 Found 303 hits with Last Name = 'patel' and Initial = 'dv'
Found 303 hits with Last Name = 'patel' and Initial = 'dv' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor X
(Homo sapiens (Human)) | BDBM12657
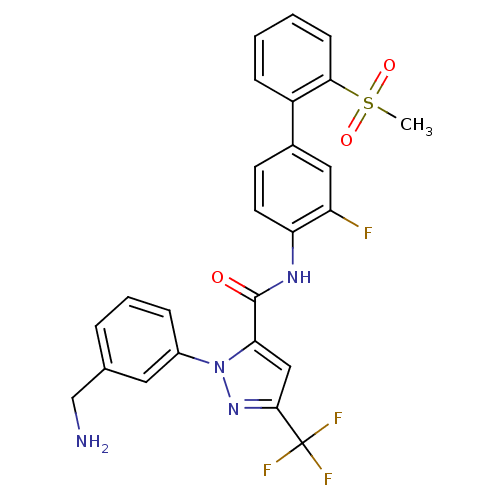
(1-[3-(Aminomethyl)phenyl]-N-[3-fluoro-2-(methylsul...)Show SMILES CS(=O)(=O)c1ccccc1-c1ccc(NC(=O)c2cc(nn2-c2cccc(CN)c2)C(F)(F)F)c(F)c1 Show InChI InChI=1S/C25H20F4N4O3S/c1-37(35,36)22-8-3-2-7-18(22)16-9-10-20(19(26)12-16)31-24(34)21-13-23(25(27,28)29)32-33(21)17-6-4-5-15(11-17)14-30/h2-13H,14,30H2,1H3,(H,31,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
| 0.150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50614408
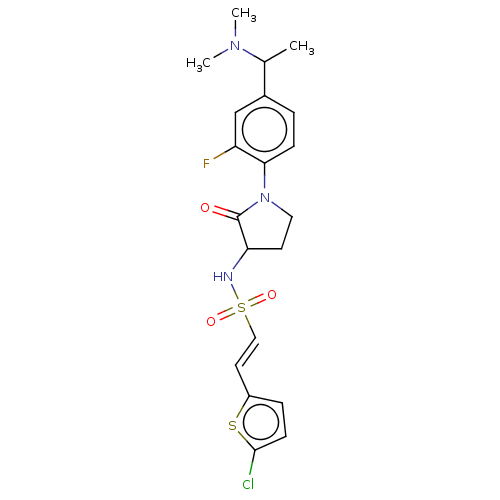
(CHEMBL5269594)Show SMILES CC(N(C)C)c1ccc(N2CCC(NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50096104

(2-(3-Aminomethyl-phenyl)-5-trifluoromethyl-2H-pyra...)Show SMILES NCc1cccc(c1)-n1nc(cc1C(=O)Nc1ccc(cc1F)-c1ccccc1S(N)(=O)=O)C(F)(F)F Show InChI InChI=1S/C24H19F4N5O3S/c25-18-11-15(17-6-1-2-7-21(17)37(30,35)36)8-9-19(18)31-23(34)20-12-22(24(26,27)28)32-33(20)16-5-3-4-14(10-16)13-29/h1-12H,13,29H2,(H,31,34)(H2,30,35,36) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50614410
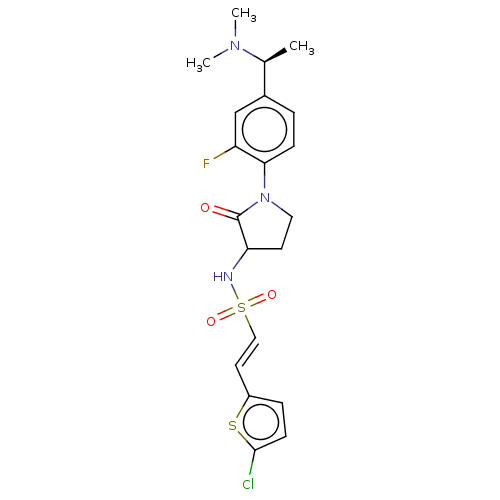
(CHEMBL5286872)Show SMILES C[C@H](N(C)C)c1ccc(N2CCC(NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50614403
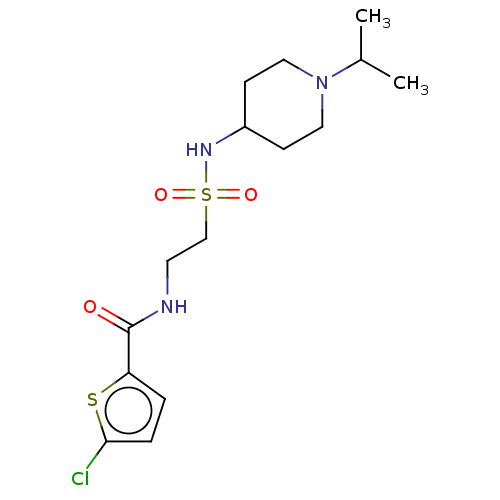
(CHEMBL5291366)Show SMILES CC(C)N1CCC(CC1)NS(=O)(=O)CCNC(=O)c1ccc(Cl)s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50614404
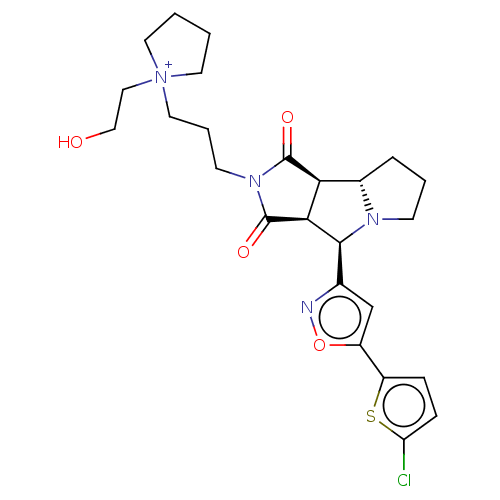
(CHEMBL5266057)Show SMILES [H][C@@]12CCCN1[C@@H](c1cc(on1)-c1ccc(Cl)s1)[C@@]1([H])C(=O)N(CCC[N+]3(CCO)CCCC3)C(=O)[C@@]21[H] |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50614409
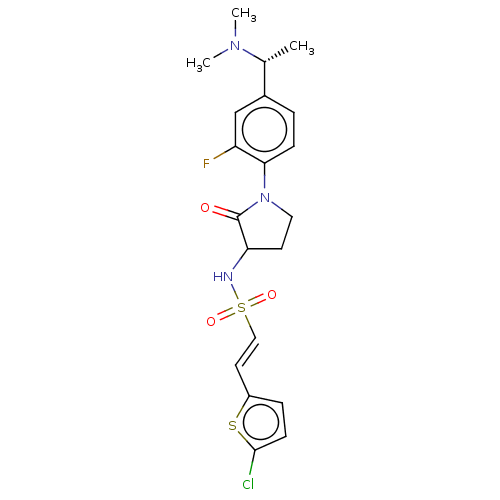
(CHEMBL5276446)Show SMILES C[C@@H](N(C)C)c1ccc(N2CCC(NS(=O)(=O)\C=C\c3ccc(Cl)s3)C2=O)c(F)c1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50614407
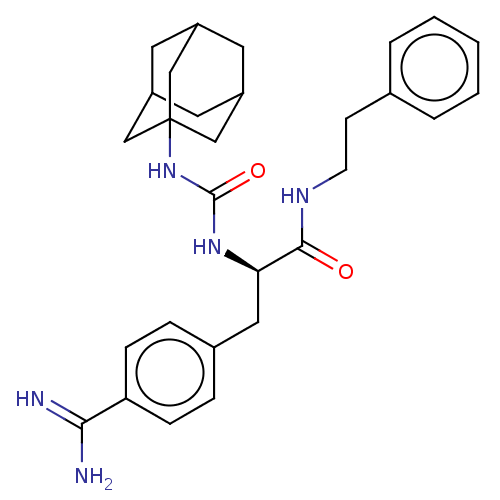
(CHEMBL5276447)Show SMILES NC(=N)c1ccc(C[C@@H](NC(=O)NC23CC4CC(CC(C4)C2)C3)C(=O)NCCc2ccccc2)cc1 |r,TLB:12:13:16:20.19.18,THB:21:13:16:20.19.18,21:19:16:22.14.13,14:15:18:22.13.21,14:13:16.15.20:18| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50614405

