 Found 641 hits with Last Name = 'pham' and Initial = 'n'
Found 641 hits with Last Name = 'pham' and Initial = 'n' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50083651
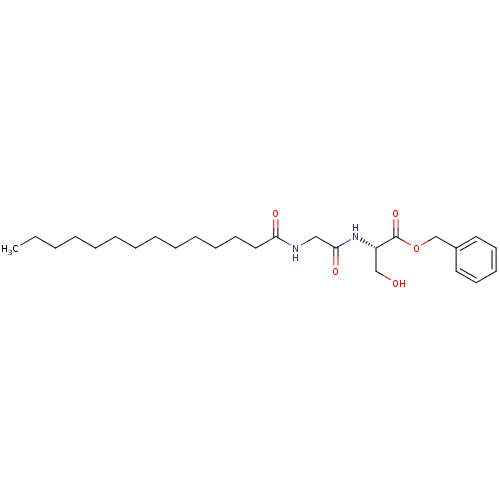
((S)-3-Hydroxy-2-(2-tetradecanoylamino-acetylamino)...)Show SMILES CCCCCCCCCCCCCC(=O)NCC(=O)N[C@@H](CO)C(=O)OCc1ccccc1 Show InChI InChI=1S/C26H42N2O5/c1-2-3-4-5-6-7-8-9-10-11-15-18-24(30)27-19-25(31)28-23(20-29)26(32)33-21-22-16-13-12-14-17-22/h12-14,16-17,23,29H,2-11,15,18-21H2,1H3,(H,27,30)(H,28,31)/t23-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo
Curated by ChEMBL
| Assay Description
Ability to displace [3H]QNB from HM1 receptor binding to acetylcholine was evaluated by ligand inhibition assay |
Bioorg Med Chem Lett 9: 3363-8 (2000)
BindingDB Entry DOI: 10.7270/Q2668CDH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50083651

((S)-3-Hydroxy-2-(2-tetradecanoylamino-acetylamino)...)Show SMILES CCCCCCCCCCCCCC(=O)NCC(=O)N[C@@H](CO)C(=O)OCc1ccccc1 Show InChI InChI=1S/C26H42N2O5/c1-2-3-4-5-6-7-8-9-10-11-15-18-24(30)27-19-25(31)28-23(20-29)26(32)33-21-22-16-13-12-14-17-22/h12-14,16-17,23,29H,2-11,15,18-21H2,1H3,(H,27,30)(H,28,31)/t23-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo
Curated by ChEMBL
| Assay Description
Ability to displace [3H]QNB from HM1 receptor binding to acetylcholine was evaluated by ligand inhibition assay |
Bioorg Med Chem Lett 9: 3363-8 (2000)
BindingDB Entry DOI: 10.7270/Q2668CDH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50083652
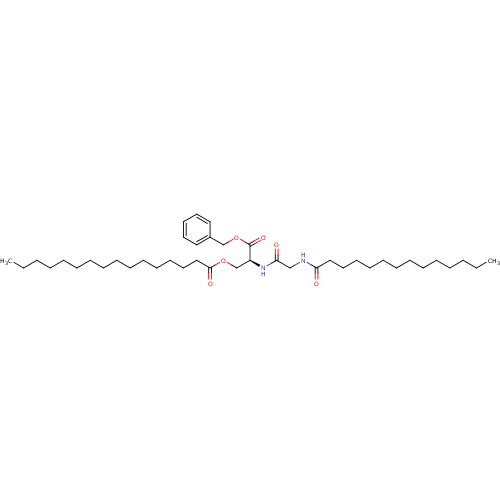
(CHEMBL112297 | Hexadecanoic acid (S)-2-benzyloxyca...)Show SMILES CCCCCCCCCCCCCCCC(=O)OC[C@H](NC(=O)CNC(=O)CCCCCCCCCCCCC)C(=O)OCc1ccccc1 Show InChI InChI=1S/C42H72N2O6/c1-3-5-7-9-11-13-15-16-18-20-22-24-29-33-41(47)49-36-38(42(48)50-35-37-30-26-25-27-31-37)44-40(46)34-43-39(45)32-28-23-21-19-17-14-12-10-8-6-4-2/h25-27,30-31,38H,3-24,28-29,32-36H2,1-2H3,(H,43,45)(H,44,46)/t38-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0129 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo
Curated by ChEMBL
| Assay Description
Ability to displace [3H]QNB from HM1 receptor binding to acetylcholine was evaluated by ligand inhibition assay |
Bioorg Med Chem Lett 9: 3363-8 (2000)
BindingDB Entry DOI: 10.7270/Q2668CDH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(Homo sapiens (Human)) | BDBM50083652

(CHEMBL112297 | Hexadecanoic acid (S)-2-benzyloxyca...)Show SMILES CCCCCCCCCCCCCCCC(=O)OC[C@H](NC(=O)CNC(=O)CCCCCCCCCCCCC)C(=O)OCc1ccccc1 Show InChI InChI=1S/C42H72N2O6/c1-3-5-7-9-11-13-15-16-18-20-22-24-29-33-41(47)49-36-38(42(48)50-35-37-30-26-25-27-31-37)44-40(46)34-43-39(45)32-28-23-21-19-17-14-12-10-8-6-4-2/h25-27,30-31,38H,3-24,28-29,32-36H2,1-2H3,(H,43,45)(H,44,46)/t38-/m0/s1 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo
Curated by ChEMBL
| Assay Description
Ability to displace [3H]QNB from HM1 receptor binding to acetylcholine was evaluated by ligand inhibition assay |
Bioorg Med Chem Lett 9: 3363-8 (2000)
BindingDB Entry DOI: 10.7270/Q2668CDH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50083651

((S)-3-Hydroxy-2-(2-tetradecanoylamino-acetylamino)...)Show SMILES CCCCCCCCCCCCCC(=O)NCC(=O)N[C@@H](CO)C(=O)OCc1ccccc1 Show InChI InChI=1S/C26H42N2O5/c1-2-3-4-5-6-7-8-9-10-11-15-18-24(30)27-19-25(31)28-23(20-29)26(32)33-21-22-16-13-12-14-17-22/h12-14,16-17,23,29H,2-11,15,18-21H2,1H3,(H,27,30)(H,28,31)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0759 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo
Curated by ChEMBL
| Assay Description
Ability to displace [3H]QNB from HM1 receptor binding to acetylcholine was evaluated by ligand inhibition assay |
Bioorg Med Chem Lett 9: 3363-8 (2000)
BindingDB Entry DOI: 10.7270/Q2668CDH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50083651

