
BindingDB Entry DOI: 10.7270/Q2M61MKQ |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50217435

(2S,4R,20S-4-{[2-carboxy-2-(2,4,6-trimethyl-benzene...)Show SMILES COc1ccnc(NC[C@@H]2C[C@H](CN2C(=O)OCc2ccccc2)OCC(=O)NC[C@H](NS(=O)(=O)c2c(C)cc(C)cc2C)C(O)=O)c1 Show InChI InChI=1S/C33H41N5O9S/c1-21-12-22(2)31(23(3)13-21)48(43,44)37-28(32(40)41)17-36-30(39)20-46-27-14-25(16-35-29-15-26(45-4)10-11-34-29)38(18-27)33(42)47-19-24-8-6-5-7-9-24/h5-13,15,25,27-28,37H,14,16-20H2,1-4H3,(H,34,35)(H,36,39)(H,40,41)/t25-,27+,28-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50423718
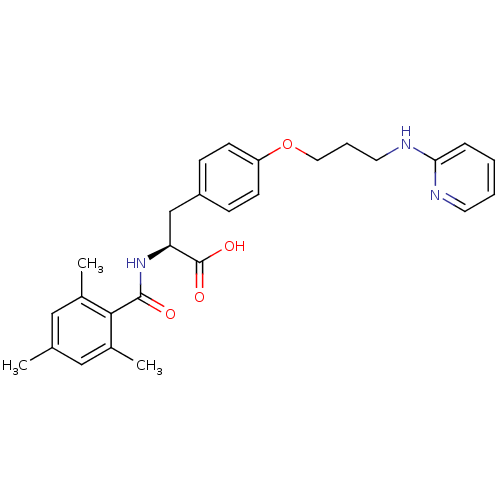
(CHEMBL606371)Show SMILES Cc1cc(C)c(C(=O)N[C@@H](Cc2ccc(OCCCNc3ccccn3)cc2)C(O)=O)c(C)c1 |r| Show InChI InChI=1S/C27H31N3O4/c1-18-15-19(2)25(20(3)16-18)26(31)30-23(27(32)33)17-21-8-10-22(11-9-21)34-14-6-13-29-24-7-4-5-12-28-24/h4-5,7-12,15-16,23H,6,13-14,17H2,1-3H3,(H,28,29)(H,30,31)(H,32,33)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to integrin alpha5beta1 receptor by ELISA |
Bioorg Med Chem Lett 20: 380-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.073
BindingDB Entry DOI: 10.7270/Q2M61MKQ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50423739
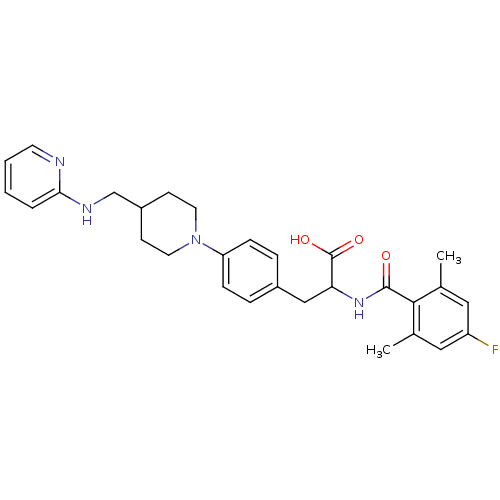
(CHEMBL591778)Show SMILES Cc1cc(F)cc(C)c1C(=O)NC(Cc1ccc(cc1)N1CCC(CNc2ccccn2)CC1)C(O)=O Show InChI InChI=1S/C29H33FN4O3/c1-19-15-23(30)16-20(2)27(19)28(35)33-25(29(36)37)17-21-6-8-24(9-7-21)34-13-10-22(11-14-34)18-32-26-5-3-4-12-31-26/h3-9,12,15-16,22,25H,10-11,13-14,17-18H2,1-2H3,(H,31,32)(H,33,35)(H,36,37) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
Bioorg Med Chem Lett 20: 65-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.030
BindingDB Entry DOI: 10.7270/Q2BR8TGJ |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466500
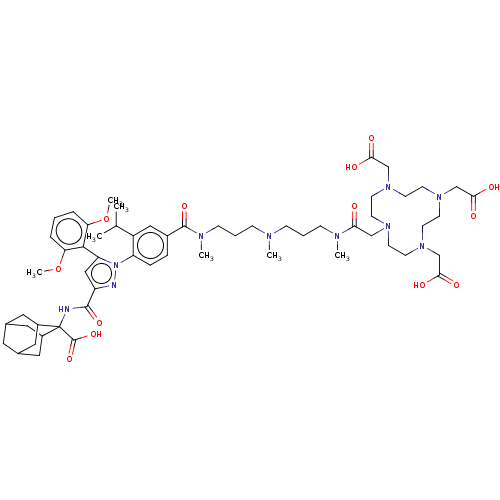
(US10799605, Example 5)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)CCCN(C)C(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:67:68:70:73.74.72,THB:75:76:70:73.74.72,75:73:70:68.76.77,78:68:70:73.74.72,68:69:76.77.75:72,68:76:70.69.74:72,(-13.42,6.92,;-12.33,5.83,;-12.73,4.34,;-14.22,3.94,;-14.62,2.45,;-13.53,1.36,;-12.04,1.76,;-10.95,.67,;-11.35,-.81,;-11.64,3.25,;-10.15,3.65,;-9.68,5.11,;-8.14,5.11,;-7.66,3.65,;-8.91,2.74,;-8.91,1.2,;-10.24,.43,;-10.24,-1.11,;-8.91,-1.88,;-7.57,-1.11,;-7.57,.43,;-6.24,1.2,;-4.91,.43,;-6.24,2.74,;-8.91,-3.42,;-10.24,-4.19,;-7.57,-4.19,;-7.57,-5.73,;-6.24,-3.42,;-4.91,-4.19,;-3.57,-3.42,;-2.24,-4.19,;-2.24,-5.73,;-.91,-3.42,;.43,-4.19,;1.76,-3.42,;3.1,-4.19,;3.1,-5.73,;4.43,-3.42,;4.43,-1.88,;5.97,-3.42,;7.3,-4.19,;8.1,-2.87,;9.68,-2.86,;10.4,-4.18,;11.17,-2.85,;10.4,-1.51,;8.86,-1.51,;11.17,-.18,;11.7,-4.95,;11.72,-6.52,;10.41,-7.28,;11.74,-8.05,;13.08,-7.28,;13.08,-5.74,;14.62,-7.28,;9.59,-8.6,;8.01,-8.61,;7.31,-7.29,;6.54,-8.62,;7.31,-9.95,;8.85,-9.95,;6.54,-11.29,;6.03,-6.49,;6.01,-4.91,;-7.05,6.2,;-7.45,7.69,;-5.56,5.8,;-4.47,6.89,;-5.12,8.29,;-6.38,9.17,;-5.87,10.62,;-4.48,11.29,;-3.16,10.64,;-3.8,9.24,;-2.64,9.19,;-3.96,8.35,;-5.23,9.22,;-3.14,6.12,;-3.14,4.58,;-1.81,6.89,)| Show InChI InChI=1S/C58H84N10O13/c1-38(2)44-32-41(13-14-46(44)68-47(54-48(80-6)11-8-12-49(54)81-7)33-45(60-68)55(76)59-58(57(78)79)42-28-39-27-40(30-42)31-43(58)29-39)56(77)63(5)18-10-16-61(3)15-9-17-62(4)50(69)34-64-19-21-65(35-51(70)71)23-25-67(37-53(74)75)26-24-66(22-20-64)36-52(72)73/h8,11-14,32-33,38-40,42-43H,9-10,15-31,34-37H2,1-7H3,(H,59,76)(H,70,71)(H,72,73)(H,74,75)(H,78,79) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
In order to determine the binding affinity of compounds comprising a radiolabel for NTR1, a radioligand binding assay was carried out. A radioligand ... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466502
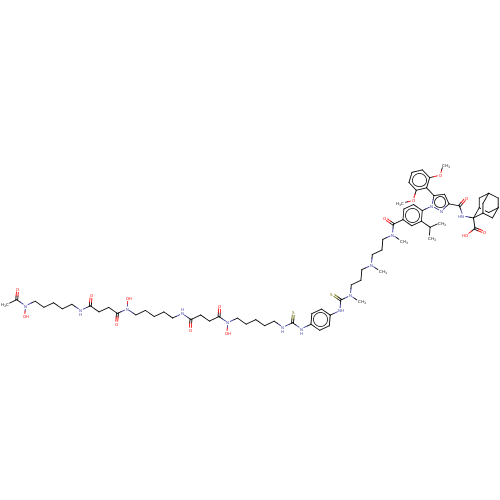
