 Found 425 hits with Last Name = 'palmer' and Initial = 'e'
Found 425 hits with Last Name = 'palmer' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50373939
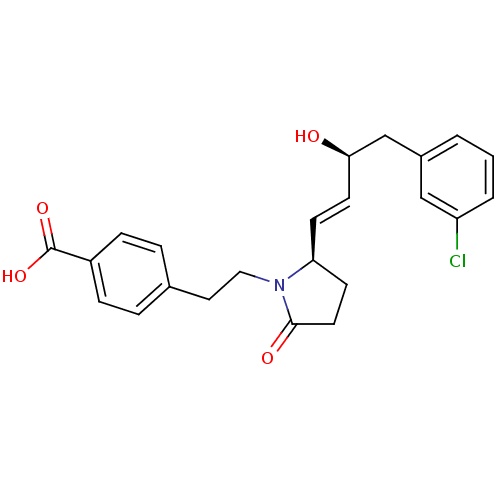
(CHEMBL258332)Show SMILES O[C@@H](Cc1cccc(Cl)c1)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H24ClNO4/c24-19-3-1-2-17(14-19)15-21(26)10-8-20-9-11-22(27)25(20)13-12-16-4-6-18(7-5-16)23(28)29/h1-8,10,14,20-21,26H,9,11-13,15H2,(H,28,29)/b10-8+/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50373942
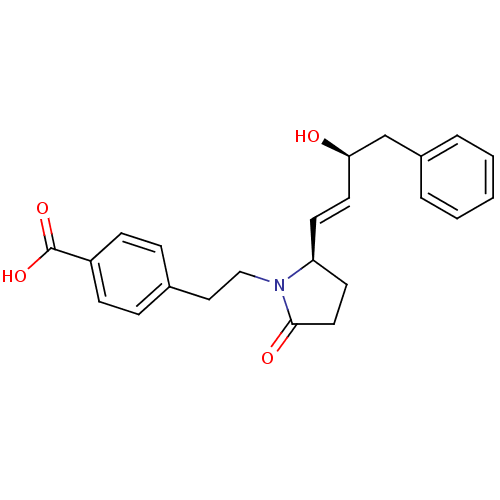
(CHEMBL272276)Show SMILES O[C@@H](Cc1ccccc1)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H25NO4/c25-21(16-18-4-2-1-3-5-18)12-10-20-11-13-22(26)24(20)15-14-17-6-8-19(9-7-17)23(27)28/h1-10,12,20-21,25H,11,13-16H2,(H,27,28)/b12-10+/t20-,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4323-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.025
BindingDB Entry DOI: 10.7270/Q2RF5VVC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP3 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50138823
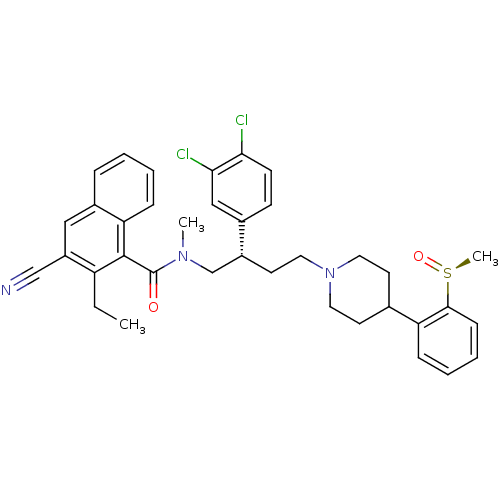
(3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...)Show SMILES CCc1c(cc2ccccc2c1C(=O)N(C)C[C@@H](CCN1CCC(CC1)c1ccccc1[S@](C)=O)c1ccc(Cl)c(Cl)c1)C#N Show InChI InChI=1S/C37H39Cl2N3O2S/c1-4-30-29(23-40)21-27-9-5-6-11-32(27)36(30)37(43)41(2)24-28(26-13-14-33(38)34(39)22-26)17-20-42-18-15-25(16-19-42)31-10-7-8-12-35(31)45(3)44/h5-14,21-22,25,28H,4,15-20,24H2,1-3H3/t28-,45+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Tachykinin receptor 1 was determined by using [3H]-SP as a radioligand |
J Med Chem 47: 519-29 (2004)
Article DOI: 10.1021/jm030197g
BindingDB Entry DOI: 10.7270/Q2FJ2G6D |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human prostaglandin EP4 receptor |
Bioorg Med Chem Lett 17: 6572-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.074
BindingDB Entry DOI: 10.7270/Q29887V9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4323-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.025
BindingDB Entry DOI: 10.7270/Q2RF5VVC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50373941
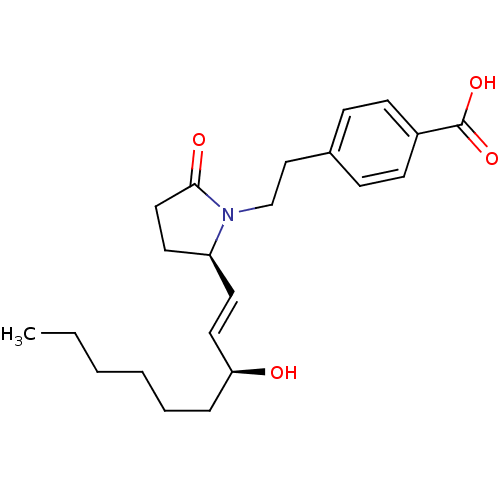
(CHEMBL257217)Show SMILES CCCCCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H31NO4/c1-2-3-4-5-6-20(24)13-11-19-12-14-21(25)23(19)16-15-17-7-9-18(10-8-17)22(26)27/h7-11,13,19-20,24H,2-6,12,14-16H2,1H3,(H,26,27)/b13-11+/t19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50213980