(CHEMBL4283525)Show SMILES [H][C@@]12CCCN1[C@@H](c1ncc(o1)-c1ccc(Cl)s1)[C@@]1([H])C(=O)N(CCC[N+](C)(C)C)C(=O)[C@@]21[H] |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| 146 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50614406

(CHEMBL4294106)Show SMILES [H][C@@]12CCCN1[C@@H](c1coc(n1)-c1ccc(Cl)s1)[C@@]1([H])C(=O)N(CCC[N+](C)(C)C)C(=O)[C@@]21[H] |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PDB
UniChem
| PDB
| 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070256
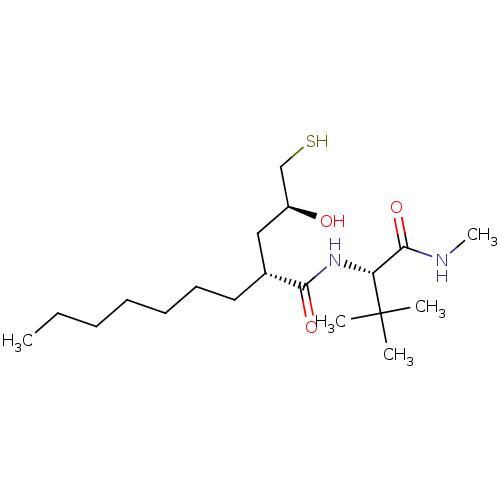
((R)-2-((S)-2-Hydroxy-3-mercapto-propyl)-nonanoic a...)Show SMILES CCCCCCC[C@H](C[C@H](O)CS)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C19H38N2O3S/c1-6-7-8-9-10-11-14(12-15(22)13-25)17(23)21-16(18(24)20-5)19(2,3)4/h14-16,22,25H,6-13H2,1-5H3,(H,20,24)(H,21,23)/t14-,15+,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-9, gelatinase-B |
Bioorg Med Chem Lett 8: 1163-8 (1999)
BindingDB Entry DOI: 10.7270/Q2348JH9 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070257
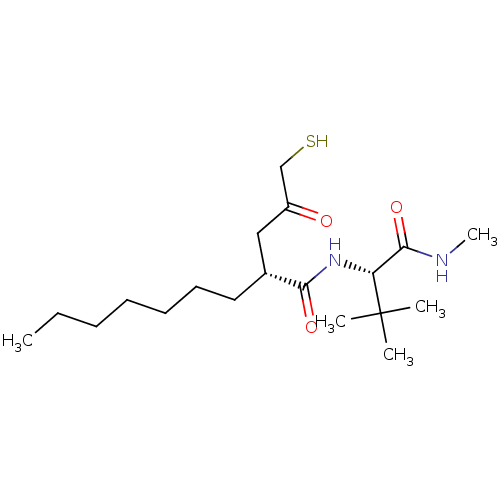
((R)-2-(3-Mercapto-2-oxo-propyl)-nonanoic acid ((S)...)Show SMILES CCCCCCC[C@H](CC(=O)CS)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C19H36N2O3S/c1-6-7-8-9-10-11-14(12-15(22)13-25)17(23)21-16(18(24)20-5)19(2,3)4/h14,16,25H,6-13H2,1-5H3,(H,20,24)(H,21,23)/t14-,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-9, gelatinase-B |
Bioorg Med Chem Lett 8: 1163-8 (1999)
BindingDB Entry DOI: 10.7270/Q2348JH9 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070228