(US10799605, Example 13)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)CCCN(C)C(=S)Nc1ccc(NC(=S)NCCCCCN(O)C(=O)CCC(=O)NCCCCCN(O)C(=O)CCC(=O)NCCCCCN(O)C(C)=O)cc1)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:91:92:94:97.98.96,THB:92:93:100.101.99:96,92:100:94.93.98:96,99:100:94:97.98.96,99:97:94:92.100.101,102:92:94:97.98.96,(-20.33,6.06,;-19.24,4.97,;-19.64,3.48,;-21.13,3.09,;-21.53,1.6,;-20.44,.51,;-18.95,.91,;-17.86,-.18,;-18.26,-1.67,;-18.55,2.39,;-17.06,2.79,;-16.59,4.26,;-15.05,4.26,;-14.57,2.79,;-15.82,1.89,;-15.82,.35,;-17.15,-.42,;-17.15,-1.96,;-15.82,-2.73,;-14.48,-1.96,;-14.48,-.42,;-13.15,.35,;-11.82,-.42,;-13.15,1.89,;-15.82,-4.27,;-17.15,-5.04,;-14.48,-5.04,;-14.48,-6.58,;-13.15,-4.27,;-11.82,-5.04,;-10.48,-4.27,;-9.15,-5.04,;-9.15,-6.58,;-7.82,-4.27,;-6.48,-5.04,;-5.15,-4.27,;-3.81,-5.04,;-3.81,-6.58,;-2.48,-4.27,;-2.48,-2.73,;-1.15,-5.04,;.19,-4.27,;1.52,-5.04,;2.85,-4.27,;2.85,-2.73,;4.19,-1.96,;5.52,-2.73,;5.52,-4.27,;6.86,-1.96,;8.19,-1.19,;9.52,-1.96,;10.86,-1.19,;12.19,-1.96,;12.19,-3.5,;13.52,-4.27,;14.86,-3.5,;13.52,-5.81,;12.19,-6.58,;14.86,-6.58,;14.86,-8.12,;16.19,-8.89,;16.19,-10.43,;17.52,-8.12,;18.86,-8.89,;20.19,-8.12,;20.19,-6.58,;21.53,-5.81,;21.53,-4.27,;20.19,-3.5,;18.86,-4.27,;20.19,-1.96,;21.53,-1.19,;18.86,-1.19,;18.86,.35,;20.19,1.12,;21.53,.35,;20.19,2.66,;18.86,3.43,;17.52,2.66,;16.19,3.43,;14.86,2.66,;14.86,1.12,;13.52,.35,;13.52,-1.19,;12.19,1.12,;10.86,.35,;12.19,2.66,;1.52,-1.96,;.19,-2.73,;-13.96,5.35,;-14.36,6.83,;-12.47,4.95,;-11.38,6.04,;-12.03,7.44,;-13.29,8.31,;-12.78,9.77,;-11.39,10.43,;-10.07,9.78,;-10.71,8.38,;-9.55,8.33,;-10.87,7.49,;-12.14,8.37,;-10.05,5.27,;-10.05,3.62,;-8.71,6.04,)| Show InChI InChI=1S/C75H110N14O14S2/c1-50(2)59-48-54(23-28-61(59)89-62(69-63(102-7)21-18-22-64(69)103-8)49-60(82-89)70(95)81-75(72(97)98)55-44-52-43-53(46-55)47-56(75)45-52)71(96)84(5)38-19-36-83(4)37-20-39-85(6)74(105)80-58-26-24-57(25-27-58)79-73(104)78-35-14-11-17-42-88(101)68(94)32-30-66(92)77-34-13-10-16-41-87(100)67(93)31-29-65(91)76-33-12-9-15-40-86(99)51(3)90/h18,21-28,48-50,52-53,55-56,99-101H,9-17,19-20,29-47H2,1-8H3,(H,76,91)(H,77,92)(H,80,105)(H,81,95)(H,97,98)(H2,78,79,104) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
In order to determine the binding affinity of compounds comprising a radiolabel for NTR1, a radioligand binding assay was carried out. A radioligand ... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466505
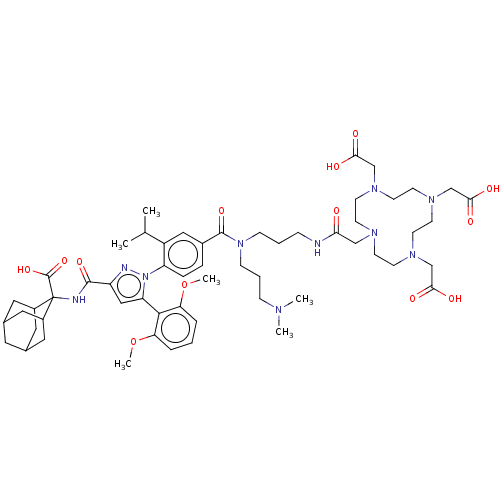
(US10799605, Example 19)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(CCCNC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)CCCN(C)C)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:77:67:76:72.74.71,73:72:76:69.67.68,73:68:76:72.74.71,THB:66:67:76:72.74.71,67:75:69.73.68:71,67:68:76.74.75:71,(-10.5,7.02,;-9.41,5.93,;-9.8,4.44,;-11.29,4.04,;-11.69,2.56,;-10.6,1.47,;-9.11,1.86,;-8.03,.78,;-8.42,-.71,;-8.72,3.35,;-7.23,3.75,;-6.75,5.22,;-5.21,5.22,;-4.74,3.75,;-5.98,2.85,;-5.98,1.31,;-7.32,.54,;-7.32,-1,;-5.98,-1.77,;-4.65,-1,;-4.65,.54,;-3.32,1.31,;-3.32,2.85,;-1.98,.54,;-5.98,-3.31,;-7.32,-4.08,;-4.65,-4.08,;-3.32,-3.31,;-1.98,-4.08,;-.65,-3.31,;.69,-4.08,;2.02,-3.31,;2.02,-1.77,;3.56,-3.31,;4.65,-4.4,;5.42,-3.07,;6.96,-3.07,;7.73,-4.4,;9.06,-3.63,;8.29,-2.3,;6.75,-2.3,;8.69,-.81,;9.06,-5.17,;9.06,-6.71,;7.73,-7.48,;8.82,-8.57,;10.15,-7.8,;10.15,-6.26,;11.69,-7.8,;6.96,-8.82,;5.42,-8.82,;4.65,-7.48,;3.88,-8.82,;4.65,-10.15,;6.19,-10.15,;3.88,-11.48,;3.31,-6.71,;3.31,-5.17,;-4.65,-5.62,;-3.32,-6.39,;-3.32,-7.93,;-1.98,-8.7,;-1.98,-10.24,;-.65,-7.93,;-4.12,6.3,;-4.52,7.79,;-2.64,5.91,;-1.55,6.99,;-1.23,8.49,;-2.5,9.25,;-3.25,10.59,;-2.16,11.48,;-.86,10.92,;-.09,9.58,;-1.23,9.42,;-2.32,8.33,;-3.65,9.11,;-.21,6.22,;-.21,4.68,;1.12,6.99,)| Show InChI InChI=1S/C57H82N10O13/c1-37(2)43-31-40(12-13-45(43)67-46(53-47(79-5)10-7-11-48(53)80-6)32-44(60-67)54(75)59-57(56(77)78)41-27-38-26-39(29-41)30-42(57)28-38)55(76)66(17-9-15-61(3)4)16-8-14-58-49(68)33-62-18-20-63(34-50(69)70)22-24-65(36-52(73)74)25-23-64(21-19-62)35-51(71)72/h7,10-13,31-32,37-39,41-42H,8-9,14-30,33-36H2,1-6H3,(H,58,68)(H,59,75)(H,69,70)(H,71,72)(H,73,74)(H,77,78) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