(4-(2-((R)-2-((S)-3-hydroxyhept-1-enyl)-5-oxopyrrol...)Show SMILES CCCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C20H27NO4/c1-2-3-4-18(22)11-9-17-10-12-19(23)21(17)14-13-15-5-7-16(8-6-15)20(24)25/h5-9,11,17-18,22H,2-4,10,12-14H2,1H3,(H,24,25)/b11-9+/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50138823

(3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...)Show SMILES CCc1c(cc2ccccc2c1C(=O)N(C)C[C@@H](CCN1CCC(CC1)c1ccccc1[S@](C)=O)c1ccc(Cl)c(Cl)c1)C#N Show InChI InChI=1S/C37H39Cl2N3O2S/c1-4-30-29(23-40)21-27-9-5-6-11-32(27)36(30)37(43)41(2)24-28(26-13-14-33(38)34(39)22-26)17-20-42-18-15-25(16-19-42)31-10-7-8-12-35(31)45(3)44/h5-14,21-22,25,28H,4,15-20,24H2,1-3H3/t28-,45+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Tachykinin receptor 1 was determined by using [3H]-SP as a radioligand |
J Med Chem 47: 519-29 (2004)
Article DOI: 10.1021/jm030197g
BindingDB Entry DOI: 10.7270/Q2FJ2G6D |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50373944
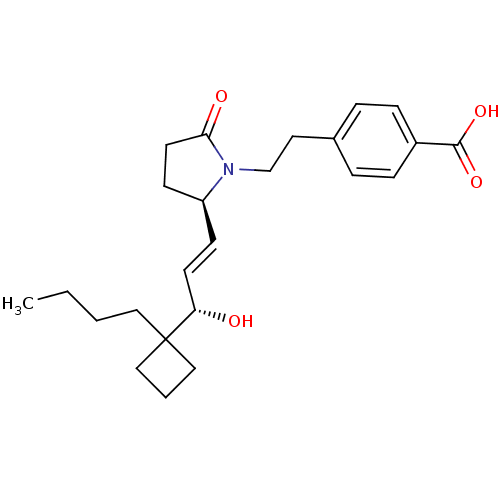
(CHEMBL272277)Show SMILES CCCCC1(CCC1)[C@@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H33NO4/c1-2-3-14-24(15-4-16-24)21(26)11-9-20-10-12-22(27)25(20)17-13-18-5-7-19(8-6-18)23(28)29/h5-9,11,20-21,26H,2-4,10,12-17H2,1H3,(H,28,29)/b11-9+/t20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50156547
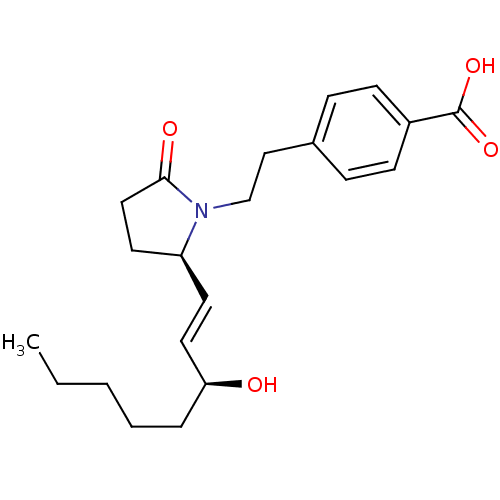
(4-(2-((R)-2-((S)-3-hydroxyoct-1-enyl)-5-oxopyrroli...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C21H29NO4/c1-2-3-4-5-19(23)12-10-18-11-13-20(24)22(18)15-14-16-6-8-17(9-7-16)21(25)26/h6-10,12,18-19,23H,2-5,11,13-15H2,1H3,(H,25,26)/b12-10+/t18-,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50213980

(4-(2-((R)-2-((S)-3-hydroxyhept-1-enyl)-5-oxopyrrol...)Show SMILES CCCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C20H27NO4/c1-2-3-4-18(22)11-9-17-10-12-19(23)21(17)14-13-15-5-7-16(8-6-15)20(24)25/h5-9,11,17-18,22H,2-4,10,12-14H2,1H3,(H,24,25)/b11-9+/t17-,18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4323-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.025
BindingDB Entry DOI: 10.7270/Q2RF5VVC |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50138823

(3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...)Show SMILES CCc1c(cc2ccccc2c1C(=O)N(C)C[C@@H](CCN1CCC(CC1)c1ccccc1[S@](C)=O)c1ccc(Cl)c(Cl)c1)C#N Show InChI InChI=1S/C37H39Cl2N3O2S/c1-4-30-29(23-40)21-27-9-5-6-11-32(27)36(30)37(43)41(2)24-28(26-13-14-33(38)34(39)22-26)17-20-42-18-15-25(16-19-42)31-10-7-8-12-35(31)45(3)44/h5-14,21-22,25,28H,4,15-20,24H2,1-3H3/t28-,45+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Tachykinin receptor 1 was determined by using [3H]-SP as a radioligand |
J Med Chem 47: 519-29 (2004)
Article DOI: 10.1021/jm030197g
BindingDB Entry DOI: 10.7270/Q2FJ2G6D |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50138823