(2-(2-Mercapto-acetyl)-nonanoic acid ((S)-2,2-dimet...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C18H34N2O3S/c1-6-7-8-9-10-11-13(14(21)12-24)16(22)20-15(17(23)19-5)18(2,3)4/h13,15,24H,6-12H2,1-5H3,(H,19,23)(H,20,22)/t13?,15-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50048982
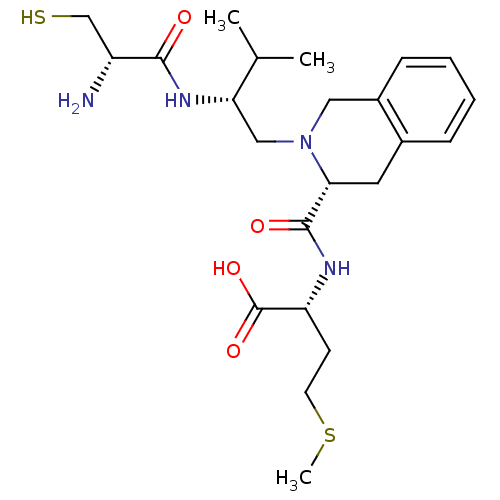
((R)-2-({(R)-2-[(R)-2-((S)-2-Amino-3-mercapto-propi...)Show SMILES CSCC[C@@H](NC(=O)[C@H]1Cc2ccccc2CN1C[C@H](NC(=O)[C@H](N)CS)C(C)C)C(O)=O Show InChI InChI=1S/C23H36N4O4S2/c1-14(2)19(26-21(28)17(24)13-32)12-27-11-16-7-5-4-6-15(16)10-20(27)22(29)25-18(23(30)31)8-9-33-3/h4-7,14,17-20,32H,8-13,24H2,1-3H3,(H,25,29)(H,26,28)(H,30,31)/t17-,18-,19+,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Farnesyltransferase |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50048970

((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...)Show SMILES CC(C)(C)[C@H](NC[C@@H](N)CS)C(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@H](CCS(C)(=O)=O)C(O)=O Show InChI InChI=1S/C24H38N4O6S2/c1-24(2,3)20(26-12-17(25)14-35)22(30)28-13-16-8-6-5-7-15(16)11-19(28)21(29)27-18(23(31)32)9-10-36(4,33)34/h5-8,17-20,26,35H,9-14,25H2,1-4H3,(H,27,29)(H,31,32)/t17-,18-,19+,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Farnesyltransferase |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50048963

((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...)Show SMILES CSCC[C@@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](NC[C@@H](N)CS)C(C)(C)C)C(O)=O Show InChI InChI=1S/C24H38N4O4S2/c1-24(2,3)20(26-12-17(25)14-33)22(30)28-13-16-8-6-5-7-15(16)11-19(28)21(29)27-18(23(31)32)9-10-34-4/h5-8,17-20,26,33H,9-14,25H2,1-4H3,(H,27,29)(H,31,32)/t17-,18-,19+,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Farnesyltransferase |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50048972
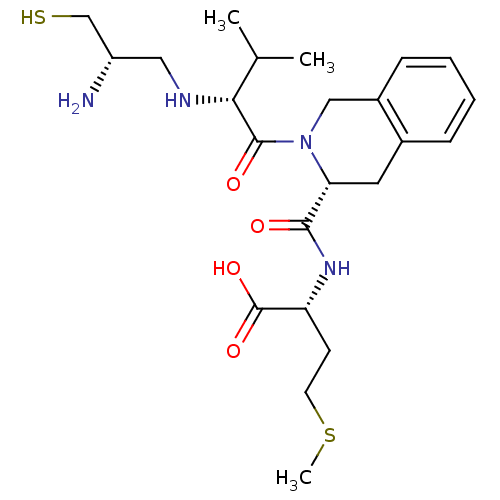
((R)-2-({(R)-2-[(R)-2-((S)-2-Amino-3-mercapto-propy...)Show SMILES CSCC[C@@H](NC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@H](NC[C@H](N)CS)C(C)C)C(O)=O Show InChI InChI=1S/C23H36N4O4S2/c1-14(2)20(25-11-17(24)13-32)22(29)27-12-16-7-5-4-6-15(16)10-19(27)21(28)26-18(23(30)31)8-9-33-3/h4-7,14,17-20,25,32H,8-13,24H2,1-3H3,(H,26,28)(H,30,31)/t17-,18+,19+,20+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Farnesyltransferase |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50048967

((R)-2-({(R)-2-[(R)-2-((S)-2-Amino-3-mercapto-propy...)Show SMILES CSCC[C@@H](NC(=O)[C@H]1Cc2ccccc2CN1C[C@H](NC[C@H](N)CS)C(C)C)C(O)=O Show InChI InChI=1S/C23H38N4O3S2/c1-15(2)20(25-11-18(24)14-31)13-27-12-17-7-5-4-6-16(17)10-21(27)22(28)26-19(23(29)30)8-9-32-3/h4-7,15,18-21,25,31H,8-14,24H2,1-3H3,(H,26,28)(H,29,30)/t18-,19+,20-,21+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Farnesyltransferase |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM25743

(1-cycloheptyl-3-(1-acetylpiperidin-4-yl)urea | US8...)Show InChI InChI=1S/C15H27N3O2/c1-12(19)18-10-8-14(9-11-18)17-15(20)16-13-6-4-2-3-5-7-13/h13-14H,2-11H2,1H3,(H2,16,17,20) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50276625