((S)-3-Hydroxy-2-(2-tetradecanoylamino-acetylamino)...)Show SMILES CCCCCCCCCCCCCC(=O)NCC(=O)N[C@@H](CO)C(=O)OCc1ccccc1 Show InChI InChI=1S/C26H42N2O5/c1-2-3-4-5-6-7-8-9-10-11-15-18-24(30)27-19-25(31)28-23(20-29)26(32)33-21-22-16-13-12-14-17-22/h12-14,16-17,23,29H,2-11,15,18-21H2,1H3,(H,27,30)(H,28,31)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo
Curated by ChEMBL
| Assay Description
Ability to displace [3H]QNB from HM2 receptor binding to acetylcholine was evaluated by ligand inhibition assay |
Bioorg Med Chem Lett 9: 3363-8 (2000)
BindingDB Entry DOI: 10.7270/Q2668CDH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50083652

(CHEMBL112297 | Hexadecanoic acid (S)-2-benzyloxyca...)Show SMILES CCCCCCCCCCCCCCCC(=O)OC[C@H](NC(=O)CNC(=O)CCCCCCCCCCCCC)C(=O)OCc1ccccc1 Show InChI InChI=1S/C42H72N2O6/c1-3-5-7-9-11-13-15-16-18-20-22-24-29-33-41(47)49-36-38(42(48)50-35-37-30-26-25-27-31-37)44-40(46)34-43-39(45)32-28-23-21-19-17-14-12-10-8-6-4-2/h25-27,30-31,38H,3-24,28-29,32-36H2,1-2H3,(H,43,45)(H,44,46)/t38-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo
Curated by ChEMBL
| Assay Description
Ability to displace [3H]QNB from HM2 receptor binding to acetylcholine was evaluated by ligand inhibition assay |
Bioorg Med Chem Lett 9: 3363-8 (2000)
BindingDB Entry DOI: 10.7270/Q2668CDH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50083652

(CHEMBL112297 | Hexadecanoic acid (S)-2-benzyloxyca...)Show SMILES CCCCCCCCCCCCCCCC(=O)OC[C@H](NC(=O)CNC(=O)CCCCCCCCCCCCC)C(=O)OCc1ccccc1 Show InChI InChI=1S/C42H72N2O6/c1-3-5-7-9-11-13-15-16-18-20-22-24-29-33-41(47)49-36-38(42(48)50-35-37-30-26-25-27-31-37)44-40(46)34-43-39(45)32-28-23-21-19-17-14-12-10-8-6-4-2/h25-27,30-31,38H,3-24,28-29,32-36H2,1-2H3,(H,43,45)(H,44,46)/t38-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.229 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo
Curated by ChEMBL
| Assay Description
Binding of [3H]-QNB to HM2 receptor was evaluated by saturation binding assay |
Bioorg Med Chem Lett 9: 3363-8 (2000)
BindingDB Entry DOI: 10.7270/Q2668CDH |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458458

(CHEMBL4210729)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C34H33ClN4O3/c1-42-24-14-11-22(12-15-24)20-39-21-28(33(40)27-8-3-5-10-31(27)39)34(41)37-18-6-17-36-32-25-7-2-4-9-29(25)38-30-19-23(35)13-16-26(30)32/h3,5,8,10-16,19,21H,2,4,6-7,9,17-18,20H2,1H3,(H,36,38)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 1.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human erythrocyte AChE assessed as enzyme-substrate-inhibitor complex using varying levels of acetylthiocholine iodide as su... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458458

(CHEMBL4210729)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C34H33ClN4O3/c1-42-24-14-11-22(12-15-24)20-39-21-28(33(40)27-8-3-5-10-31(27)39)34(41)37-18-6-17-36-32-25-7-2-4-9-29(25)38-30-19-23(35)13-16-26(30)32/h3,5,8,10-16,19,21H,2,4,6-7,9,17-18,20H2,1H3,(H,36,38)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human erythrocyte AChE assessed as enzyme-inhibitor complex using varying levels of acetylthiocholine iodide as substrate pr... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50083651

((S)-3-Hydroxy-2-(2-tetradecanoylamino-acetylamino)...)Show SMILES CCCCCCCCCCCCCC(=O)NCC(=O)N[C@@H](CO)C(=O)OCc1ccccc1 Show InChI InChI=1S/C26H42N2O5/c1-2-3-4-5-6-7-8-9-10-11-15-18-24(30)27-19-25(31)28-23(20-29)26(32)33-21-22-16-13-12-14-17-22/h12-14,16-17,23,29H,2-11,15,18-21H2,1H3,(H,27,30)(H,28,31)/t23-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 562 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo
Curated by ChEMBL
| Assay Description
Ability to displace [3H]QNB from HM1 receptor binding to acetylcholine was evaluated by ligand inhibition assay |
Bioorg Med Chem Lett 9: 3363-8 (2000)
BindingDB Entry DOI: 10.7270/Q2668CDH |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(Homo sapiens (Human)) | BDBM50083652

(CHEMBL112297 | Hexadecanoic acid (S)-2-benzyloxyca...)Show SMILES CCCCCCCCCCCCCCCC(=O)OC[C@H](NC(=O)CNC(=O)CCCCCCCCCCCCC)C(=O)OCc1ccccc1 Show InChI InChI=1S/C42H72N2O6/c1-3-5-7-9-11-13-15-16-18-20-22-24-29-33-41(47)49-36-38(42(48)50-35-37-30-26-25-27-31-37)44-40(46)34-43-39(45)32-28-23-21-19-17-14-12-10-8-6-4-2/h25-27,30-31,38H,3-24,28-29,32-36H2,1-2H3,(H,43,45)(H,44,46)/t38-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 851 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toledo
Curated by ChEMBL
| Assay Description
Ability to displace [3H]QNB from HM2 receptor binding to acetylcholine was evaluated by ligand inhibition assay |
Bioorg Med Chem Lett 9: 3363-8 (2000)
BindingDB Entry DOI: 10.7270/Q2668CDH |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151638