(3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...)Show SMILES CCc1c(cc2ccccc2c1C(=O)N(C)C[C@@H](CCN1CCC(CC1)c1ccccc1[S@](C)=O)c1ccc(Cl)c(Cl)c1)C#N Show InChI InChI=1S/C37H39Cl2N3O2S/c1-4-30-29(23-40)21-27-9-5-6-11-32(27)36(30)37(43)41(2)24-28(26-13-14-33(38)34(39)22-26)17-20-42-18-15-25(16-19-42)31-10-7-8-12-35(31)45(3)44/h5-14,21-22,25,28H,4,15-20,24H2,1-3H3/t28-,45+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Tachykinin receptor 1 was determined by using [3H]-SP as a radioligand |
J Med Chem 47: 519-29 (2004)
Article DOI: 10.1021/jm030197g
BindingDB Entry DOI: 10.7270/Q2FJ2G6D |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50213978
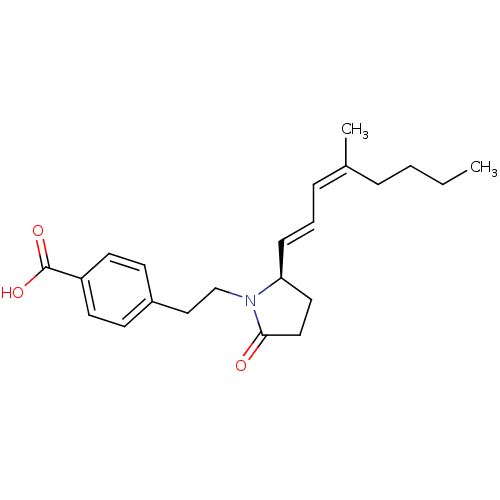
(4-(2-((R)-2-(4-methylocta-1,3-dienyl)-5-oxopyrroli...)Show SMILES CCCC\C(C)=C/C=C/[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H29NO3/c1-3-4-6-17(2)7-5-8-20-13-14-21(24)23(20)16-15-18-9-11-19(12-10-18)22(25)26/h5,7-12,20H,3-4,6,13-16H2,1-2H3,(H,25,26)/b8-5+,17-7-/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4323-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.025
BindingDB Entry DOI: 10.7270/Q2RF5VVC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4323-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.025
BindingDB Entry DOI: 10.7270/Q2RF5VVC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human prostaglandin EP2 receptor |
Bioorg Med Chem Lett 17: 6572-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.074
BindingDB Entry DOI: 10.7270/Q29887V9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 4.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Melanocortin receptor 3
(Homo sapiens (Human)) | BDBM50230827
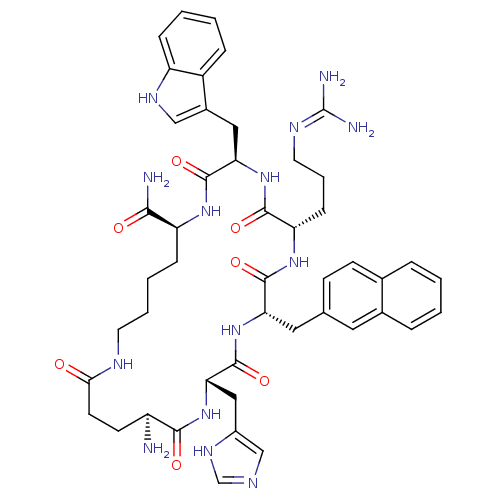
(CHEMBL253364 | MK-9)Show SMILES N[C@H]1CCC(=O)NCCCC[C@H](NC(=O)[C@@H](Cc2c[nH]c3ccccc23)NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@H](Cc2ccc3ccccc3c2)NC(=O)[C@H](Cc2cnc[nH]2)NC1=O)C(N)=O |wU:15.15,11.70,1.0,wD:55.59,29.31,40.42,(27.17,-1.13,;27.07,-2.65,;28.33,-3.52,;29.72,-2.86,;30.99,-3.74,;30.88,-5.27,;34.1,-3.27,;34.1,-4.94,;32.82,-5.79,;32.91,-7.32,;31.62,-8.17,;31.71,-9.71,;30.43,-10.56,;29.05,-9.87,;28.96,-8.33,;27.77,-10.72,;27.85,-12.26,;29.23,-12.95,;30.52,-12.09,;31.55,-13.24,;31.01,-14.69,;31.57,-16.11,;30.62,-17.3,;29.09,-17.08,;28.54,-15.65,;29.5,-14.45,;26.39,-10.03,;25.1,-10.87,;25.19,-12.41,;23.31,-10.55,;22.46,-11.84,;23.15,-13.22,;22.31,-14.51,;22.98,-15.89,;22.14,-17.18,;22.82,-18.57,;20.6,-17.1,;23.48,-9.02,;22.63,-7.74,;24.03,-7.12,;21.38,-6.85,;19.97,-7.47,;19.82,-9.01,;21.06,-9.91,;20.91,-11.42,;19.5,-12.06,;19.36,-13.58,;17.96,-14.22,;16.7,-13.32,;16.85,-11.79,;18.26,-11.15,;18.41,-9.62,;21.5,-5.31,;22.89,-4.64,;24.16,-5.51,;23.01,-3.11,;21.74,-2.23,;20.35,-2.9,;20.06,-4.41,;18.55,-4.61,;17.87,-3.22,;18.99,-2.17,;24.4,-2.45,;25.68,-3.32,;25.55,-4.85,;33.09,-10.4,;33.18,-11.94,;34.38,-9.55,)| Show InChI InChI=1S/C47H60N14O7/c48-33-16-17-40(62)53-18-6-5-12-35(41(49)63)57-45(67)38(22-30-24-55-34-11-4-3-10-32(30)34)61-43(65)36(13-7-19-54-47(50)51)58-44(66)37(21-27-14-15-28-8-1-2-9-29(28)20-27)60-46(68)39(59-42(33)64)23-31-25-52-26-56-31/h1-4,8-11,14-15,20,24-26,33,35-39,55H,5-7,12-13,16-19,21-23,48H2,(H2,49,63)(H,52,56)(H,53,62)(H,57,67)(H,58,66)(H,59,64)(H,60,68)(H,61,65)(H4,50,51,54)/t33-,35-,36-,37-,38+,39-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Arizona
Curated by ChEMBL
| Assay Description
Displacement of [125I][Nle4,D-Phe7]alpha-MSH from human MC3 receptor expressed in HEK293 cells |
J Med Chem 51: 187-95 (2008)
Article DOI: 10.1021/jm070461w
BindingDB Entry DOI: 10.7270/Q2V125N2 |
More data for this
Ligand-Target Pair | |
Substance-P receptor
(Homo sapiens (Human)) | BDBM50138823