In order to determine the binding affinity of compounds comprising a radiolabel for NTR1, a radioligand binding assay was carried out. A radioligand ... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50217434
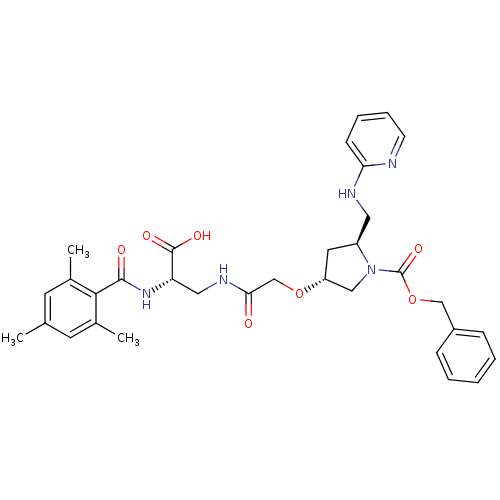
(2S,4R,20S-4-{[2-carboxy-2-(2,4,6-trimethyl-benzoyl...)Show SMILES Cc1cc(C)c(C(=O)N[C@@H](CNC(=O)CO[C@@H]2C[C@@H](CNc3ccccn3)N(C2)C(=O)OCc2ccccc2)C(O)=O)c(C)c1 Show InChI InChI=1S/C33H39N5O7/c1-21-13-22(2)30(23(3)14-21)31(40)37-27(32(41)42)17-36-29(39)20-44-26-15-25(16-35-28-11-7-8-12-34-28)38(18-26)33(43)45-19-24-9-5-4-6-10-24/h4-14,25-27H,15-20H2,1-3H3,(H,34,35)(H,36,39)(H,37,40)(H,41,42)/t25-,26+,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50217420
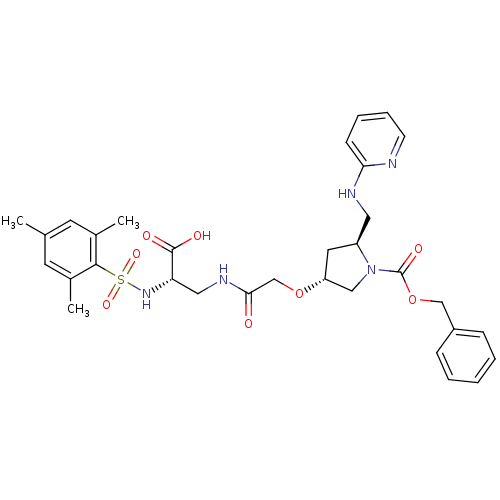
(12S,14R,30S-4-{[2-carboxy-2-(2,4,6-trimethylbenzen...)Show SMILES Cc1cc(C)c(c(C)c1)S(=O)(=O)N[C@@H](CNC(=O)CO[C@@H]1C[C@@H](CNc2ccccn2)N(C1)C(=O)OCc1ccccc1)C(O)=O Show InChI InChI=1S/C32H39N5O8S/c1-21-13-22(2)30(23(3)14-21)46(42,43)36-27(31(39)40)17-35-29(38)20-44-26-15-25(16-34-28-11-7-8-12-33-28)37(18-26)32(41)45-19-24-9-5-4-6-10-24/h4-14,25-27,36H,15-20H2,1-3H3,(H,33,34)(H,35,38)(H,39,40)/t25-,26+,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50217422

(2S,3R,20S-3-(2-{1-(3,3-dimethyl-butyryl)-5-[(4-met...)Show SMILES COc1ccnc(NC[C@@H]2C[C@H](CN2C(=O)CC(C)(C)C)OCC(=O)NC[C@H](NS(=O)(=O)c2c(C)cc(C)cc2C)C(O)=O)c1 Show InChI InChI=1S/C31H45N5O8S/c1-19-10-20(2)29(21(3)11-19)45(41,42)35-25(30(39)40)16-34-27(37)18-44-24-12-22(36(17-24)28(38)14-31(4,5)6)15-33-26-13-23(43-7)8-9-32-26/h8-11,13,22,24-25,35H,12,14-18H2,1-7H3,(H,32,33)(H,34,37)(H,39,40)/t22-,24+,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50423733
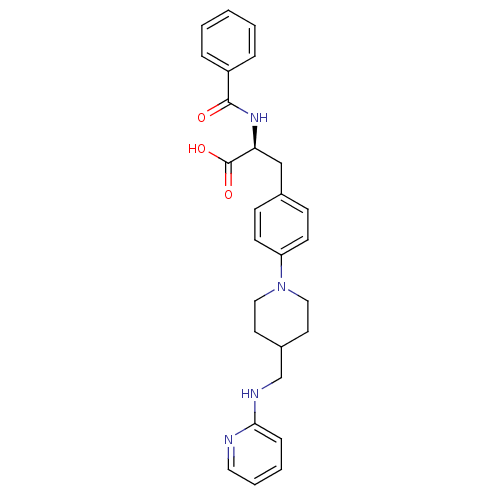
(CHEMBL599877)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)N1CCC(CNc2ccccn2)CC1)NC(=O)c1ccccc1 |r| Show InChI InChI=1S/C27H30N4O3/c32-26(22-6-2-1-3-7-22)30-24(27(33)34)18-20-9-11-23(12-10-20)31-16-13-21(14-17-31)19-29-25-8-4-5-15-28-25/h1-12,15,21,24H,13-14,16-19H2,(H,28,29)(H,30,32)(H,33,34)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta3 receptor by ELISA |
Bioorg Med Chem Lett 20: 65-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.030
BindingDB Entry DOI: 10.7270/Q2BR8TGJ |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466501
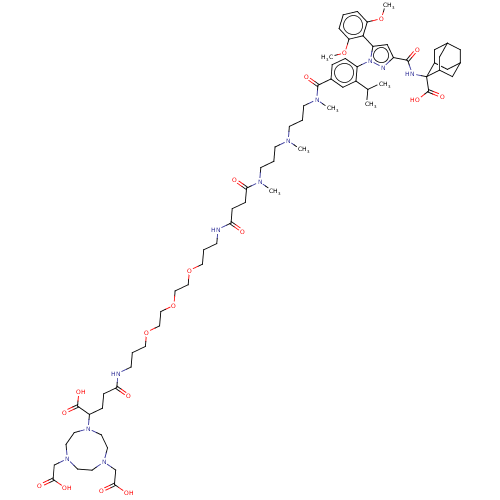
(US10799605, Example 12)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)CCCN(C)C(=O)CCC(=O)NCCCOCCOCCOCCCNC(=O)CCC(N1CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(O)=O)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:86:87:89:92.93.91,THB:87:88:95.96.94:91,87:95:89.88.93:91,94:95:89:92.93.91,94:92:89:87.95.96,97:87:89:92.93.91,(-12.72,2.45,;-11.95,1.11,;-12.72,-.22,;-14.26,-.22,;-15.03,-1.56,;-14.26,-2.89,;-12.72,-2.89,;-11.95,-4.22,;-12.72,-5.56,;-11.95,-1.56,;-10.41,-1.56,;-9.94,-.09,;-8.4,-.09,;-7.92,-1.56,;-9.17,-2.46,;-9.17,-4,;-10.5,-4.77,;-10.5,-6.31,;-9.17,-7.08,;-7.84,-6.31,;-7.84,-4.77,;-6.5,-4,;-5.17,-4.77,;-6.5,-2.46,;-9.17,-8.62,;-10.5,-9.39,;-7.84,-9.39,;-7.84,-10.93,;-6.5,-8.62,;-5.17,-9.39,;-3.83,-8.62,;-2.5,-9.39,;-2.5,-10.93,;-1.17,-8.62,;.17,-9.39,;1.5,-8.62,;2.83,-9.39,;2.83,-10.93,;4.17,-8.62,;5.5,-9.39,;4.17,-7.08,;5.5,-6.31,;6.84,-7.08,;6.84,-8.62,;8.17,-6.31,;8.17,-4.77,;6.84,-4,;5.5,-4.77,;4.17,-4,;2.83,-4.77,;1.5,-4,;1.5,-2.46,;2.83,-1.69,;4.17,-2.46,;5.5,-1.69,;6.84,-2.46,;8.17,-1.69,;9.5,-2.46,;10.84,-1.69,;10.84,-.15,;12.38,-.15,;10.07,1.18,;8.53,1.18,;7.76,2.52,;8.52,3.85,;7.18,4.62,;7.17,6.16,;8.5,6.93,;7.72,8.26,;8.49,9.6,;7.71,10.93,;10.03,9.6,;9.83,7.71,;11.17,6.95,;11.18,5.41,;12.72,5.42,;13.49,4.09,;15.03,4.09,;12.73,2.75,;11.19,3.87,;9.86,3.09,;6.22,2.53,;5.45,1.19,;5.44,3.85,;-7.31,1,;-7.71,2.49,;-5.82,.6,;-4.73,1.69,;-5.38,3.09,;-6.64,3.96,;-6.13,5.42,;-4.74,6.08,;-3.42,5.43,;-4.06,4.03,;-2.9,3.98,;-4.22,3.14,;-5.49,4.02,;-3.4,.92,;-2.63,-.42,;-2.07,1.69,)| Show