(1-Adamantan-1-yl-3-[3-(3-morpholin-4-yl-propoxy)-p...)Show SMILES O=C(Nc1cccc(OCCCN2CCOCC2)c1)NC12CC3CC(CC(C3)C1)C2 |TLB:19:20:23:27.26.25,THB:21:22:25:29.20.28,21:20:23.22.27:25,28:20:23:27.26.25,28:26:23:29.21.20| Show InChI InChI=1S/C24H35N3O3/c28-23(26-24-15-18-11-19(16-24)13-20(12-18)17-24)25-21-3-1-4-22(14-21)30-8-2-5-27-6-9-29-10-7-27/h1,3-4,14,18-20H,2,5-13,15-17H2,(H2,25,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Arête Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 19: 1066-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.013
BindingDB Entry DOI: 10.7270/Q2C24WBG |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50048964
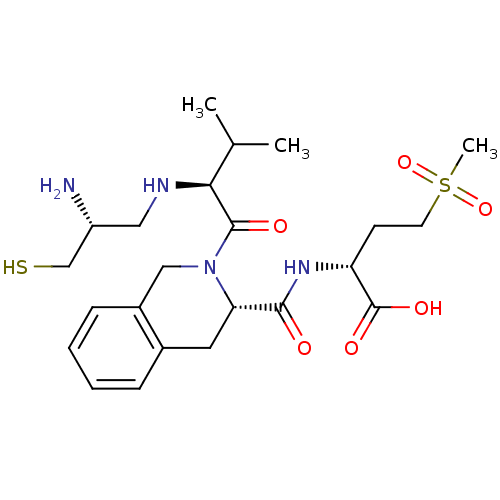
((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...)Show SMILES CC(C)[C@H](NC[C@@H](N)CS)C(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@H](CCS(C)(=O)=O)C(O)=O Show InChI InChI=1S/C23H36N4O6S2/c1-14(2)20(25-11-17(24)13-34)22(29)27-12-16-7-5-4-6-15(16)10-19(27)21(28)26-18(23(30)31)8-9-35(3,32)33/h4-7,14,17-20,25,34H,8-13,24H2,1-3H3,(H,26,28)(H,30,31)/t17-,18-,19+,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Farnesyltransferase |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50048981
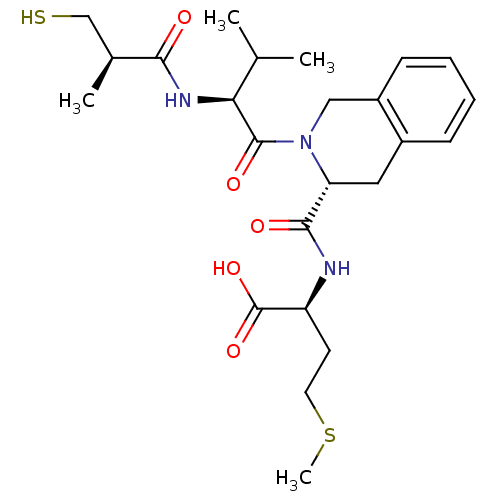
((S)-2-({(R)-2-[(S)-2-((R)-3-Mercapto-2-methyl-prop...)Show SMILES CSCC[C@H](NC(=O)[C@H]1Cc2ccccc2CN1C(=O)[C@@H](NC(=O)[C@@H](C)CS)C(C)C)C(O)=O Show InChI InChI=1S/C24H35N3O5S2/c1-14(2)20(26-21(28)15(3)13-33)23(30)27-12-17-8-6-5-7-16(17)11-19(27)22(29)25-18(24(31)32)9-10-34-4/h5-8,14-15,18-20,33H,9-13H2,1-4H3,(H,25,29)(H,26,28)(H,31,32)/t15-,18-,19+,20-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Farnesyltransferase |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50276673

(1-(3-(3-morpholinopropoxy)phenyl)-3-(4-(trifluorom...)Show SMILES FC(F)(F)c1ccc(NC(=O)Nc2cccc(OCCCN3CCOCC3)c2)cc1 Show InChI InChI=1S/C21H24F3N3O3/c22-21(23,24)16-5-7-17(8-6-16)25-20(28)26-18-3-1-4-19(15-18)30-12-2-9-27-10-13-29-14-11-27/h1,3-8,15H,2,9-14H2,(H2,25,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Arête Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 19: 1066-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.013
BindingDB Entry DOI: 10.7270/Q2C24WBG |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50048966

((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...)Show SMILES CCCC[C@@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](NC[C@@H](N)CS)C(C)C)C(O)=O Show InChI InChI=1S/C24H38N4O4S/c1-4-5-10-19(24(31)32)27-22(29)20-11-16-8-6-7-9-17(16)13-28(20)23(30)21(15(2)3)26-12-18(25)14-33/h6-9,15,18-21,26,33H,4-5,10-14,25H2,1-3H3,(H,27,29)(H,31,32)/t18-,19-,20+,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of Geranylgeranyl transferase type I |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070247

(2-(2-Mercapto-acetyl)-nonanoic acid ((S)-2-cyclohe...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C28H44N2O3S/c1-2-3-4-5-12-17-24(26(31)21-34)27(32)30-25(20-23-15-10-7-11-16-23)28(33)29-19-18-22-13-8-6-9-14-22/h6,8-9,13-14,23-25,34H,2-5,7,10-12,15-21H2,1H3,(H,29,33)(H,30,32)/t24?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070227
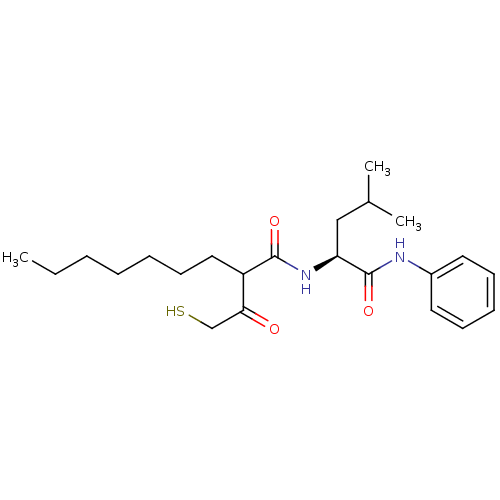
(2-(2-Mercapto-acetyl)-nonanoic acid ((S)-3-methyl-...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CC(C)C)C(=O)Nc1ccccc1 Show InChI InChI=1S/C23H36N2O3S/c1-4-5-6-7-11-14-19(21(26)16-29)22(27)25-20(15-17(2)3)23(28)24-18-12-9-8-10-13-18/h8-10,12-13,17,19-20,29H,4-7,11,14-16H2,1-3H3,(H,24,28)(H,25,27)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50048968

((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...)Show SMILES COCC[C@@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](NC[C@@H](N)CS)C(C)(C)C)C(O)=O Show InChI InChI=1S/C24H38N4O5S/c1-24(2,3)20(26-12-17(25)14-34)22(30)28-13-16-8-6-5-7-15(16)11-19(28)21(29)27-18(23(31)32)9-10-33-4/h5-8,17-20,26,34H,9-14,25H2,1-4H3,(H,27,29)(H,31,32)/t17-,18-,19+,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Farnesyltransferase |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335965
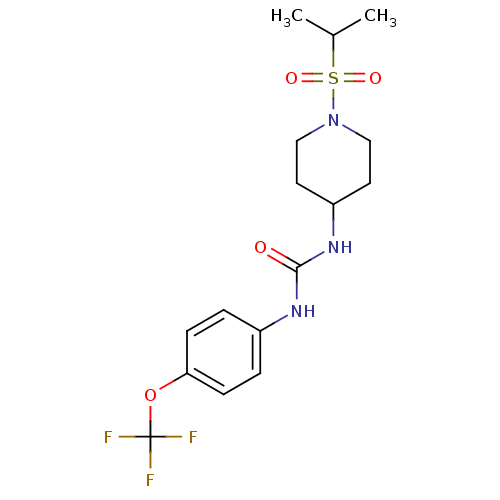
(1-(1-(isopropylsulfonyl)piperidin-4-yl)-3-(4-(trif...)Show SMILES CC(C)S(=O)(=O)N1CCC(CC1)NC(=O)Nc1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C16H22F3N3O4S/c1-11(2)27(24,25)22-9-7-13(8-10-22)21-15(23)20-12-3-5-14(6-4-12)26-16(17,18)19/h3-6,11,13H,7-10H2,1-2H3,(H2,20,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335964