(8''-chloro-5''-(5-hydroxy-1,2,4-oxadiazol-3-ylmeth...)Show SMILES Clc1ccc(OCc2nc(=O)o[nH]2)c2c1NC(=O)NC21CCCCC1 Show InChI InChI=1S/C16H17ClN4O4/c17-9-4-5-10(24-8-11-18-15(23)25-21-11)12-13(9)19-14(22)20-16(12)6-2-1-3-7-16/h4-5H,1-3,6-8H2,(H,18,21,23)(H2,19,20,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4627-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.010
BindingDB Entry DOI: 10.7270/Q2TT4QDX |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151562

(7-{5-[(Z)-Cyclohexylimino]-4-methyl-4,5-dihydro-[1...)Show SMILES Cc1nc(N)c2ccc(cc2n1)-c1nn(C)\c(=N\C2CCCCC2)s1 Show InChI InChI=1S/C18H22N6S/c1-11-20-15-10-12(8-9-14(15)16(19)21-11)17-23-24(2)18(25-17)22-13-6-4-3-5-7-13/h8-10,13H,3-7H2,1-2H3,(H2,19,20,21)/b22-18- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4607-13 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.008
BindingDB Entry DOI: 10.7270/Q2377855 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151635
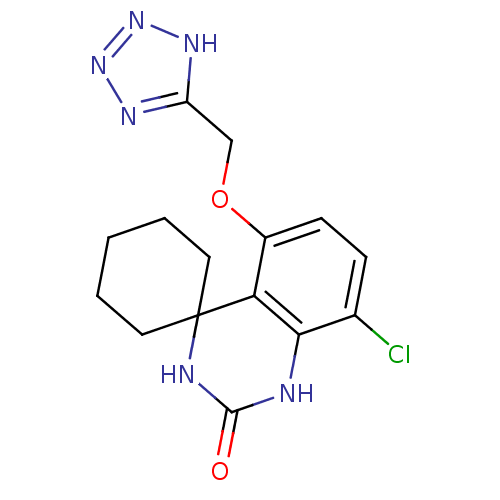
(8''-chloro-5''-(1H-1,2,3,4-tetraazol-5-ylmethoxy)s...)Show InChI InChI=1S/C15H17ClN6O2/c16-9-4-5-10(24-8-11-19-21-22-20-11)12-13(9)17-14(23)18-15(12)6-2-1-3-7-15/h4-5H,1-3,6-8H2,(H2,17,18,23)(H,19,20,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4627-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.010
BindingDB Entry DOI: 10.7270/Q2TT4QDX |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151636

(3-[8''-chloro-2''-oxospiro[cyclohexane-1,4''-(1'',...)Show SMILES OS(=O)(=O)CCCOc1ccc(Cl)c2NC(=O)NC3(CCCCC3)c12 Show InChI InChI=1S/C16H21ClN2O5S/c17-11-5-6-12(24-9-4-10-25(21,22)23)13-14(11)18-15(20)19-16(13)7-2-1-3-8-16/h5-6H,1-4,7-10H2,(H2,18,19,20)(H,21,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4627-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.010
BindingDB Entry DOI: 10.7270/Q2TT4QDX |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151640

(8''-chloro-5''-methoxyspiro[cyclohexane-1,4''-(1''...)Show InChI InChI=1S/C14H17ClN2O2/c1-19-10-6-5-9(15)12-11(10)14(17-13(18)16-12)7-3-2-4-8-14/h5-6H,2-4,7-8H2,1H3,(H2,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4627-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.010
BindingDB Entry DOI: 10.7270/Q2TT4QDX |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151634
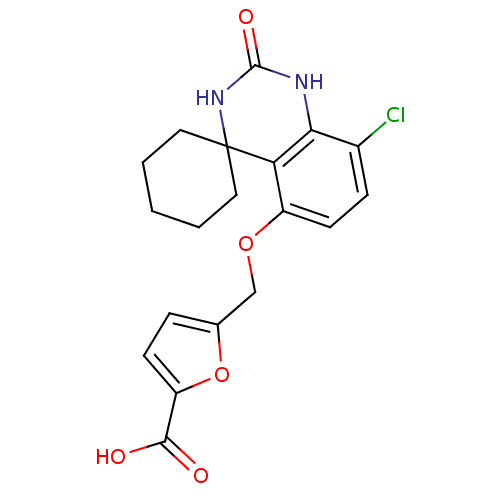
(5-[8''-chloro-2''-oxospiro[cyclohexane-1,4''-(1'',...)Show SMILES OC(=O)c1ccc(COc2ccc(Cl)c3NC(=O)NC4(CCCCC4)c23)o1 Show InChI InChI=1S/C19H19ClN2O5/c20-12-5-7-13(26-10-11-4-6-14(27-11)17(23)24)15-16(12)21-18(25)22-19(15)8-2-1-3-9-19/h4-7H,1-3,8-10H2,(H,23,24)(H2,21,22,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4627-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.010
BindingDB Entry DOI: 10.7270/Q2TT4QDX |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151609
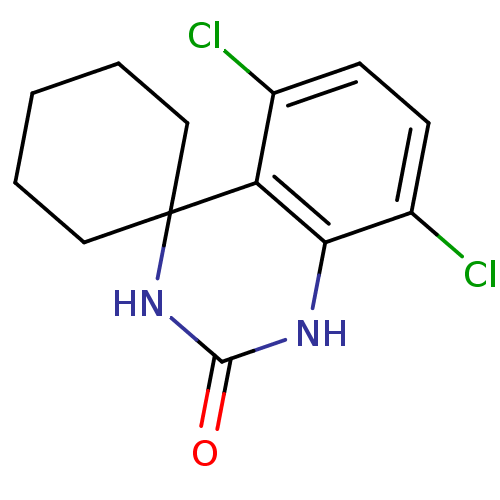
(5'',8''-dichlorospiro[cyclohexane-1,4''-(1'',2'',3...)Show InChI InChI=1S/C13H14Cl2N2O/c14-8-4-5-9(15)11-10(8)13(17-12(18)16-11)6-2-1-3-7-13/h4-5H,1-3,6-7H2,(H2,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4627-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.010
BindingDB Entry DOI: 10.7270/Q2TT4QDX |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151644