InChI InChI=1S/C71H107N11O18/c1-48(2)54-44-51(15-16-56(54)82-58(66-59(96-6)13-8-14-60(66)97-7)45-55(75-82)67(90)74-71(70(94)95)52-40-49-39-50(42-52)43-53(71)41-49)68(91)78(5)26-12-24-76(3)23-11-25-77(4)63(85)20-19-62(84)73-22-10-34-99-36-38-100-37-35-98-33-9-21-72-61(83)18-17-57(69(92)93)81-31-29-79(46-64(86)87)27-28-80(30-32-81)47-65(88)89/h8,13-16,44-45,48-50,52-53,57H,9-12,17-43,46-47H2,1-7H3,(H,72,83)(H,73,84)(H,74,90)(H,86,87)(H,88,89)(H,92,93)(H,94,95) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
In order to determine the binding affinity of compounds comprising a radiolabel for NTR1, a radioligand binding assay was carried out. A radioligand ... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466504
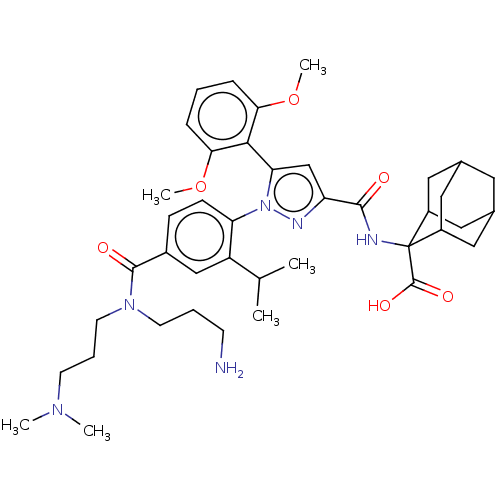
(US10799605, Example 18)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(CCCN)CCCN(C)C)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:50:40:49:45.47.44,46:45:49:42.40.41,46:41:49:45.47.44,THB:39:40:49:45.47.44,40:48:42.46.41:44,40:41:49.47.48:44,(-5.21,6.4,;-4.12,5.31,;-4.52,3.82,;-6.01,3.42,;-6.41,1.94,;-5.32,.85,;-3.83,1.24,;-2.74,.16,;-3.14,-1.33,;-3.43,2.73,;-1.94,3.13,;-1.47,4.6,;.07,4.6,;.55,3.13,;-.7,2.23,;-.7,.69,;-2.03,-.08,;-2.03,-1.62,;-.7,-2.39,;.64,-1.62,;.64,-.08,;1.97,.69,;1.97,2.23,;3.3,-.08,;-.7,-3.93,;-2.03,-4.7,;.64,-4.7,;1.97,-3.93,;3.3,-4.7,;4.64,-3.93,;5.97,-4.7,;.64,-6.24,;1.97,-7.01,;1.97,-8.55,;3.3,-9.32,;3.3,-10.86,;4.64,-8.55,;1.16,5.68,;.76,7.17,;2.65,5.29,;3.74,6.37,;4.05,7.87,;2.79,8.63,;2.04,9.97,;3.13,10.86,;4.43,10.3,;5.2,8.96,;4.05,8.8,;2.97,7.71,;1.64,8.49,;5.07,5.6,;5.07,4.06,;6.41,6.37,)| Show InChI InChI=1S/C41H56N6O6/c1-25(2)31-23-28(39(49)46(16-8-14-42)17-9-15-45(3)4)12-13-33(31)47-34(37-35(52-5)10-7-11-36(37)53-6)24-32(44-47)38(48)43-41(40(50)51)29-19-26-18-27(21-29)22-30(41)20-26/h7,10-13,23-27,29-30H,8-9,14-22,42H2,1-6H3,(H,43,48)(H,50,51) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
In order to determine the binding affinity of compounds comprising a radiolabel for NTR1, a radioligand binding assay was carried out. A radioligand ... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50217430
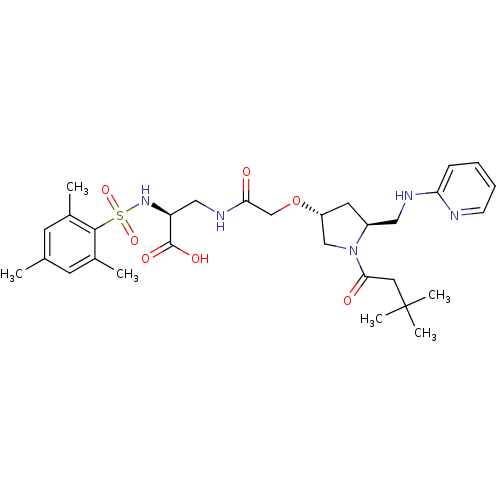
(2S,3R,5S-3-{2-[1-(3,3-dimethylbutyryl)-5-(pyridin-...)Show SMILES Cc1cc(C)c(c(C)c1)S(=O)(=O)N[C@@H](CNC(=O)CO[C@@H]1C[C@@H](CNc2ccccn2)N(C1)C(=O)CC(C)(C)C)C(O)=O Show InChI InChI=1S/C30H43N5O7S/c1-19-11-20(2)28(21(3)12-19)43(40,41)34-24(29(38)39)16-33-26(36)18-42-23-13-22(15-32-25-9-7-8-10-31-25)35(17-23)27(37)14-30(4,5)6/h7-12,22-24,34H,13-18H2,1-6H3,(H,31,32)(H,33,36)(H,38,39)/t22-,23+,24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50423724
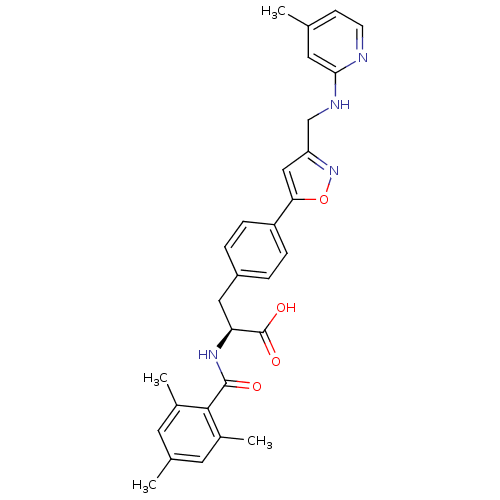
(CHEMBL596248)Show SMILES Cc1ccnc(NCc2cc(on2)-c2ccc(C[C@H](NC(=O)c3c(C)cc(C)cc3C)C(O)=O)cc2)c1 |r| Show InChI InChI=1S/C29H30N4O4/c1-17-9-10-30-26(13-17)31-16-23-15-25(37-33-23)22-7-5-21(6-8-22)14-24(29(35)36)32-28(34)27-19(3)11-18(2)12-20(27)4/h5-13,15,24H,14,16H2,1-4H3,(H,30,31)(H,32,34)(H,35,36)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to integrin alpha5beta1 receptor by ELISA |
Bioorg Med Chem Lett 20: 380-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.073
BindingDB Entry DOI: 10.7270/Q2M61MKQ |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50217431
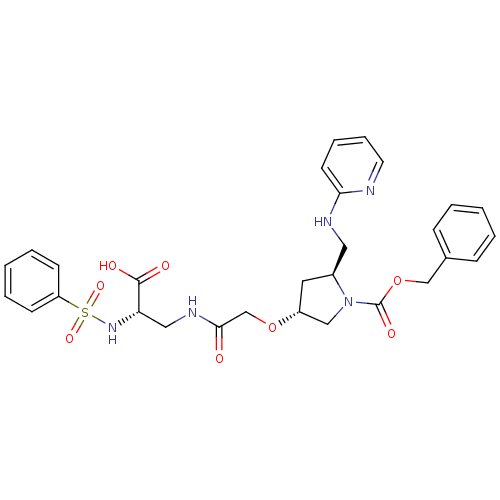