(1-(1-nicotinoylpiperidin-4-yl)-3-(4-(trifluorometh...)Show SMILES FC(F)(F)Oc1ccc(NC(=O)NC2CCN(CC2)C(=O)c2cccnc2)cc1 Show InChI InChI=1S/C19H19F3N4O3/c20-19(21,22)29-16-5-3-14(4-6-16)24-18(28)25-15-7-10-26(11-8-15)17(27)13-2-1-9-23-12-13/h1-6,9,12,15H,7-8,10-11H2,(H2,24,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50048969
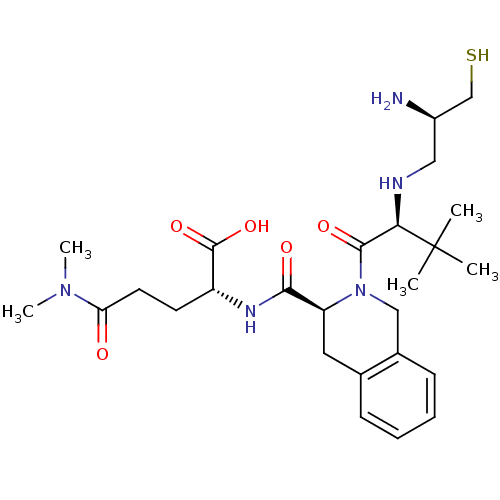
((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...)Show SMILES CN(C)C(=O)CC[C@@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](NC[C@@H](N)CS)C(C)(C)C)C(O)=O Show InChI InChI=1S/C26H41N5O5S/c1-26(2,3)22(28-13-18(27)15-37)24(34)31-14-17-9-7-6-8-16(17)12-20(31)23(33)29-19(25(35)36)10-11-21(32)30(4)5/h6-9,18-20,22,28,37H,10-15,27H2,1-5H3,(H,29,33)(H,35,36)/t18-,19-,20+,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Farnesyltransferase |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335966
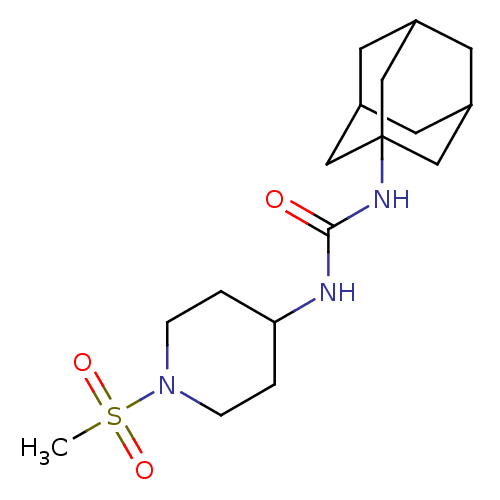
(1-Adamantan-1-yl-3-(1-methanesulfonyl-piperidin-4-...)Show SMILES CS(=O)(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:17:18:16.21.15:22,THB:19:18:15:21.20.22,19:20:17.18.23:15,17:16:18.19.23:22| Show InChI InChI=1S/C17H29N3O3S/c1-24(22,23)20-4-2-15(3-5-20)18-16(21)19-17-9-12-6-13(10-17)8-14(7-12)11-17/h12-15H,2-11H2,1H3,(H2,18,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50070250

(2-(1-Hydroxy-2-mercapto-ethyl)-5-phenyl-pentanoic ...)Show SMILES OC(CS)C(CCCc1ccccc1)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C30H42N2O3S/c33-28(22-36)26(18-10-17-23-11-4-1-5-12-23)29(34)32-27(21-25-15-8-3-9-16-25)30(35)31-20-19-24-13-6-2-7-14-24/h1-2,4-7,11-14,25-28,33,36H,3,8-10,15-22H2,(H,31,35)(H,32,34)/t26?,27-,28?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against stromelysin-3 (MMP-3) |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50276676