(CHEMBL359890 | ethyl 5-[8''-chloro-2''-oxospiro[cy...)Show SMILES CCOC(=O)c1ccc(COc2ccc(Cl)c3NC(=O)NC4(CCCCC4)c23)o1 Show InChI InChI=1S/C21H23ClN2O5/c1-2-27-19(25)16-8-6-13(29-16)12-28-15-9-7-14(22)18-17(15)21(24-20(26)23-18)10-4-3-5-11-21/h6-9H,2-5,10-12H2,1H3,(H2,23,24,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4627-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.010
BindingDB Entry DOI: 10.7270/Q2TT4QDX |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151649

(8''-chloro-5''-hydroxyspiro[cyclohexane-1,4''-(1''...)Show InChI InChI=1S/C13H15ClN2O2/c14-8-4-5-9(17)10-11(8)15-12(18)16-13(10)6-2-1-3-7-13/h4-5,17H,1-3,6-7H2,(H2,15,16,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4627-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.010
BindingDB Entry DOI: 10.7270/Q2TT4QDX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM8987

(6-chloro-1,2,3,4-tetrahydroacridin-9-amine | 6-chl...)Show InChI InChI=1S/C13H13ClN2/c14-8-5-6-10-12(7-8)16-11-4-2-1-3-9(11)13(10)15/h5-7H,1-4H2,(H2,15,16) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151572
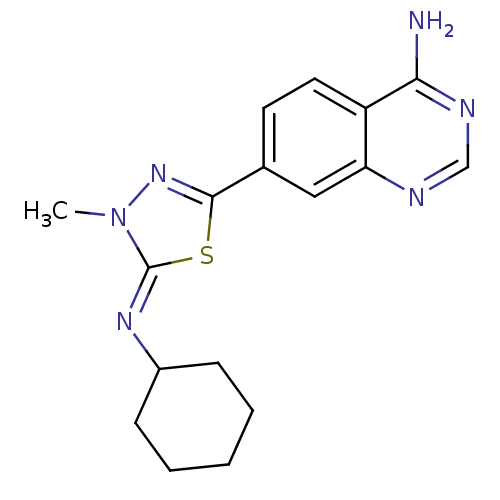
(7-{5-[(Z)-Cyclohexylimino]-4-methyl-4,5-dihydro-[1...)Show InChI InChI=1S/C17H20N6S/c1-23-17(21-12-5-3-2-4-6-12)24-16(22-23)11-7-8-13-14(9-11)19-10-20-15(13)18/h7-10,12H,2-6H2,1H3,(H2,18,19,20)/b21-17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4607-13 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.008
BindingDB Entry DOI: 10.7270/Q2377855 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151538
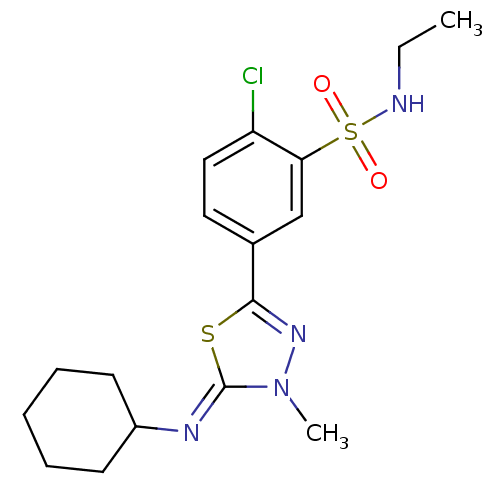
(2-Chloro-5-{5-[(Z)-cyclohexylimino]-4-methyl-4,5-d...)Show SMILES CCNS(=O)(=O)c1cc(ccc1Cl)-c1nn(C)\c(=N\C2CCCCC2)s1 Show InChI InChI=1S/C17H23ClN4O2S2/c1-3-19-26(23,24)15-11-12(9-10-14(15)18)16-21-22(2)17(25-16)20-13-7-5-4-6-8-13/h9-11,13,19H,3-8H2,1-2H3/b20-17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4607-13 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.008
BindingDB Entry DOI: 10.7270/Q2377855 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151631

(2-[8''-chloro-2''-oxospiro[cyclohexane-1,4''-(1'',...)Show InChI InChI=1S/C15H16ClN3O2/c16-10-4-5-11(21-9-8-17)12-13(10)18-14(20)19-15(12)6-2-1-3-7-15/h4-5H,1-3,6-7,9H2,(H2,18,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4627-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.010
BindingDB Entry DOI: 10.7270/Q2TT4QDX |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151645
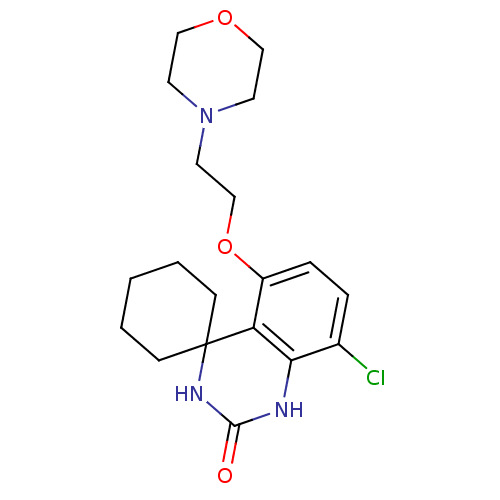
(8''-chloro-5''-[2-(1,4-oxazinan-4-yl)ethoxy]spiro[...)Show InChI InChI=1S/C19H26ClN3O3/c20-14-4-5-15(26-13-10-23-8-11-25-12-9-23)16-17(14)21-18(24)22-19(16)6-2-1-3-7-19/h4-5H,1-3,6-13H2,(H2,21,22,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4627-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.010
BindingDB Entry DOI: 10.7270/Q2TT4QDX |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151527