(2S,4R,20S-4-[(2-benzenesulfonylamino-2-carboxy-eth...)Show SMILES OC(=O)[C@H](CNC(=O)CO[C@@H]1C[C@@H](CNc2ccccn2)N(C1)C(=O)OCc1ccccc1)NS(=O)(=O)c1ccccc1 Show InChI InChI=1S/C29H33N5O8S/c35-27(32-17-25(28(36)37)33-43(39,40)24-11-5-2-6-12-24)20-41-23-15-22(16-31-26-13-7-8-14-30-26)34(18-23)29(38)42-19-21-9-3-1-4-10-21/h1-14,22-23,25,33H,15-20H2,(H,30,31)(H,32,35)(H,36,37)/t22-,23+,25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466499

(US10799605, Example 4)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)CCCN(C)C(=O)CCCC(O)=O)C(=O)NC1(C2CC3CC(C2)CC1C3)C(=O)OC(C)(C)C |TLB:48:49:51:54.55.53,THB:49:50:57.58.56:53,49:57:51.50.55:53,56:57:51:54.55.53,56:54:51:49.57.58,59:49:51:54.55.53,(-8.99,4.14,;-7.9,3.05,;-8.3,1.56,;-9.79,1.16,;-10.19,-.33,;-9.1,-1.42,;-7.61,-1.02,;-6.52,-2.11,;-6.92,-3.59,;-7.21,.47,;-5.73,.87,;-5.25,2.33,;-3.71,2.33,;-3.24,.87,;-4.48,-.04,;-4.48,-1.58,;-5.81,-2.35,;-5.81,-3.89,;-4.48,-4.66,;-3.15,-3.89,;-3.15,-2.35,;-1.81,-1.58,;-.48,-2.35,;-1.81,-.04,;-4.48,-6.2,;-5.81,-6.97,;-3.15,-6.97,;-3.15,-8.51,;-1.81,-6.2,;-.48,-6.97,;.85,-6.2,;2.19,-6.97,;2.19,-8.51,;3.52,-6.2,;4.85,-6.97,;6.19,-6.2,;7.52,-6.97,;7.52,-8.51,;8.86,-6.2,;10.19,-6.97,;8.86,-4.66,;7.52,-3.89,;7.52,-2.35,;6.19,-1.58,;6.19,-.04,;4.85,-2.35,;-2.62,3.42,;-3.02,4.91,;-1.13,3.02,;-.05,4.11,;-.69,5.51,;-1.96,6.39,;-1.45,7.84,;-.06,8.51,;1.27,7.86,;.63,6.46,;1.78,6.41,;.46,5.57,;-.8,6.44,;1.29,3.34,;2.62,4.11,;1.29,1.8,;2.62,1.03,;2.62,-.51,;3.96,1.8,;3.96,.26,)| Show InChI InChI=1S/C51H72N6O9/c1-32(2)38-30-35(48(62)56(8)24-14-22-54(6)21-13-23-55(7)44(58)17-12-18-45(59)60)19-20-40(38)57-41(46-42(64-9)15-11-16-43(46)65-10)31-39(53-57)47(61)52-51(49(63)66-50(3,4)5)36-26-33-25-34(28-36)29-37(51)27-33/h11,15-16,19-20,30-34,36-37H,12-14,17-18,21-29H2,1-10H3,(H,52,61)(H,59,60) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 7.54 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
Ca2+ ions are usually kept at nanomolar levels in the cytosol of cells, and act in a number of signal transduction pathways as second messengers. Man... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Integrin alpha-IIb/beta-3
(Homo sapiens (Human)) | BDBM50217435

(2S,4R,20S-4-{[2-carboxy-2-(2,4,6-trimethyl-benzene...)Show SMILES COc1ccnc(NC[C@@H]2C[C@H](CN2C(=O)OCc2ccccc2)OCC(=O)NC[C@H](NS(=O)(=O)c2c(C)cc(C)cc2C)C(O)=O)c1 Show InChI InChI=1S/C33H41N5O9S/c1-21-12-22(2)31(23(3)13-21)48(43,44)37-28(32(40)41)17-36-30(39)20-46-27-14-25(16-35-29-15-26(45-4)10-11-34-29)38(18-27)33(42)47-19-24-8-6-5-7-9-24/h5-13,15,25,27-28,37H,14,16-20H2,1-4H3,(H,34,35)(H,36,39)(H,40,41)/t25-,27+,28-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha2bbeta3 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466504

(US10799605, Example 18)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(CCCN)CCCN(C)C)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:50:40:49:45.47.44,46:45:49:42.40.41,46:41:49:45.47.44,THB:39:40:49:45.47.44,40:48:42.46.41:44,40:41:49.47.48:44,(-5.21,6.4,;-4.12,5.31,;-4.52,3.82,;-6.01,3.42,;-6.41,1.94,;-5.32,.85,;-3.83,1.24,;-2.74,.16,;-3.14,-1.33,;-3.43,2.73,;-1.94,3.13,;-1.47,4.6,;.07,4.6,;.55,3.13,;-.7,2.23,;-.7,.69,;-2.03,-.08,;-2.03,-1.62,;-.7,-2.39,;.64,-1.62,;.64,-.08,;1.97,.69,;1.97,2.23,;3.3,-.08,;-.7,-3.93,;-2.03,-4.7,;.64,-4.7,;1.97,-3.93,;3.3,-4.7,;4.64,-3.93,;5.97,-4.7,;.64,-6.24,;1.97,-7.01,;1.97,-8.55,;3.3,-9.32,;3.3,-10.86,;4.64,-8.55,;1.16,5.68,;.76,7.17,;2.65,5.29,;3.74,6.37,;4.05,7.87,;2.79,8.63,;2.04,9.97,;3.13,10.86,;4.43,10.3,;5.2,8.96,;4.05,8.8,;2.97,7.71,;1.64,8.49,;5.07,5.6,;5.07,4.06,;6.41,6.37,)| Show InChI InChI=1S/C41H56N6O6/c1-25(2)31-23-28(39(49)46(16-8-14-42)17-9-15-45(3)4)12-13-33(31)47-34(37-35(52-5)10-7-11-36(37)53-6)24-32(44-47)38(48)43-41(40(50)51)29-19-26-18-27(21-29)22-30(41)20-26/h7,10-13,23-27,29-30H,8-9,14-22,42H2,1-6H3,(H,43,48)(H,50,51) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 8.95 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