(2-Adamantan-1-yl-N-[3-(3-morpholin-4-yl-propoxy)-p...)Show SMILES O=C(CC12CC3CC(CC(C3)C1)C2)Nc1cccc(OCCCN2CCOCC2)c1 |TLB:2:3:6:10.9.8,THB:4:5:8:12.3.11,4:3:6.5.10:8,11:3:6:10.9.8,11:9:6:12.4.3| Show InChI InChI=1S/C25H36N2O3/c28-24(18-25-15-19-11-20(16-25)13-21(12-19)17-25)26-22-3-1-4-23(14-22)30-8-2-5-27-6-9-29-10-7-27/h1,3-4,14,19-21H,2,5-13,15-18H2,(H,26,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Arête Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 19: 1066-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.013
BindingDB Entry DOI: 10.7270/Q2C24WBG |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50048974
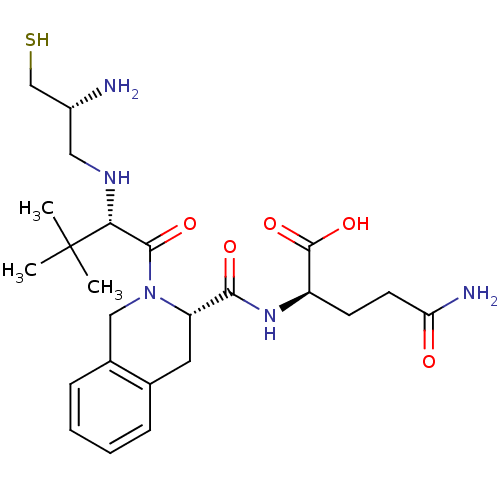
((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...)Show SMILES CC(C)(C)[C@H](NC[C@@H](N)CS)C(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@H](CCC(N)=O)C(O)=O Show InChI InChI=1S/C24H37N5O5S/c1-24(2,3)20(27-11-16(25)13-35)22(32)29-12-15-7-5-4-6-14(15)10-18(29)21(31)28-17(23(33)34)8-9-19(26)30/h4-7,16-18,20,27,35H,8-13,25H2,1-3H3,(H2,26,30)(H,28,31)(H,33,34)/t16-,17-,18+,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Farnesyltransferase |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
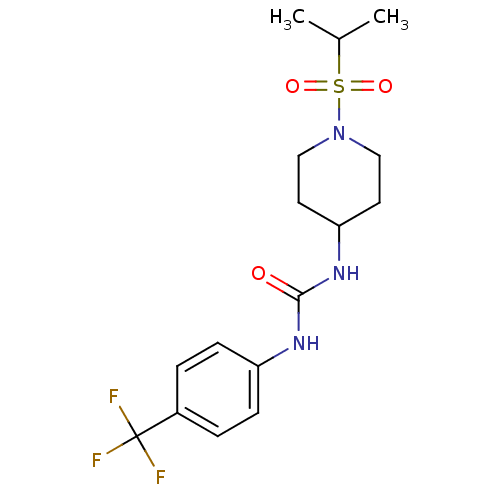
(Homo sapiens (Human)) | BDBM50335967
(1-(1-(isopropylsulfonyl)piperidin-4-yl)-3-(4-(trif...)Show SMILES CC(C)S(=O)(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C16H22F3N3O3S/c1-11(2)26(24,25)22-9-7-14(8-10-22)21-15(23)20-13-5-3-12(4-6-13)16(17,18)19/h3-6,11,14H,7-10H2,1-2H3,(H2,20,21,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Mus musculus (Mouse)) | BDBM50191854
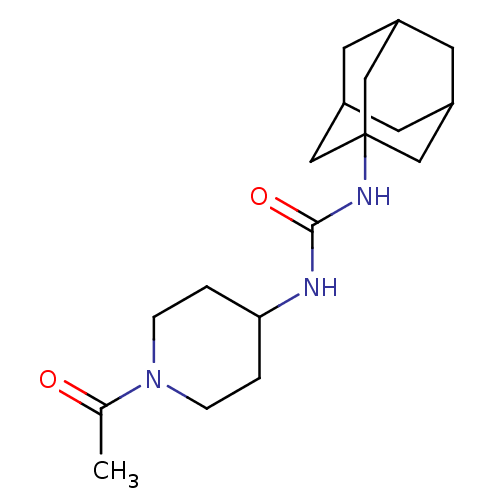
(CHEMBL436774 | N-(1-acetyl-piperidin-4-yl)-N'-(ada...)Show SMILES CC(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:12:13:16.15.20:18,THB:14:15:18:22.13.21,14:13:16.15.20:18,21:13:16:20.19.18,21:19:16:22.14.13,12:13:16:20.19.18| Show InChI InChI=1S/C18H29N3O2/c1-12(22)21-4-2-16(3-5-21)19-17(23)20-18-9-13-6-14(10-18)8-15(7-13)11-18/h13-16H,2-11H2,1H3,(H2,19,20,23) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of mouse soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335968
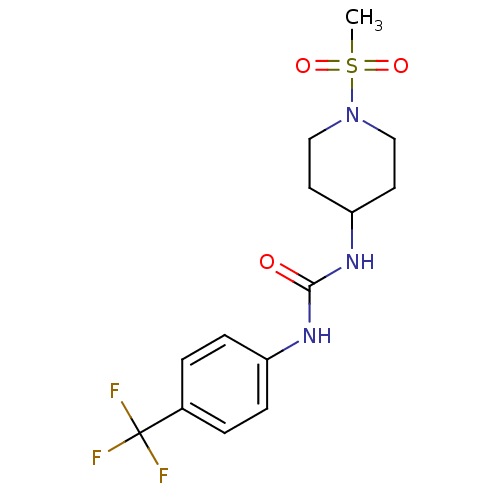
(1-(1-(methylsulfonyl)piperidin-4-yl)-3-(4-(trifluo...)Show SMILES CS(=O)(=O)N1CCC(CC1)NC(=O)Nc1ccc(cc1)C(F)(F)F Show InChI InChI=1S/C14H18F3N3O3S/c1-24(22,23)20-8-6-12(7-9-20)19-13(21)18-11-4-2-10(3-5-11)14(15,16)17/h2-5,12H,6-9H2,1H3,(H2,18,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335966