(4-{5-[(Z)-Cyclohexylimino]-4-methyl-4,5-dihydro-[1...)Show SMILES Cn1nc(s\c1=N/C1CCCCC1)-c1ccc(cc1)C(=O)NCCN1CCOCC1 Show InChI InChI=1S/C22H31N5O2S/c1-26-22(24-19-5-3-2-4-6-19)30-21(25-26)18-9-7-17(8-10-18)20(28)23-11-12-27-13-15-29-16-14-27/h7-10,19H,2-6,11-16H2,1H3,(H,23,28)/b24-22- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4607-13 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.008
BindingDB Entry DOI: 10.7270/Q2377855 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151641

(2-[8''-chloro-2''-oxospiro[cyclohexane-1,4''-(1'',...)Show InChI InChI=1S/C15H17ClN2O4/c16-9-4-5-10(22-8-11(19)20)12-13(9)17-14(21)18-15(12)6-2-1-3-7-15/h4-5H,1-3,6-8H2,(H,19,20)(H2,17,18,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4627-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.010
BindingDB Entry DOI: 10.7270/Q2TT4QDX |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151630
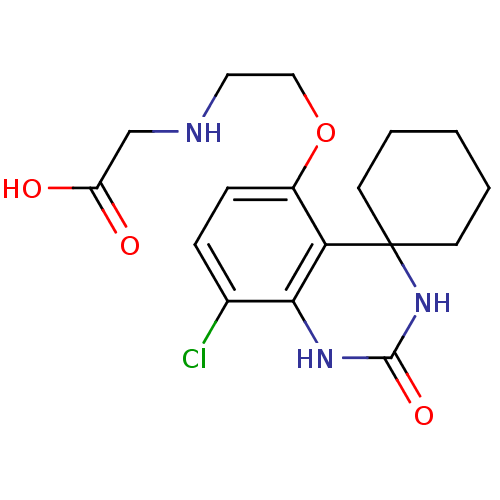
(2-{2-[8''-chloro-2''-oxospiro[cyclohexane-1,4''-(1...)Show InChI InChI=1S/C17H22ClN3O4/c18-11-4-5-12(25-9-8-19-10-13(22)23)14-15(11)20-16(24)21-17(14)6-2-1-3-7-17/h4-5,19H,1-3,6-10H2,(H,22,23)(H2,20,21,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4627-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.010
BindingDB Entry DOI: 10.7270/Q2TT4QDX |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458458