(3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...)Show SMILES CCc1c(cc2ccccc2c1C(=O)N(C)C[C@@H](CCN1CCC(CC1)c1ccccc1[S@](C)=O)c1ccc(Cl)c(Cl)c1)C#N Show InChI InChI=1S/C37H39Cl2N3O2S/c1-4-30-29(23-40)21-27-9-5-6-11-32(27)36(30)37(43)41(2)24-28(26-13-14-33(38)34(39)22-26)17-20-42-18-15-25(16-19-42)31-10-7-8-12-35(31)45(3)44/h5-14,21-22,25,28H,4,15-20,24H2,1-3H3/t28-,45+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Tachykinin receptor 1 was determined by using [3H]-SP as a radioligand |
J Med Chem 47: 519-29 (2004)
Article DOI: 10.1021/jm030197g
BindingDB Entry DOI: 10.7270/Q2FJ2G6D |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50373940
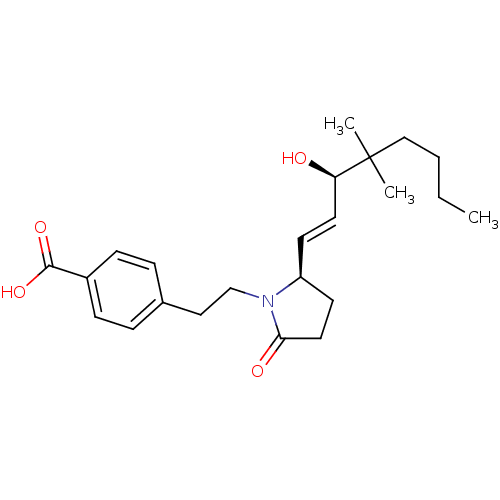
(CHEMBL257658)Show SMILES CCCCC(C)(C)[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C23H33NO4/c1-4-5-15-23(2,3)20(25)12-10-19-11-13-21(26)24(19)16-14-17-6-8-18(9-7-17)22(27)28/h6-10,12,19-20,25H,4-5,11,13-16H2,1-3H3,(H,27,28)/b12-10+/t19-,20+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 9.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP1 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4323-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.025
BindingDB Entry DOI: 10.7270/Q2RF5VVC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50224577
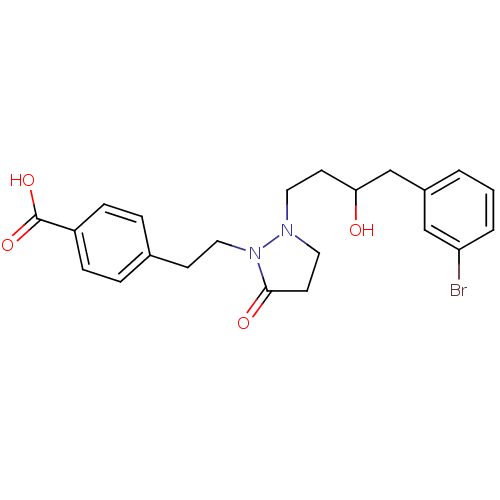
(4-(2-(2-(4-(3-bromophenyl)-3-hydroxybutyl)-5-oxopy...)Show SMILES OC(CCN1CCC(=O)N1CCc1ccc(cc1)C(O)=O)Cc1cccc(Br)c1 Show InChI InChI=1S/C22H25BrN2O4/c23-19-3-1-2-17(14-19)15-20(26)9-11-24-12-10-21(27)25(24)13-8-16-4-6-18(7-5-16)22(28)29/h1-7,14,20,26H,8-13,15H2,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human prostaglandin EP4 receptor |
Bioorg Med Chem Lett 17: 6572-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.074
BindingDB Entry DOI: 10.7270/Q29887V9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50224575
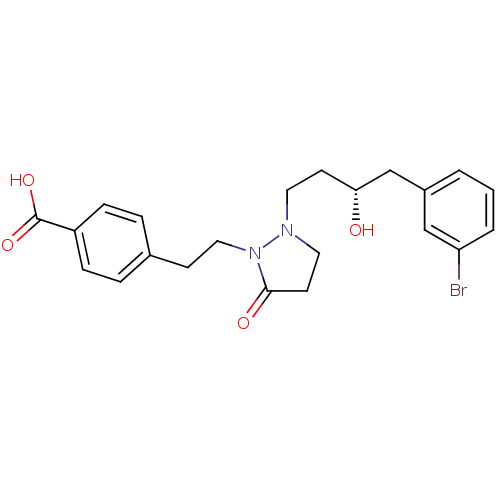
((S)-4-(2-(2-(4-(3-bromophenyl)-3-hydroxybutyl)-5-o...)Show SMILES O[C@H](CCN1CCC(=O)N1CCc1ccc(cc1)C(O)=O)Cc1cccc(Br)c1 Show InChI InChI=1S/C22H25BrN2O4/c23-19-3-1-2-17(14-19)15-20(26)9-11-24-12-10-21(27)25(24)13-8-16-4-6-18(7-5-16)22(28)29/h1-7,14,20,26H,8-13,15H2,(H,28,29)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human prostaglandin EP4 receptor |
Bioorg Med Chem Lett 17: 6572-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.074
BindingDB Entry DOI: 10.7270/Q29887V9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50224572

(4-(2-(2-(4-(3-chlorophenyl)-3-hydroxybutyl)-5-oxop...)Show SMILES OC(CCN1CCC(=O)N1CCc1ccc(cc1)C(O)=O)Cc1cccc(Cl)c1 Show InChI InChI=1S/C22H25ClN2O4/c23-19-3-1-2-17(14-19)15-20(26)9-11-24-12-10-21(27)25(24)13-8-16-4-6-18(7-5-16)22(28)29/h1-7,14,20,26H,8-13,15H2,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human prostaglandin EP4 receptor |
Bioorg Med Chem Lett 17: 6572-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.074
BindingDB Entry DOI: 10.7270/Q29887V9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50373944