(1-Adamantan-1-yl-3-(1-methanesulfonyl-piperidin-4-...)Show SMILES CS(=O)(=O)N1CCC(CC1)NC(=O)NC12CC3CC(CC(C3)C1)C2 |TLB:17:18:16.21.15:22,THB:19:18:15:21.20.22,19:20:17.18.23:15,17:16:18.19.23:22| Show InChI InChI=1S/C17H29N3O3S/c1-24(22,23)20-4-2-15(3-5-20)18-16(21)19-17-9-12-6-13(10-17)8-14(7-12)11-17/h12-15H,2-11H2,1H3,(H2,18,19,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase in HUVEC assessed inhibition of as conversion of 14, 15-EET to 14, 15-DHET |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
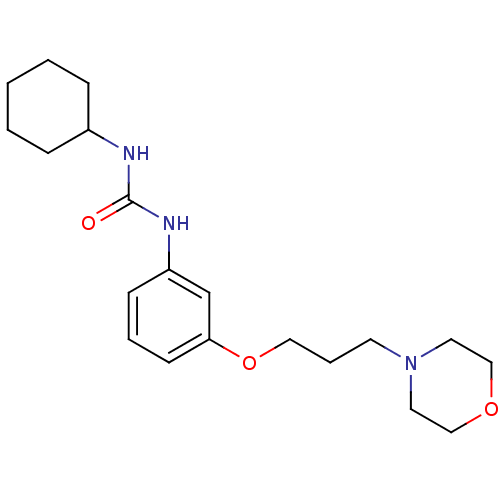
(Homo sapiens (Human)) | BDBM50276626
(1-cyclohexyl-3-(3-(3-morpholinopropoxy)phenyl)urea...)Show InChI InChI=1S/C20H31N3O3/c24-20(21-17-6-2-1-3-7-17)22-18-8-4-9-19(16-18)26-13-5-10-23-11-14-25-15-12-23/h4,8-9,16-17H,1-3,5-7,10-15H2,(H2,21,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Arête Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of soluble epoxide hydrolase (unknown origin) by fluorescence assay |
Bioorg Med Chem Lett 19: 1066-70 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.013
BindingDB Entry DOI: 10.7270/Q2C24WBG |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
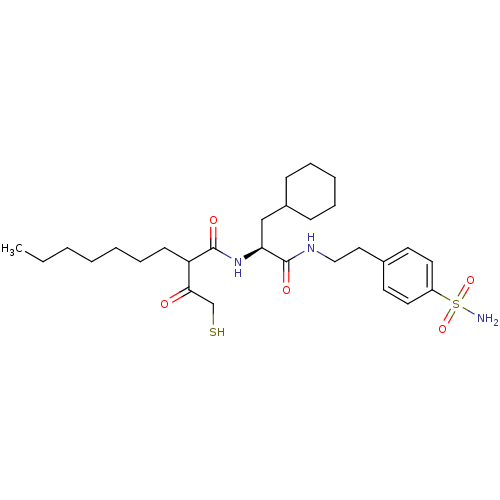
(Homo sapiens (Human)) | BDBM50070226
(2-(2-Mercapto-acetyl)-nonanoic acid {(S)-2-cyclohe...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CC1CCCCC1)C(=O)NCCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C28H45N3O5S2/c1-2-3-4-5-9-12-24(26(32)20-37)27(33)31-25(19-22-10-7-6-8-11-22)28(34)30-18-17-21-13-15-23(16-14-21)38(29,35)36/h13-16,22,24-25,37H,2-12,17-20H2,1H3,(H,30,34)(H,31,33)(H2,29,35,36)/t24?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
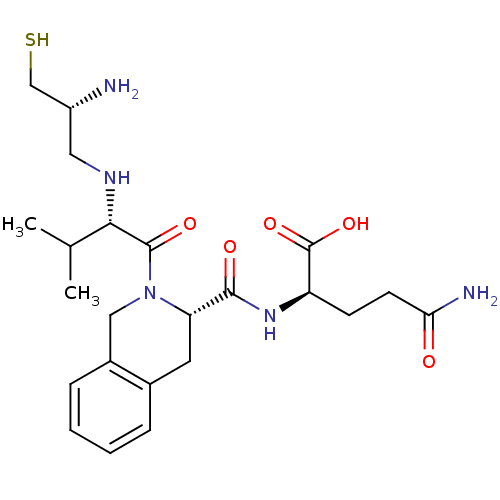
(Homo sapiens (Human)) | BDBM50048975
((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...)Show SMILES CC(C)[C@H](NC[C@@H](N)CS)C(=O)N1Cc2ccccc2C[C@H]1C(=O)N[C@H](CCC(N)=O)C(O)=O Show InChI InChI=1S/C23H35N5O5S/c1-13(2)20(26-10-16(24)12-34)22(31)28-11-15-6-4-3-5-14(15)9-18(28)21(30)27-17(23(32)33)7-8-19(25)29/h3-6,13,16-18,20,26,34H,7-12,24H2,1-2H3,(H2,25,29)(H,27,30)(H,32,33)/t16-,17-,18+,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against Farnesyltransferase |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50048963

((R)-2-({(S)-2-[(S)-2-((R)-2-Amino-3-mercapto-propy...)Show SMILES CSCC[C@@H](NC(=O)[C@@H]1Cc2ccccc2CN1C(=O)[C@@H](NC[C@@H](N)CS)C(C)(C)C)C(O)=O Show InChI InChI=1S/C24H38N4O4S2/c1-24(2,3)20(26-12-17(25)14-33)22(30)28-13-16-8-6-5-7-15(16)11-19(28)21(29)27-18(23(31)32)9-10-34-4/h5-8,17-20,26,33H,9-14,25H2,1-4H3,(H,27,29)(H,31,32)/t17-,18-,19+,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibition of Geranylgeranyl transferase type I |
J Med Chem 39: 224-36 (1996)
Article DOI: 10.1021/jm950642a
BindingDB Entry DOI: 10.7270/Q2610ZDS |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50029588
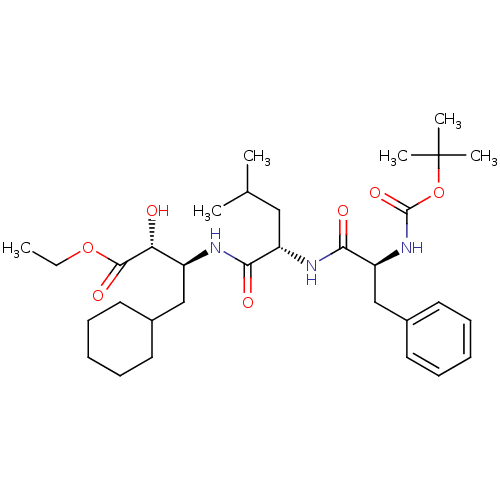
((2R,3S)-3-[(S)-2-((S)-2-tert-Butoxycarbonylamino-3...)Show SMILES CCOC(=O)[C@H](O)[C@H](CC1CCCCC1)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](Cc1ccccc1)NC(=O)OC(C)(C)C Show InChI InChI=1S/C32H51N3O7/c1-7-41-30(39)27(36)24(19-22-14-10-8-11-15-22)33-28(37)25(18-21(2)3)34-29(38)26(20-23-16-12-9-13-17-23)35-31(40)42-32(4,5)6/h9,12-13,16-17,21-22,24-27,36H,7-8,10-11,14-15,18-20H2,1-6H3,(H,33,37)(H,34,38)(H,35,40)/t24-,25-,26-,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
| Assay Description
Evaluation of inhibitory activity of the compound against human renin |
J Med Chem 38: 4557-69 (1995)
BindingDB Entry DOI: 10.7270/Q27D2T5V |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070223
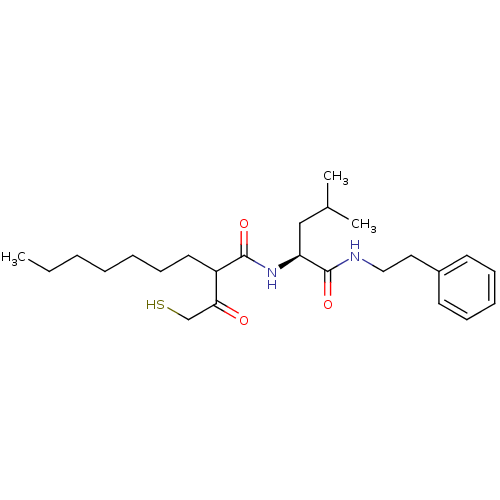
(2-(2-Mercapto-acetyl)-nonanoic acid ((S)-3-methyl-...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CC(C)C)C(=O)NCCc1ccccc1 Show InChI InChI=1S/C25H40N2O3S/c1-4-5-6-7-11-14-21(23(28)18-31)24(29)27-22(17-19(2)3)25(30)26-16-15-20-12-9-8-10-13-20/h8-10,12-13,19,21-22,31H,4-7,11,14-18H2,1-3H3,(H,26,30)(H,27,29)/t21?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50070256