(CHEMBL4210729)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C34H33ClN4O3/c1-42-24-14-11-22(12-15-24)20-39-21-28(33(40)27-8-3-5-10-31(27)39)34(41)37-18-6-17-36-32-25-7-2-4-9-29(25)38-30-19-23(35)13-16-26(30)32/h3,5,8,10-16,19,21H,2,4,6-7,9,17-18,20H2,1H3,(H,36,38)(H,37,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151647
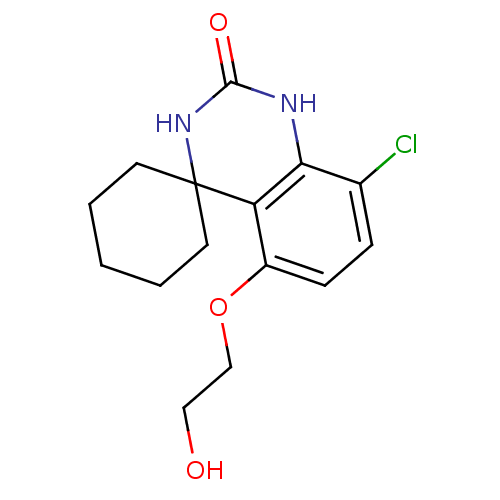
(8''-chloro-5''-(2-hydroxyethoxy)spiro[cyclohexane-...)Show InChI InChI=1S/C15H19ClN2O3/c16-10-4-5-11(21-9-8-19)12-13(10)17-14(20)18-15(12)6-2-1-3-7-15/h4-5,19H,1-3,6-9H2,(H2,17,18,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human PDE7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4627-31 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.010
BindingDB Entry DOI: 10.7270/Q2TT4QDX |
More data for this
Ligand-Target Pair | |
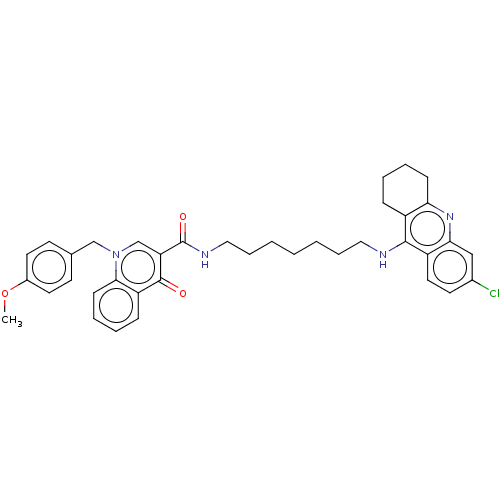
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458457
(CHEMBL4213591)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C38H41ClN4O3/c1-46-28-18-15-26(16-19-28)24-43-25-32(37(44)31-12-6-8-14-35(31)43)38(45)41-22-10-4-2-3-9-21-40-36-29-11-5-7-13-33(29)42-34-23-27(39)17-20-30(34)36/h6,8,12,14-20,23,25H,2-5,7,9-11,13,21-22,24H2,1H3,(H,40,42)(H,41,45) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50458451
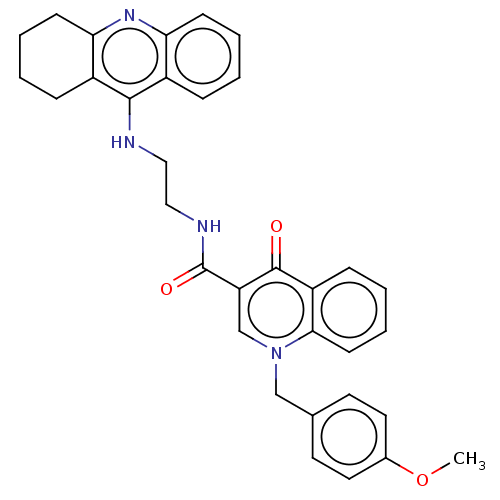
(CHEMBL4205374)Show SMILES COc1ccc(Cn2cc(C(=O)NCCNc3c4CCCCc4nc4ccccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C33H32N4O3/c1-40-23-16-14-22(15-17-23)20-37-21-27(32(38)26-10-4-7-13-30(26)37)33(39)35-19-18-34-31-24-8-2-5-11-28(24)36-29-12-6-3-9-25(29)31/h2,4-5,7-8,10-11,13-17,21H,3,6,9,12,18-20H2,1H3,(H,34,36)(H,35,39) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151546
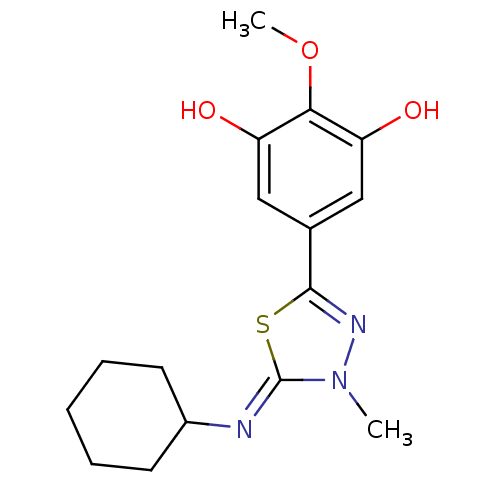
(5-{5-[(Z)-Cyclohexylimino]-4-methyl-4,5-dihydro-[1...)Show InChI InChI=1S/C16H21N3O3S/c1-19-16(17-11-6-4-3-5-7-11)23-15(18-19)10-8-12(20)14(22-2)13(21)9-10/h8-9,11,20-21H,3-7H2,1-2H3/b17-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4607-13 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.008
BindingDB Entry DOI: 10.7270/Q2377855 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151526
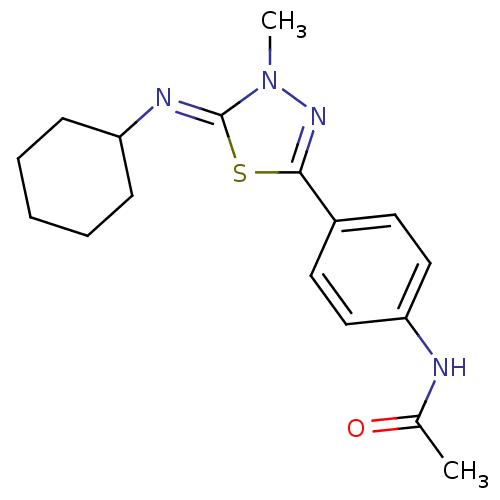
(CHEMBL363097 | N-(4-{5-[(Z)-Cyclohexylimino]-4-met...)Show InChI InChI=1S/C17H22N4OS/c1-12(22)18-15-10-8-13(9-11-15)16-20-21(2)17(23-16)19-14-6-4-3-5-7-14/h8-11,14H,3-7H2,1-2H3,(H,18,22)/b19-17- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4607-13 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.008
BindingDB Entry DOI: 10.7270/Q2377855 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151569
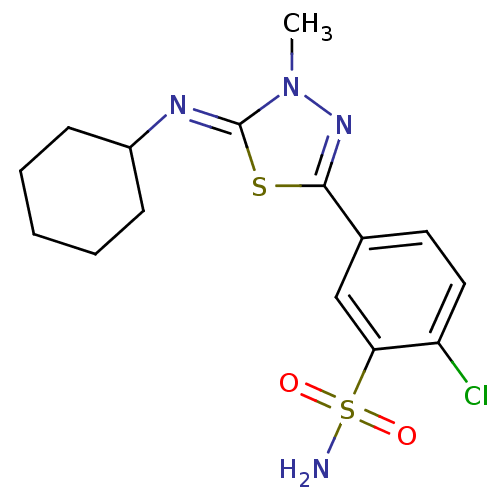
(2-Chloro-5-{5-[(Z)-cyclohexylimino]-4-methyl-4,5-d...)Show SMILES Cn1nc(s\c1=N/C1CCCCC1)-c1ccc(Cl)c(c1)S(N)(=O)=O Show InChI InChI=1S/C15H19ClN4O2S2/c1-20-15(18-11-5-3-2-4-6-11)23-14(19-20)10-7-8-12(16)13(9-10)24(17,21)22/h7-9,11H,2-6H2,1H3,(H2,17,21,22)/b18-15- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4607-13 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.008
BindingDB Entry DOI: 10.7270/Q2377855 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
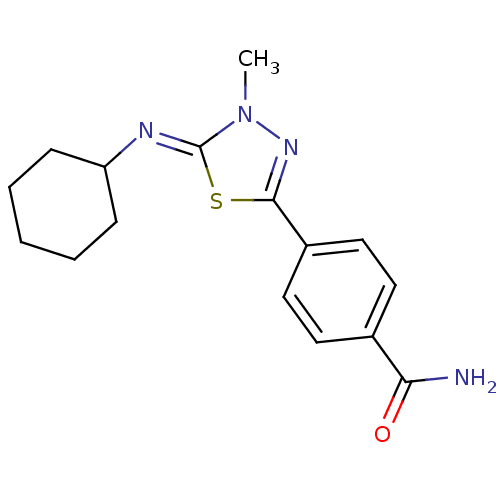
(Homo sapiens (Human)) | BDBM50151533
(4-{5-[(Z)-Cyclohexylimino]-4-methyl-4,5-dihydro-[1...)Show InChI InChI=1S/C16H20N4OS/c1-20-16(18-13-5-3-2-4-6-13)22-15(19-20)12-9-7-11(8-10-12)14(17)21/h7-10,13H,2-6H2,1H3,(H2,17,21)/b18-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4607-13 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.008
BindingDB Entry DOI: 10.7270/Q2377855 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151560
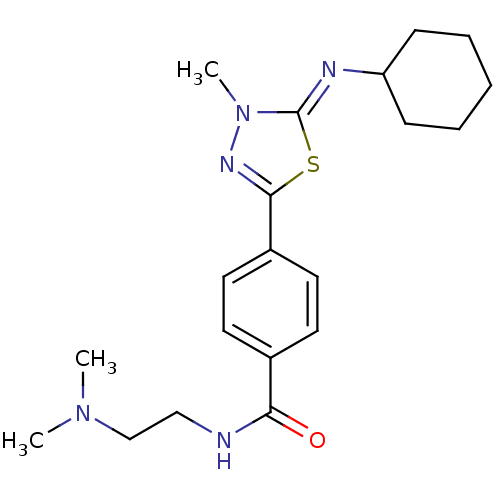
(4-{5-[(Z)-Cyclohexylimino]-4-methyl-4,5-dihydro-[1...)Show SMILES CN(C)CCNC(=O)c1ccc(cc1)-c1nn(C)\c(=N\C2CCCCC2)s1 Show InChI InChI=1S/C20H29N5OS/c1-24(2)14-13-21-18(26)15-9-11-16(12-10-15)19-23-25(3)20(27-19)22-17-7-5-4-6-8-17/h9-12,17H,4-8,13-14H2,1-3H3,(H,21,26)/b22-20- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4607-13 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.008
BindingDB Entry DOI: 10.7270/Q2377855 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 4
(Homo sapiens (Human)) | BDBM50577661