(CHEMBL272277)Show SMILES CCCCC1(CCC1)[C@@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H33NO4/c1-2-3-14-24(15-4-16-24)21(26)11-9-20-10-12-22(27)25(20)17-13-18-5-7-19(8-6-18)23(28)29/h5-9,11,20-21,26H,2-4,10,12-17H2,1H3,(H,28,29)/b11-9+/t20-,21-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50138823

(3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...)Show SMILES CCc1c(cc2ccccc2c1C(=O)N(C)C[C@@H](CCN1CCC(CC1)c1ccccc1[S@](C)=O)c1ccc(Cl)c(Cl)c1)C#N Show InChI InChI=1S/C37H39Cl2N3O2S/c1-4-30-29(23-40)21-27-9-5-6-11-32(27)36(30)37(43)41(2)24-28(26-13-14-33(38)34(39)22-26)17-20-42-18-15-25(16-19-42)31-10-7-8-12-35(31)45(3)44/h5-14,21-22,25,28H,4,15-20,24H2,1-3H3/t28-,45+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Tachykinin receptor 2 was determined by using [125I]-NKA as a radioligand |
J Med Chem 47: 519-29 (2004)
Article DOI: 10.1021/jm030197g
BindingDB Entry DOI: 10.7270/Q2FJ2G6D |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50138823

(3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...)Show SMILES CCc1c(cc2ccccc2c1C(=O)N(C)C[C@@H](CCN1CCC(CC1)c1ccccc1[S@](C)=O)c1ccc(Cl)c(Cl)c1)C#N Show InChI InChI=1S/C37H39Cl2N3O2S/c1-4-30-29(23-40)21-27-9-5-6-11-32(27)36(30)37(43)41(2)24-28(26-13-14-33(38)34(39)22-26)17-20-42-18-15-25(16-19-42)31-10-7-8-12-35(31)45(3)44/h5-14,21-22,25,28H,4,15-20,24H2,1-3H3/t28-,45+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Tachykinin receptor 2 was determined by using [125I]-NKA as a radioligand |
J Med Chem 47: 519-29 (2004)
Article DOI: 10.1021/jm030197g
BindingDB Entry DOI: 10.7270/Q2FJ2G6D |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50138823

(3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...)Show SMILES CCc1c(cc2ccccc2c1C(=O)N(C)C[C@@H](CCN1CCC(CC1)c1ccccc1[S@](C)=O)c1ccc(Cl)c(Cl)c1)C#N Show InChI InChI=1S/C37H39Cl2N3O2S/c1-4-30-29(23-40)21-27-9-5-6-11-32(27)36(30)37(43)41(2)24-28(26-13-14-33(38)34(39)22-26)17-20-42-18-15-25(16-19-42)31-10-7-8-12-35(31)45(3)44/h5-14,21-22,25,28H,4,15-20,24H2,1-3H3/t28-,45+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Tachykinin receptor 2 was determined by using [125I]-NKA as a radioligand |
J Med Chem 47: 519-29 (2004)
Article DOI: 10.1021/jm030197g
BindingDB Entry DOI: 10.7270/Q2FJ2G6D |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50138823

(3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...)Show SMILES CCc1c(cc2ccccc2c1C(=O)N(C)C[C@@H](CCN1CCC(CC1)c1ccccc1[S@](C)=O)c1ccc(Cl)c(Cl)c1)C#N Show InChI InChI=1S/C37H39Cl2N3O2S/c1-4-30-29(23-40)21-27-9-5-6-11-32(27)36(30)37(43)41(2)24-28(26-13-14-33(38)34(39)22-26)17-20-42-18-15-25(16-19-42)31-10-7-8-12-35(31)45(3)44/h5-14,21-22,25,28H,4,15-20,24H2,1-3H3/t28-,45+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Tachykinin receptor 2 was determined by using [125I]-NKA as a radioligand |
J Med Chem 47: 519-29 (2004)
Article DOI: 10.1021/jm030197g
BindingDB Entry DOI: 10.7270/Q2FJ2G6D |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50213977
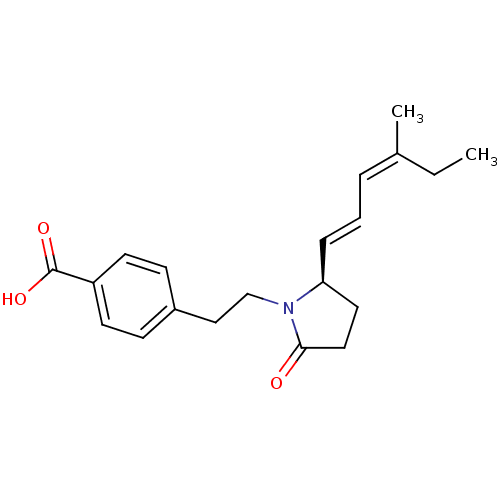
(4-(2-((R)-2-(4-methylhexa-1,3-dienyl)-5-oxopyrroli...)Show SMILES CC\C(C)=C/C=C/[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C20H25NO3/c1-3-15(2)5-4-6-18-11-12-19(22)21(18)14-13-16-7-9-17(10-8-16)20(23)24/h4-10,18H,3,11-14H2,1-2H3,(H,23,24)/b6-4+,15-5-/t18-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4323-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.025
BindingDB Entry DOI: 10.7270/Q2RF5VVC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50213981
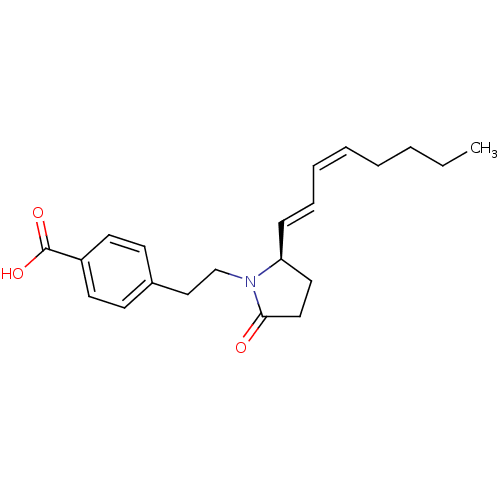
(4-(2-((R)-2-(octa-1,3-dienyl)-5-oxopyrrolidin-1-yl...)Show SMILES CCCC\C=C/C=C/[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C21H27NO3/c1-2-3-4-5-6-7-8-19-13-14-20(23)22(19)16-15-17-9-11-18(12-10-17)21(24)25/h5-12,19H,2-4,13-16H2,1H3,(H,24,25)/b6-5-,8-7+/t19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4323-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.025
BindingDB Entry DOI: 10.7270/Q2RF5VVC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50224570
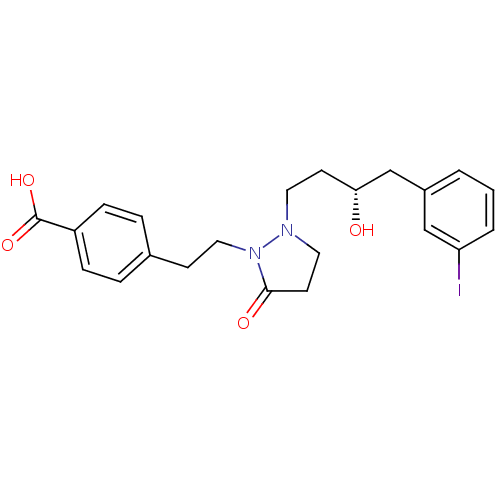
((S)-4-(2-(2-(3-hydroxy-4-(3-iodophenyl)butyl)-5-ox...)Show SMILES O[C@H](CCN1CCC(=O)N1CCc1ccc(cc1)C(O)=O)Cc1cccc(I)c1 Show InChI InChI=1S/C22H25IN2O4/c23-19-3-1-2-17(14-19)15-20(26)9-11-24-12-10-21(27)25(24)13-8-16-4-6-18(7-5-16)22(28)29/h1-7,14,20,26H,8-13,15H2,(H,28,29)/t20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 26 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human prostaglandin EP4 receptor |
Bioorg Med Chem Lett 17: 6572-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.074
BindingDB Entry DOI: 10.7270/Q29887V9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50373945