Ca2+ ions are usually kept at nanomolar levels in the cytosol of cells, and act in a number of signal transduction pathways as second messengers. Man... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50217421
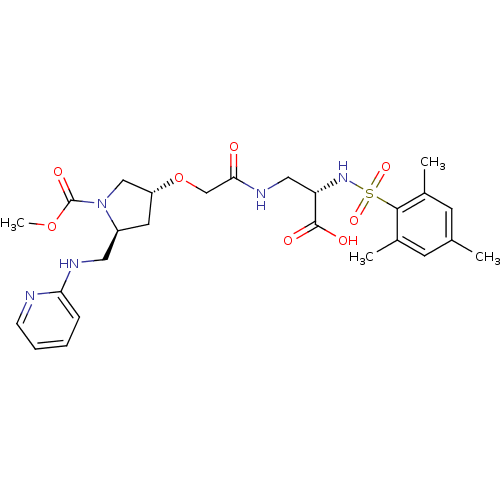
((S)-3-(2-((3R,5S)-1-(methoxycarbonyl)-5-((pyridin-...)Show SMILES COC(=O)N1C[C@@H](C[C@H]1CNc1ccccn1)OCC(=O)NC[C@H](NS(=O)(=O)c1c(C)cc(C)cc1C)C(O)=O Show InChI InChI=1S/C26H35N5O8S/c1-16-9-17(2)24(18(3)10-16)40(36,37)30-21(25(33)34)13-29-23(32)15-39-20-11-19(31(14-20)26(35)38-4)12-28-22-7-5-6-8-27-22/h5-10,19-21,30H,11-15H2,1-4H3,(H,27,28)(H,29,32)(H,33,34)/t19-,20+,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466505

(US10799605, Example 19)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(CCCNC(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)CCCN(C)C)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:77:67:76:72.74.71,73:72:76:69.67.68,73:68:76:72.74.71,THB:66:67:76:72.74.71,67:75:69.73.68:71,67:68:76.74.75:71,(-10.5,7.02,;-9.41,5.93,;-9.8,4.44,;-11.29,4.04,;-11.69,2.56,;-10.6,1.47,;-9.11,1.86,;-8.03,.78,;-8.42,-.71,;-8.72,3.35,;-7.23,3.75,;-6.75,5.22,;-5.21,5.22,;-4.74,3.75,;-5.98,2.85,;-5.98,1.31,;-7.32,.54,;-7.32,-1,;-5.98,-1.77,;-4.65,-1,;-4.65,.54,;-3.32,1.31,;-3.32,2.85,;-1.98,.54,;-5.98,-3.31,;-7.32,-4.08,;-4.65,-4.08,;-3.32,-3.31,;-1.98,-4.08,;-.65,-3.31,;.69,-4.08,;2.02,-3.31,;2.02,-1.77,;3.56,-3.31,;4.65,-4.4,;5.42,-3.07,;6.96,-3.07,;7.73,-4.4,;9.06,-3.63,;8.29,-2.3,;6.75,-2.3,;8.69,-.81,;9.06,-5.17,;9.06,-6.71,;7.73,-7.48,;8.82,-8.57,;10.15,-7.8,;10.15,-6.26,;11.69,-7.8,;6.96,-8.82,;5.42,-8.82,;4.65,-7.48,;3.88,-8.82,;4.65,-10.15,;6.19,-10.15,;3.88,-11.48,;3.31,-6.71,;3.31,-5.17,;-4.65,-5.62,;-3.32,-6.39,;-3.32,-7.93,;-1.98,-8.7,;-1.98,-10.24,;-.65,-7.93,;-4.12,6.3,;-4.52,7.79,;-2.64,5.91,;-1.55,6.99,;-1.23,8.49,;-2.5,9.25,;-3.25,10.59,;-2.16,11.48,;-.86,10.92,;-.09,9.58,;-1.23,9.42,;-2.32,8.33,;-3.65,9.11,;-.21,6.22,;-.21,4.68,;1.12,6.99,)| Show InChI InChI=1S/C57H82N10O13/c1-37(2)43-31-40(12-13-45(43)67-46(53-47(79-5)10-7-11-48(53)80-6)32-44(60-67)54(75)59-57(56(77)78)41-27-38-26-39(29-41)30-42(57)28-38)55(76)66(17-9-15-61(3)4)16-8-14-58-49(68)33-62-18-20-63(34-50(69)70)22-24-65(36-52(73)74)25-23-64(21-19-62)35-51(71)72/h7,10-13,31-32,37-39,41-42H,8-9,14-30,33-36H2,1-6H3,(H,58,68)(H,59,75)(H,69,70)(H,71,72)(H,73,74)(H,77,78) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 12.6 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
Ca2+ ions are usually kept at nanomolar levels in the cytosol of cells, and act in a number of signal transduction pathways as second messengers. Man... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50423727
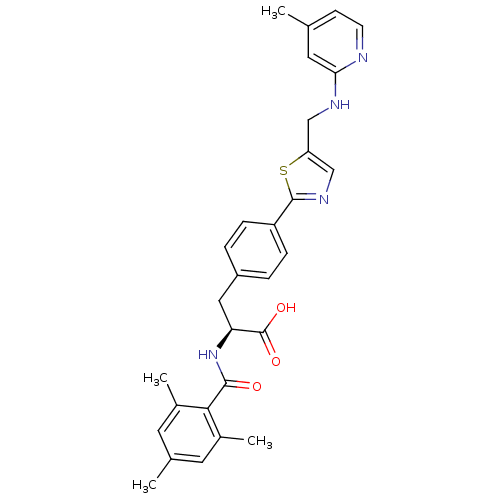
(CHEMBL593226)Show SMILES Cc1ccnc(NCc2cnc(s2)-c2ccc(C[C@H](NC(=O)c3c(C)cc(C)cc3C)C(O)=O)cc2)c1 |r| Show InChI InChI=1S/C29H30N4O3S/c1-17-9-10-30-25(13-17)31-15-23-16-32-28(37-23)22-7-5-21(6-8-22)14-24(29(35)36)33-27(34)26-19(3)11-18(2)12-20(26)4/h5-13,16,24H,14-15H2,1-4H3,(H,30,31)(H,33,34)(H,35,36)/t24-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to integrin alpha5beta1 receptor by ELISA |
Bioorg Med Chem Lett 20: 380-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.073
BindingDB Entry DOI: 10.7270/Q2M61MKQ |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50423734

(CHEMBL591779)Show SMILES OC(=O)[C@H](Cc1ccc(cc1)N1CCC(CNc2ccccn2)CC1)N1Cc2ccccc2C1=O |r| Show InChI InChI=1S/C28H30N4O3/c33-27-24-6-2-1-5-22(24)19-32(27)25(28(34)35)17-20-8-10-23(11-9-20)31-15-12-21(13-16-31)18-30-26-7-3-4-14-29-26/h1-11,14,21,25H,12-13,15-19H2,(H,29,30)(H,34,35)/t25-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta3 receptor by ELISA |
Bioorg Med Chem Lett 20: 65-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.030
BindingDB Entry DOI: 10.7270/Q2BR8TGJ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50217421