(CHEMBL4879048) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant full length N-terminal GST tagged MEK4 expressed in Sf21 cells using p38 alpha as substrate pre-incubated for 30 mins... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00376
BindingDB Entry DOI: 10.7270/Q20C50MC |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50126143

(Epacadostat | INCB-024360)Show SMILES NS(=O)(=O)NCCNc1nonc1\C(Nc1ccc(F)c(Br)c1)=N\O Show InChI InChI=1S/C11H13BrFN7O4S/c12-7-5-6(1-2-8(7)13)17-11(18-21)9-10(20-24-19-9)15-3-4-16-25(14,22)23/h1-2,5,16,21H,3-4H2,(H,15,20)(H,17,18)(H2,14,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Bryn Mawr College
Curated by ChEMBL
| Assay Description
Inhibition of IDO1 in human HeLa cells using L-tryptophan as substrate after 20 hrs |
Eur J Med Chem 162: 455-464 (2019)
Article DOI: 10.1016/j.ejmech.2018.11.010
BindingDB Entry DOI: 10.7270/Q2959MV6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458459
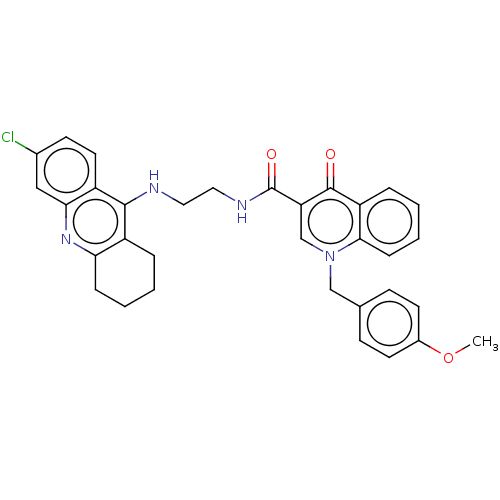
(CHEMBL4215217)Show SMILES COc1ccc(Cn2cc(C(=O)NCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C33H31ClN4O3/c1-41-23-13-10-21(11-14-23)19-38-20-27(32(39)26-7-3-5-9-30(26)38)33(40)36-17-16-35-31-24-6-2-4-8-28(24)37-29-18-22(34)12-15-25(29)31/h3,5,7,9-15,18,20H,2,4,6,8,16-17,19H2,1H3,(H,35,37)(H,36,40) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151531
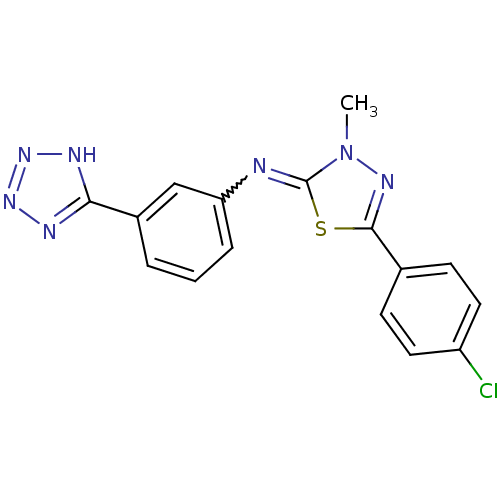
(CHEMBL185203 | [5-(4-Chloro-phenyl)-3-methyl-3H-[1...)Show SMILES Cn1nc(sc1=Nc1cccc(c1)-c1nnn[nH]1)-c1ccc(Cl)cc1 |w:6.7| Show InChI InChI=1S/C16H12ClN7S/c1-24-16(25-15(21-24)10-5-7-12(17)8-6-10)18-13-4-2-3-11(9-13)14-19-22-23-20-14/h2-9H,1H3,(H,19,20,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4607-13 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.008
BindingDB Entry DOI: 10.7270/Q2377855 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151561
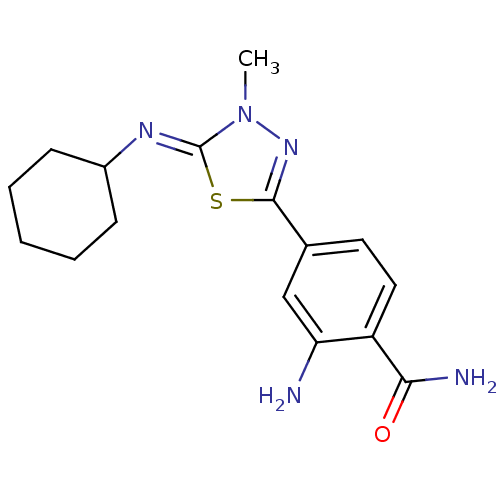
(2-Amino-4-{5-[(Z)-cyclohexylimino]-4-methyl-4,5-di...)Show InChI InChI=1S/C16H21N5OS/c1-21-16(19-11-5-3-2-4-6-11)23-15(20-21)10-7-8-12(14(18)22)13(17)9-10/h7-9,11H,2-6,17H2,1H3,(H2,18,22)/b19-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4607-13 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.008
BindingDB Entry DOI: 10.7270/Q2377855 |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM50458459