(CHEMBL271488)Show SMILES CCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C19H25NO4/c1-2-3-17(21)10-8-16-9-11-18(22)20(16)13-12-14-4-6-15(7-5-14)19(23)24/h4-8,10,16-17,21H,2-3,9,11-13H2,1H3,(H,23,24)/b10-8+/t16-,17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE4 from human EP4 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50224574

(4-(2-(2-(3-hydroxy-4-(3-iodophenyl)butyl)-5-oxopyr...)Show SMILES OC(CCN1CCC(=O)N1CCc1ccc(cc1)C(O)=O)Cc1cccc(I)c1 Show InChI InChI=1S/C22H25IN2O4/c23-19-3-1-2-17(14-19)15-20(26)9-11-24-12-10-21(27)25(24)13-8-16-4-6-18(7-5-16)22(28)29/h1-7,14,20,26H,8-13,15H2,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human prostaglandin EP4 receptor |
Bioorg Med Chem Lett 17: 6572-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.074
BindingDB Entry DOI: 10.7270/Q29887V9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50213974
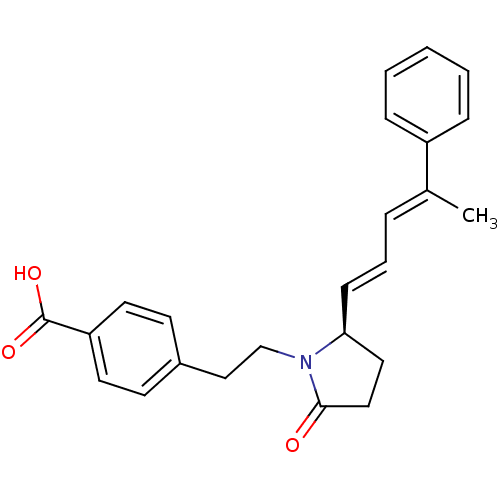
(4-(2-((R)-2-oxo-5-(4-phenylpenta-1,3-dienyl)pyrrol...)Show SMILES C\C(=C/C=C/[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O)c1ccccc1 Show InChI InChI=1S/C24H25NO3/c1-18(20-7-3-2-4-8-20)6-5-9-22-14-15-23(26)25(22)17-16-19-10-12-21(13-11-19)24(27)28/h2-13,22H,14-17H2,1H3,(H,27,28)/b9-5+,18-6+/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4323-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.025
BindingDB Entry DOI: 10.7270/Q2RF5VVC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50213979

(4-(2-((R)-2-(3-methylocta-1,3-dienyl)-5-oxopyrroli...)Show SMILES CCCC\C=C(/C)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H29NO3/c1-3-4-5-6-17(2)7-12-20-13-14-21(24)23(20)16-15-18-8-10-19(11-9-18)22(25)26/h6-12,20H,3-5,13-16H2,1-2H3,(H,25,26)/b12-7+,17-6+/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4323-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.025
BindingDB Entry DOI: 10.7270/Q2RF5VVC |
More data for this
Ligand-Target Pair | |
Substance-K receptor
(Homo sapiens (Human)) | BDBM50138823