((S)-3-(2-((3R,5S)-1-(methoxycarbonyl)-5-((pyridin-...)Show SMILES COC(=O)N1C[C@@H](C[C@H]1CNc1ccccn1)OCC(=O)NC[C@H](NS(=O)(=O)c1c(C)cc(C)cc1C)C(O)=O Show InChI InChI=1S/C26H35N5O8S/c1-16-9-17(2)24(18(3)10-16)40(36,37)30-21(25(33)34)13-29-23(32)15-39-20-11-19(31(14-20)26(35)38-4)12-28-22-7-5-6-8-27-22/h5-10,19-21,30H,11-15H2,1-4H3,(H,27,28)(H,29,32)(H,33,34)/t19-,20+,21-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-3
(Homo sapiens (Human)) | BDBM50217420

(12S,14R,30S-4-{[2-carboxy-2-(2,4,6-trimethylbenzen...)Show SMILES Cc1cc(C)c(c(C)c1)S(=O)(=O)N[C@@H](CNC(=O)CO[C@@H]1C[C@@H](CNc2ccccn2)N(C1)C(=O)OCc1ccccc1)C(O)=O Show InChI InChI=1S/C32H39N5O8S/c1-21-13-22(2)30(23(3)14-21)46(42,43)36-27(31(39)40)17-35-29(38)20-44-26-15-25(16-34-28-11-7-8-12-33-28)37(18-26)32(41)45-19-24-9-5-4-6-10-24/h4-14,25-27,36H,15-20H2,1-3H3,(H,33,34)(H,35,38)(H,39,40)/t25-,26+,27-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta3 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466502

(US10799605, Example 13)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)CCCN(C)C(=S)Nc1ccc(NC(=S)NCCCCCN(O)C(=O)CCC(=O)NCCCCCN(O)C(=O)CCC(=O)NCCCCCN(O)C(C)=O)cc1)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:91:92:94:97.98.96,THB:92:93:100.101.99:96,92:100:94.93.98:96,99:100:94:97.98.96,99:97:94:92.100.101,102:92:94:97.98.96,(-20.33,6.06,;-19.24,4.97,;-19.64,3.48,;-21.13,3.09,;-21.53,1.6,;-20.44,.51,;-18.95,.91,;-17.86,-.18,;-18.26,-1.67,;-18.55,2.39,;-17.06,2.79,;-16.59,4.26,;-15.05,4.26,;-14.57,2.79,;-15.82,1.89,;-15.82,.35,;-17.15,-.42,;-17.15,-1.96,;-15.82,-2.73,;-14.48,-1.96,;-14.48,-.42,;-13.15,.35,;-11.82,-.42,;-13.15,1.89,;-15.82,-4.27,;-17.15,-5.04,;-14.48,-5.04,;-14.48,-6.58,;-13.15,-4.27,;-11.82,-5.04,;-10.48,-4.27,;-9.15,-5.04,;-9.15,-6.58,;-7.82,-4.27,;-6.48,-5.04,;-5.15,-4.27,;-3.81,-5.04,;-3.81,-6.58,;-2.48,-4.27,;-2.48,-2.73,;-1.15,-5.04,;.19,-4.27,;1.52,-5.04,;2.85,-4.27,;2.85,-2.73,;4.19,-1.96,;5.52,-2.73,;5.52,-4.27,;6.86,-1.96,;8.19,-1.19,;9.52,-1.96,;10.86,-1.19,;12.19,-1.96,;12.19,-3.5,;13.52,-4.27,;14.86,-3.5,;13.52,-5.81,;12.19,-6.58,;14.86,-6.58,;14.86,-8.12,;16.19,-8.89,;16.19,-10.43,;17.52,-8.12,;18.86,-8.89,;20.19,-8.12,;20.19,-6.58,;21.53,-5.81,;21.53,-4.27,;20.19,-3.5,;18.86,-4.27,;20.19,-1.96,;21.53,-1.19,;18.86,-1.19,;18.86,.35,;20.19,1.12,;21.53,.35,;20.19,2.66,;18.86,3.43,;17.52,2.66,;16.19,3.43,;14.86,2.66,;14.86,1.12,;13.52,.35,;13.52,-1.19,;12.19,1.12,;10.86,.35,;12.19,2.66,;1.52,-1.96,;.19,-2.73,;-13.96,5.35,;-14.36,6.83,;-12.47,4.95,;-11.38,6.04,;-12.03,7.44,;-13.29,8.31,;-12.78,9.77,;-11.39,10.43,;-10.07,9.78,;-10.71,8.38,;-9.55,8.33,;-10.87,7.49,;-12.14,8.37,;-10.05,5.27,;-10.05,3.62,;-8.71,6.04,)| Show InChI InChI=1S/C75H110N14O14S2/c1-50(2)59-48-54(23-28-61(59)89-62(69-63(102-7)21-18-22-64(69)103-8)49-60(82-89)70(95)81-75(72(97)98)55-44-52-43-53(46-55)47-56(75)45-52)71(96)84(5)38-19-36-83(4)37-20-39-85(6)74(105)80-58-26-24-57(25-27-58)79-73(104)78-35-14-11-17-42-88(101)68(94)32-30-66(92)77-34-13-10-16-41-87(100)67(93)31-29-65(91)76-33-12-9-15-40-86(99)51(3)90/h18,21-28,48-50,52-53,55-56,99-101H,9-17,19-20,29-47H2,1-8H3,(H,76,91)(H,77,92)(H,80,105)(H,81,95)(H,97,98)(H2,78,79,104) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 17.5 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
Ca2+ ions are usually kept at nanomolar levels in the cytosol of cells, and act in a number of signal transduction pathways as second messengers. Man... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50217435

(2S,4R,20S-4-{[2-carboxy-2-(2,4,6-trimethyl-benzene...)Show SMILES COc1ccnc(NC[C@@H]2C[C@H](CN2C(=O)OCc2ccccc2)OCC(=O)NC[C@H](NS(=O)(=O)c2c(C)cc(C)cc2C)C(O)=O)c1 Show InChI InChI=1S/C33H41N5O9S/c1-21-12-22(2)31(23(3)13-21)48(43,44)37-28(32(40)41)17-36-30(39)20-46-27-14-25(16-35-29-15-26(45-4)10-11-34-29)38(18-27)33(42)47-19-24-8-6-5-7-9-24/h5-13,15,25,27-28,37H,14,16-20H2,1-4H3,(H,34,35)(H,36,39)(H,40,41)/t25-,27+,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alphaVbeta5 receptor by ELISA |