(CHEMBL4215217)Show SMILES COc1ccc(Cn2cc(C(=O)NCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C33H31ClN4O3/c1-41-23-13-10-21(11-14-23)19-38-20-27(32(39)26-7-3-5-9-30(26)38)33(40)36-17-16-35-31-24-6-2-4-8-28(24)37-29-18-22(34)12-15-25(29)31/h3,5,7,9-15,18,20H,2,4,6,8,16-17,19H2,1H3,(H,35,37)(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 4
(Homo sapiens (Human)) | BDBM50577689

(CHEMBL4866649)Show SMILES NC(=O)c1ccc(NS(=O)(=O)c2cccc(c2)-c2n[nH]c3cc(F)ccc23)cc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant full length N-terminal GST tagged MEK4 expressed in Sf21 cells using p38 alpha as substrate pre-incubated for 30 mins... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00376
BindingDB Entry DOI: 10.7270/Q20C50MC |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human plasmatic BuChE using butyrylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458448
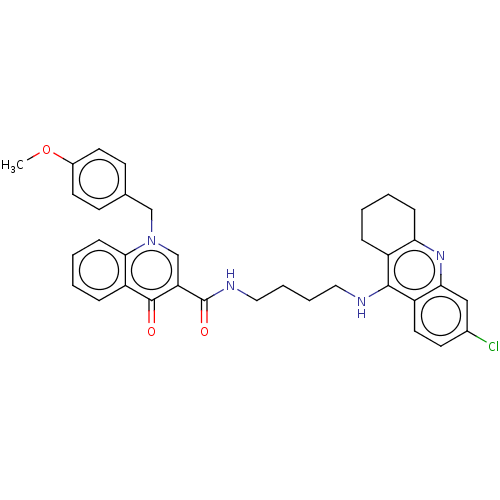
(CHEMBL4215154)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C35H35ClN4O3/c1-43-25-15-12-23(13-16-25)21-40-22-29(34(41)28-9-3-5-11-32(28)40)35(42)38-19-7-6-18-37-33-26-8-2-4-10-30(26)39-31-20-24(36)14-17-27(31)33/h3,5,9,11-17,20,22H,2,4,6-8,10,18-19,21H2,1H3,(H,37,39)(H,38,42) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
High affinity cAMP-specific 3',5'-cyclic phosphodiesterase 7A
(Homo sapiens (Human)) | BDBM50151575
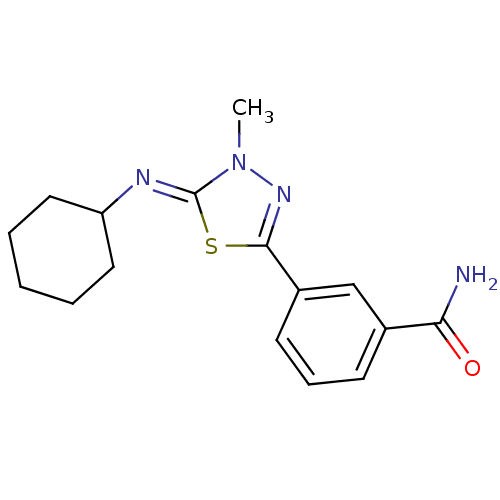
(3-{5-[(Z)-Cyclohexylimino]-4-methyl-4,5-dihydro-[1...)Show InChI InChI=1S/C16H20N4OS/c1-20-16(18-13-8-3-2-4-9-13)22-15(19-20)12-7-5-6-11(10-12)14(17)21/h5-7,10,13H,2-4,8-9H2,1H3,(H2,17,21)/b18-16- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of human Phosphodiesterase 7A1 expressed in baculovirus infected Sf9 cells |
Bioorg Med Chem Lett 14: 4607-13 (2004)
Article DOI: 10.1016/j.bmcl.2004.07.008
BindingDB Entry DOI: 10.7270/Q2377855 |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM50458444

(CHEMBL4217346)Show SMILES COc1ccc(Cn2cc(C(=O)NCCCCCCNc3c4CCCCc4nc4cc(Cl)ccc34)c(=O)c3ccccc23)cc1 Show InChI InChI=1S/C37H39ClN4O3/c1-45-27-17-14-25(15-18-27)23-42-24-31(36(43)30-11-5-7-13-34(30)42)37(44)40-21-9-3-2-8-20-39-35-28-10-4-6-12-32(28)41-33-22-26(38)16-19-29(33)35/h5,7,11,13-19,22,24H,2-4,6,8-10,12,20-21,23H2,1H3,(H,39,41)(H,40,44) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Defence
Curated by ChEMBL
| Assay Description
Inhibition of human erythrocyte AChE using acetylthiocholine iodide as substrate preincubated for 5 mins followed by substrate addition measured at 2... |
Eur J Med Chem 150: 292-306 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.083
BindingDB Entry DOI: 10.7270/Q2W95CT0 |
More data for this
Ligand-Target Pair | |
Dual specificity mitogen-activated protein kinase kinase 4
(Homo sapiens (Human)) | BDBM50577657
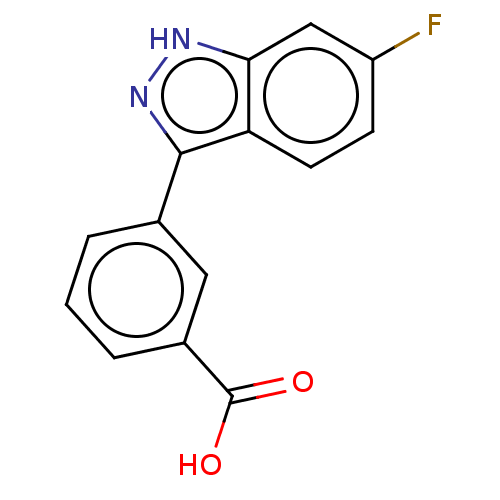
(CHEMBL4848865) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant full length N-terminal GST tagged MEK4 expressed in Sf21 cells using p38 alpha as substrate pre-incubated for 30 mins... |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00376
BindingDB Entry DOI: 10.7270/Q20C50MC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data