(3-Cyano-2-ethyl-naphthalene-1-carboxylic acid ((S)...)Show SMILES CCc1c(cc2ccccc2c1C(=O)N(C)C[C@@H](CCN1CCC(CC1)c1ccccc1[S@](C)=O)c1ccc(Cl)c(Cl)c1)C#N Show InChI InChI=1S/C37H39Cl2N3O2S/c1-4-30-29(23-40)21-27-9-5-6-11-32(27)36(30)37(43)41(2)24-28(26-13-14-33(38)34(39)22-26)17-20-42-18-15-25(16-19-42)31-10-7-8-12-35(31)45(3)44/h5-14,21-22,25,28H,4,15-20,24H2,1-3H3/t28-,45+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Pharmaceuticals LP
Curated by ChEMBL
| Assay Description
In vitro binding affinity towards human Tachykinin receptor 2 was determined by using [125I]-NKA as a radioligand |
J Med Chem 47: 519-29 (2004)
Article DOI: 10.1021/jm030197g
BindingDB Entry DOI: 10.7270/Q2FJ2G6D |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50224586
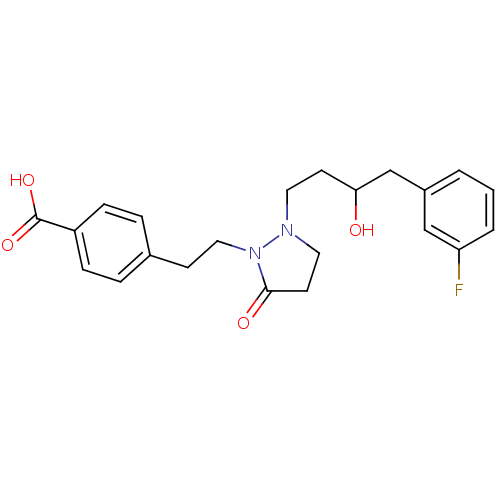
(4-(2-(2-(4-(3-fluorophenyl)-3-hydroxybutyl)-5-oxop...)Show SMILES OC(CCN1CCC(=O)N1CCc1ccc(cc1)C(O)=O)Cc1cccc(F)c1 Show InChI InChI=1S/C22H25FN2O4/c23-19-3-1-2-17(14-19)15-20(26)9-11-24-12-10-21(27)25(24)13-8-16-4-6-18(7-5-16)22(28)29/h1-7,14,20,26H,8-13,15H2,(H,28,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human prostaglandin EP4 receptor |
Bioorg Med Chem Lett 17: 6572-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.074
BindingDB Entry DOI: 10.7270/Q29887V9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50224583
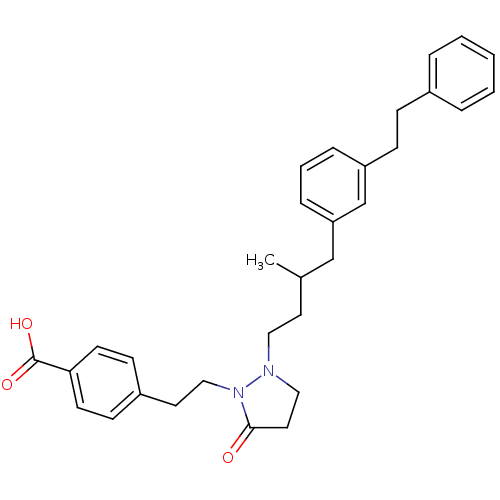
(4-(2-(2-(3-methyl-4-(3-phenethylphenyl)butyl)-5-ox...)Show SMILES CC(CCN1CCC(=O)N1CCc1ccc(cc1)C(O)=O)Cc1cccc(CCc2ccccc2)c1 Show InChI InChI=1S/C31H36N2O3/c1-24(22-28-9-5-8-27(23-28)11-10-25-6-3-2-4-7-25)16-19-32-20-18-30(34)33(32)21-17-26-12-14-29(15-13-26)31(35)36/h2-9,12-15,23-24H,10-11,16-22H2,1H3,(H,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human prostaglandin EP4 receptor |
Bioorg Med Chem Lett 17: 6572-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.074
BindingDB Entry DOI: 10.7270/Q29887V9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50213976
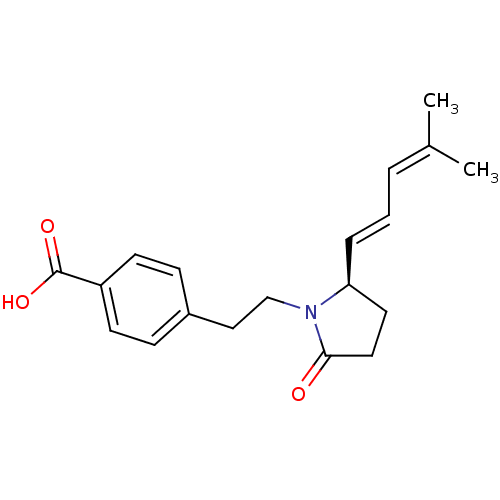
((R)-4-(2-(2-(4-methylpenta-1,3-dienyl)-5-oxopyrrol...)Show SMILES [#6]\[#6](-[#6])=[#6]/[#6]=[#6]/[#6@H]-1-[#6]-[#6]-[#6](=O)-[#7]1-[#6]-[#6]-c1ccc(cc1)-[#6](-[#8])=O Show InChI InChI=1S/C19H23NO3/c1-14(2)4-3-5-17-10-11-18(21)20(17)13-12-15-6-8-16(9-7-15)19(22)23/h3-9,17H,10-13H2,1-2H3,(H,22,23)/b5-3+/t17-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP4 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4323-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.025
BindingDB Entry DOI: 10.7270/Q2RF5VVC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50373941