((R)-2-((S)-2-Hydroxy-3-mercapto-propyl)-nonanoic a...)Show SMILES CCCCCCC[C@H](C[C@H](O)CS)C(=O)N[C@H](C(=O)NC)C(C)(C)C Show InChI InChI=1S/C19H38N2O3S/c1-6-7-8-9-10-11-14(12-15(22)13-25)17(23)21-16(18(24)20-5)19(2,3)4/h14-16,22,25H,6-13H2,1-5H3,(H,20,24)(H,21,23)/t14-,15+,16-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-1, Collagenase-1 |
Bioorg Med Chem Lett 8: 1163-8 (1999)
BindingDB Entry DOI: 10.7270/Q2348JH9 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063920
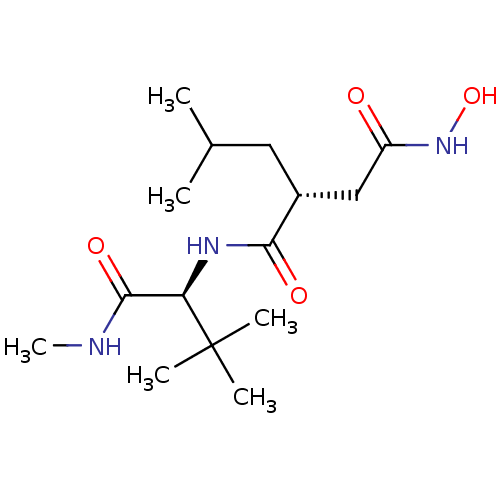
((R)-N*1*-((S)-2,2-Dimethyl-1-methylcarbamoyl-propy...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)CC(=O)NO)C(C)(C)C Show InChI InChI=1S/C15H29N3O4/c1-9(2)7-10(8-11(19)18-22)13(20)17-12(14(21)16-6)15(3,4)5/h9-10,12,22H,7-8H2,1-6H3,(H,16,21)(H,17,20)(H,18,19)/t10-,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research
Curated by ChEMBL
| Assay Description
Inhibition of matrix metalloproteinase-1, Collagenase-1 |
Bioorg Med Chem Lett 8: 1163-8 (1999)
BindingDB Entry DOI: 10.7270/Q2348JH9 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335969

(1-(1-acetylpiperidin-4-yl)-3-(4,4-dimethylcyclohex...)Show InChI InChI=1S/C16H29N3O2/c1-12(20)19-10-6-14(7-11-19)18-15(21)17-13-4-8-16(2,3)9-5-13/h13-14H,4-11H2,1-3H3,(H2,17,18,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070239
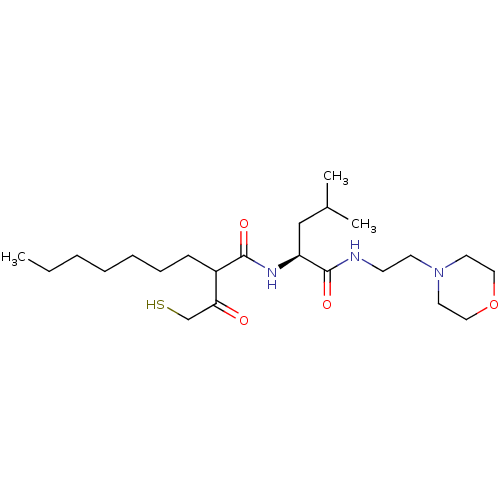
(2-(2-Mercapto-acetyl)-nonanoic acid [(S)-3-methyl-...)Show SMILES CCCCCCCC(C(=O)CS)C(=O)N[C@@H](CC(C)C)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C23H43N3O4S/c1-4-5-6-7-8-9-19(21(27)17-31)22(28)25-20(16-18(2)3)23(29)24-10-11-26-12-14-30-15-13-26/h18-20,31H,4-17H2,1-3H3,(H,24,29)(H,25,28)/t19?,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50070229

(2-(2-Mercapto-acetyl)-4-methyl-pentanoic acid ((S)...)Show SMILES CNC(=O)[C@@H](NC(=O)C(CC(C)C)C(=O)CS)C(C)(C)C Show InChI InChI=1S/C15H28N2O3S/c1-9(2)7-10(11(18)8-21)13(19)17-12(14(20)16-6)15(3,4)5/h9-10,12,21H,7-8H2,1-6H3,(H,16,20)(H,17,19)/t10?,12-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Affymax Research Institute
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity against gelatinase-B(MMP-9). |
Bioorg Med Chem Lett 8: 1157-62 (1999)
BindingDB Entry DOI: 10.7270/Q26W997Q |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50335970

(1-cyclohexyl-3-(1-picolinoylpiperidin-4-yl)urea | ...)Show InChI InChI=1S/C18H26N4O2/c23-17(16-8-4-5-11-19-16)22-12-9-15(10-13-22)21-18(24)20-14-6-2-1-3-7-14/h4-5,8,11,14-15H,1-3,6-7,9-10,12-13H2,(H2,20,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Ar£te Therapeutics, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 21: 983-8 (2011)
Article DOI: 10.1016/j.bmcl.2010.12.042
BindingDB Entry DOI: 10.7270/Q20865KB |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data