J Med Chem 50: 3786-94 (2007)
Article DOI: 10.1021/jm070002v
BindingDB Entry DOI: 10.7270/Q2PG1RFZ |
More data for this
Ligand-Target Pair | |
Neurotensin receptor type 1
(Homo sapiens (Human)) | BDBM466500

(US10799605, Example 5)Show SMILES COc1cccc(OC)c1-c1cc(nn1-c1ccc(cc1C(C)C)C(=O)N(C)CCCN(C)CCCN(C)C(=O)CN1CCN(CC(O)=O)CCN(CC(O)=O)CCN(CC(O)=O)CC1)C(=O)NC1(C2CC3CC(C2)CC1C3)C(O)=O |TLB:67:68:70:73.74.72,THB:75:76:70:73.74.72,75:73:70:68.76.77,78:68:70:73.74.72,68:69:76.77.75:72,68:76:70.69.74:72,(-13.42,6.92,;-12.33,5.83,;-12.73,4.34,;-14.22,3.94,;-14.62,2.45,;-13.53,1.36,;-12.04,1.76,;-10.95,.67,;-11.35,-.81,;-11.64,3.25,;-10.15,3.65,;-9.68,5.11,;-8.14,5.11,;-7.66,3.65,;-8.91,2.74,;-8.91,1.2,;-10.24,.43,;-10.24,-1.11,;-8.91,-1.88,;-7.57,-1.11,;-7.57,.43,;-6.24,1.2,;-4.91,.43,;-6.24,2.74,;-8.91,-3.42,;-10.24,-4.19,;-7.57,-4.19,;-7.57,-5.73,;-6.24,-3.42,;-4.91,-4.19,;-3.57,-3.42,;-2.24,-4.19,;-2.24,-5.73,;-.91,-3.42,;.43,-4.19,;1.76,-3.42,;3.1,-4.19,;3.1,-5.73,;4.43,-3.42,;4.43,-1.88,;5.97,-3.42,;7.3,-4.19,;8.1,-2.87,;9.68,-2.86,;10.4,-4.18,;11.17,-2.85,;10.4,-1.51,;8.86,-1.51,;11.17,-.18,;11.7,-4.95,;11.72,-6.52,;10.41,-7.28,;11.74,-8.05,;13.08,-7.28,;13.08,-5.74,;14.62,-7.28,;9.59,-8.6,;8.01,-8.61,;7.31,-7.29,;6.54,-8.62,;7.31,-9.95,;8.85,-9.95,;6.54,-11.29,;6.03,-6.49,;6.01,-4.91,;-7.05,6.2,;-7.45,7.69,;-5.56,5.8,;-4.47,6.89,;-5.12,8.29,;-6.38,9.17,;-5.87,10.62,;-4.48,11.29,;-3.16,10.64,;-3.8,9.24,;-2.64,9.19,;-3.96,8.35,;-5.23,9.22,;-3.14,6.12,;-3.14,4.58,;-1.81,6.89,)| Show InChI InChI=1S/C58H84N10O13/c1-38(2)44-32-41(13-14-46(44)68-47(54-48(80-6)11-8-12-49(54)81-7)33-45(60-68)55(76)59-58(57(78)79)42-28-39-27-40(30-42)31-43(58)29-39)56(77)63(5)18-10-16-61(3)15-9-17-62(4)50(69)34-64-19-21-65(35-51(70)71)23-25-67(37-53(74)75)26-24-66(22-20-64)36-52(72)73/h8,11-14,32-33,38-40,42-43H,9-10,15-31,34-37H2,1-7H3,(H,59,76)(H,70,71)(H,72,73)(H,74,75)(H,78,79) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
3B Pharmaceuticals GMBH
US Patent
| Assay Description
Ca2+ ions are usually kept at nanomolar levels in the cytosol of cells, and act in a number of signal transduction pathways as second messengers. Man... |
US Patent US10799605 (2020)
BindingDB Entry DOI: 10.7270/Q2M32ZTB |
More data for this
Ligand-Target Pair | |
Integrin alpha-V/beta-5
(Homo sapiens (Human)) | BDBM50423720
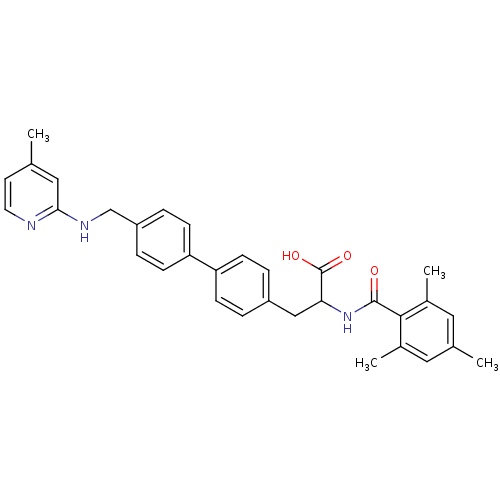
(CHEMBL606866)Show SMILES Cc1ccnc(NCc2ccc(cc2)-c2ccc(CC(NC(=O)c3c(C)cc(C)cc3C)C(O)=O)cc2)c1 Show InChI InChI=1S/C32H33N3O3/c1-20-13-14-33-29(17-20)34-19-25-7-11-27(12-8-25)26-9-5-24(6-10-26)18-28(32(37)38)35-31(36)30-22(3)15-21(2)16-23(30)4/h5-17,28H,18-19H2,1-4H3,(H,33,34)(H,35,36)(H,37,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Binding affinity to integrin alpha5beta5 receptor by ELISA |
Bioorg Med Chem Lett 20: 380-2 (2010)
Article DOI: 10.1016/j.bmcl.2009.10.073
BindingDB Entry DOI: 10.7270/Q2M61MKQ |
More data for this
Ligand-Target Pair | |
Integrin alpha-5/beta-1
(Homo sapiens (Human)) | BDBM50423732

(CHEMBL590389)Show SMILES CCc1cc(F)cc(C)c1C(=O)N[C@@H](Cc1ccc(cc1)N1CCC(CNc2ccccn2)CC1)C(O)=O |r| Show InChI InChI=1S/C30H35FN4O3/c1-3-23-18-24(31)16-20(2)28(23)29(36)34-26(30(37)38)17-21-7-9-25(10-8-21)35-14-11-22(12-15-35)19-33-27-6-4-5-13-32-27/h4-10,13,16,18,22,26H,3,11-12,14-15,17,19H2,1-2H3,(H,32,33)(H,34,36)(H,37,38)/t26-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Jerini AG
Curated by ChEMBL
| Assay Description
Inhibition of integrin alpha5beta1 receptor-mediated HEK293 cell adhesion |
Bioorg Med Chem Lett 20: 65-8 (2010)
Article DOI: 10.1016/j.bmcl.2009.11.030
BindingDB Entry DOI: 10.7270/Q2BR8TGJ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data