(CHEMBL257217)Show SMILES CCCCCC[C@H](O)\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H31NO4/c1-2-3-4-5-6-20(24)13-11-19-12-14-21(25)23(19)16-15-17-7-9-18(10-8-17)22(26)27/h7-11,13,19-20,24H,2-6,12,14-16H2,1H3,(H,26,27)/b13-11+/t19-,20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 54 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute, Inc.
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2 receptor |
Bioorg Med Chem Lett 18: 821-4 (2008)
Article DOI: 10.1016/j.bmcl.2007.11.020
BindingDB Entry DOI: 10.7270/Q2W096SW |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50224584
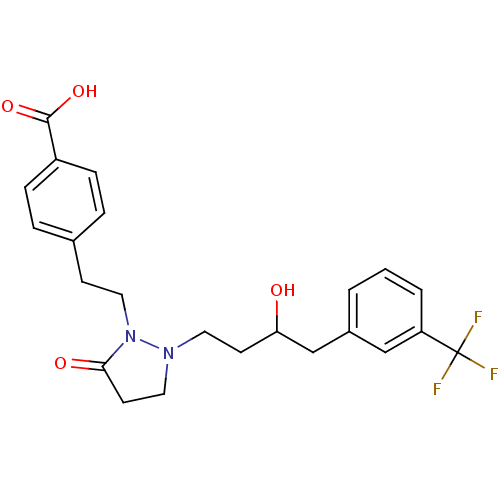
(4-(2-(2-(3-hydroxy-4-(3-(trifluoromethyl)phenyl)bu...)Show SMILES OC(CCN1CCC(=O)N1CCc1ccc(cc1)C(O)=O)Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H25F3N2O4/c24-23(25,26)19-3-1-2-17(14-19)15-20(29)9-11-27-12-10-21(30)28(27)13-8-16-4-6-18(7-5-16)22(31)32/h1-7,14,20,29H,8-13,15H2,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human prostaglandin EP4 receptor |
Bioorg Med Chem Lett 17: 6572-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.074
BindingDB Entry DOI: 10.7270/Q29887V9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50224581
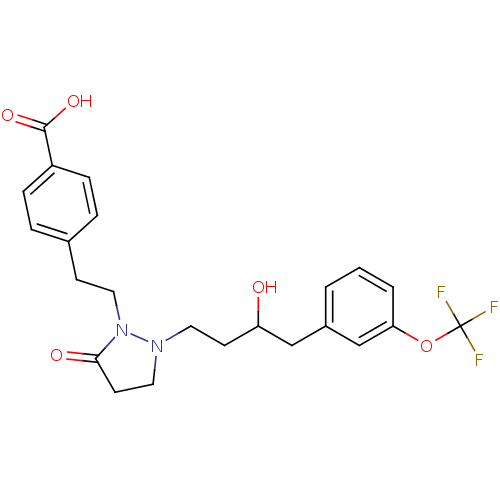
(4-(2-(2-(3-hydroxy-4-(3-(trifluoromethoxy)phenyl)b...)Show SMILES OC(CCN1CCC(=O)N1CCc1ccc(cc1)C(O)=O)Cc1cccc(OC(F)(F)F)c1 Show InChI InChI=1S/C23H25F3N2O5/c24-23(25,26)33-20-3-1-2-17(15-20)14-19(29)9-11-27-12-10-21(30)28(27)13-8-16-4-6-18(7-5-16)22(31)32/h1-7,15,19,29H,8-14H2,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human prostaglandin EP4 receptor |
Bioorg Med Chem Lett 17: 6572-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.074
BindingDB Entry DOI: 10.7270/Q29887V9 |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50213978

(4-(2-((R)-2-(4-methylocta-1,3-dienyl)-5-oxopyrroli...)Show SMILES CCCC\C(C)=C/C=C/[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C22H29NO3/c1-3-4-6-17(2)7-5-8-20-13-14-21(24)23(20)16-15-18-9-11-19(12-10-18)22(25)26/h5,7-12,20H,3-4,6,13-16H2,1-2H3,(H,25,26)/b8-5+,17-7-/t20-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4323-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.025
BindingDB Entry DOI: 10.7270/Q2RF5VVC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP2 subtype
(Homo sapiens (Human)) | BDBM50213983

(4-(2-((R)-2-(4-(cyclopentylmethyl)penta-1,3-dienyl...)Show SMILES C\C(CC1CCCC1)=C\C=C\[C@H]1CCC(=O)N1CCc1ccc(cc1)C(O)=O Show InChI InChI=1S/C24H31NO3/c1-18(17-20-6-2-3-7-20)5-4-8-22-13-14-23(26)25(22)16-15-19-9-11-21(12-10-19)24(27)28/h4-5,8-12,20,22H,2-3,6-7,13-17H2,1H3,(H,27,28)/b8-4+,18-5-/t22-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research Institute
Curated by ChEMBL
| Assay Description
Displacement of [3H]PGE2 from human EP2 receptor expressed in HEK293 cells |
Bioorg Med Chem Lett 17: 4323-7 (2007)
Article DOI: 10.1016/j.bmcl.2007.05.025
BindingDB Entry DOI: 10.7270/Q2RF5VVC |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM50224580
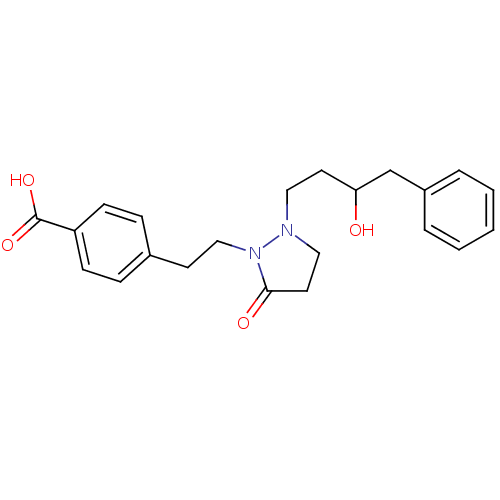
(4-(2-(2-(3-hydroxy-4-phenylbutyl)-5-oxopyrazolidin...)Show SMILES OC(CCN1CCC(=O)N1CCc1ccc(cc1)C(O)=O)Cc1ccccc1 Show InChI InChI=1S/C22H26N2O4/c25-20(16-18-4-2-1-3-5-18)11-13-23-14-12-21(26)24(23)15-10-17-6-8-19(9-7-17)22(27)28/h1-9,20,25H,10-16H2,(H,27,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
EMD-Serono Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity at human prostaglandin EP4 receptor |
Bioorg Med Chem Lett 17: 6572-5 (2007)
Article DOI: 10.1016/j.bmcl.2007.09.074
BindingDB Entry DOI: 10.7270/Q29887V9 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data