

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003699 (CHEMBL3234952) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003710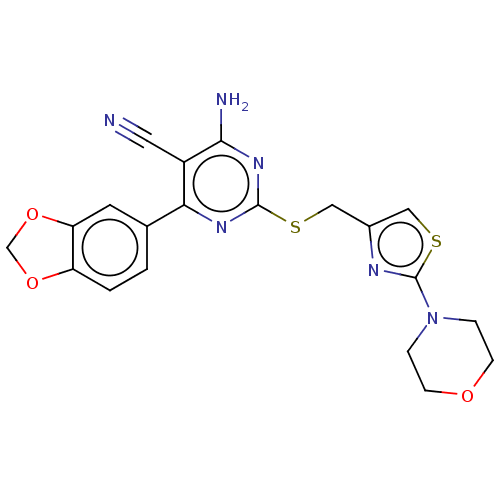 (CHEMBL3234964) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003708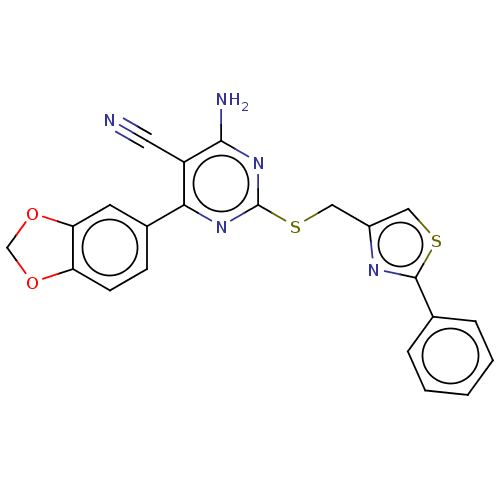 (CHEMBL3234962) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003700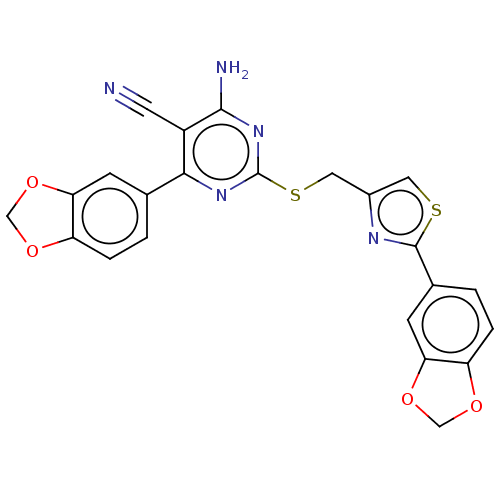 (CHEMBL3234953) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003698 (CHEMBL3234951) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50176494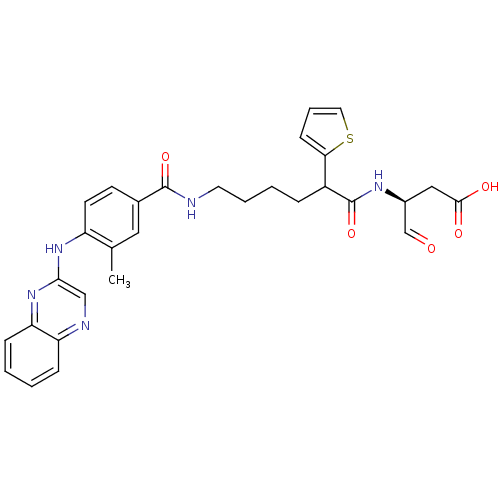 ((3S)-3-(6-(3-methyl-4-(quinoxalin-2-ylamino)benzam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human Caspase 1 | Bioorg Med Chem Lett 16: 559-62 (2005) Article DOI: 10.1016/j.bmcl.2005.10.048 BindingDB Entry DOI: 10.7270/Q2251HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50176493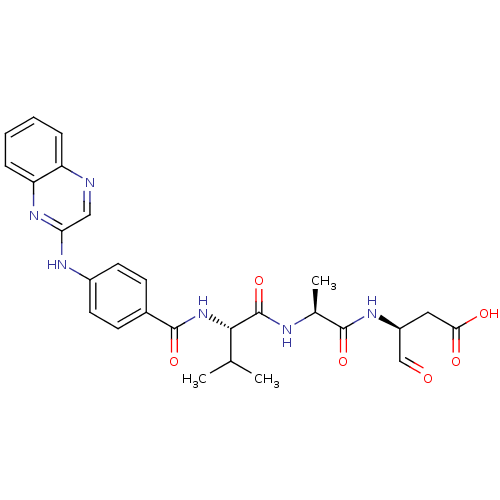 ((S)-3-((S)-2-((S)-3-methyl-2-(4-(quinoxalin-2-ylam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human Caspase 1 | Bioorg Med Chem Lett 16: 559-62 (2005) Article DOI: 10.1016/j.bmcl.2005.10.048 BindingDB Entry DOI: 10.7270/Q2251HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003648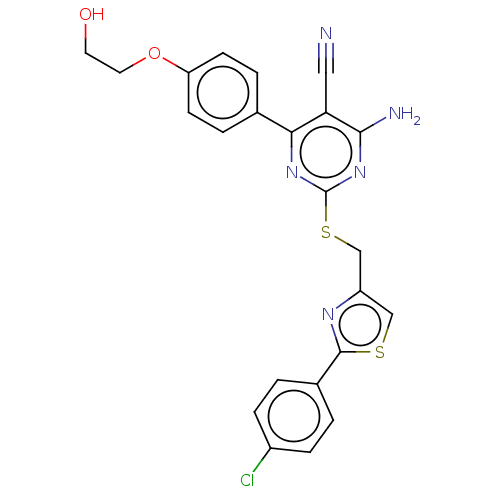 (CHEMBL3234936) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50176495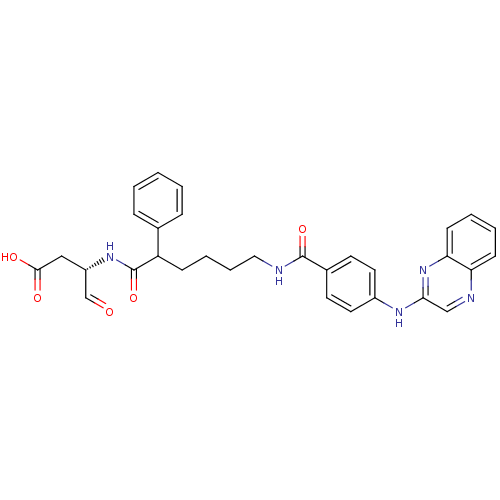 ((3S)-4-oxo-3-(2-phenyl-6-(4-(quinoxalin-2-ylamino)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human Caspase 1 | Bioorg Med Chem Lett 16: 559-62 (2005) Article DOI: 10.1016/j.bmcl.2005.10.048 BindingDB Entry DOI: 10.7270/Q2251HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50176496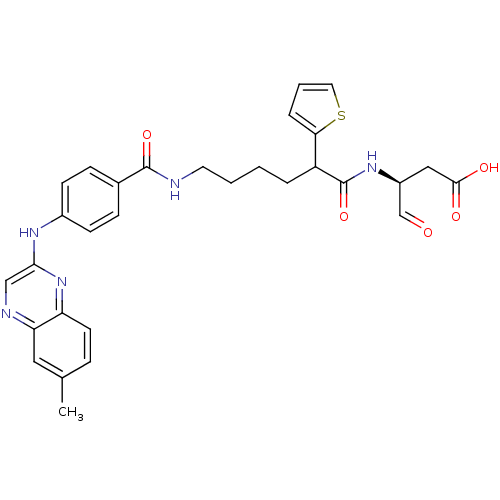 ((3S)-3-(6-(4-(6-methylquinoxalin-2-ylamino)benzami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human Caspase 1 | Bioorg Med Chem Lett 16: 559-62 (2005) Article DOI: 10.1016/j.bmcl.2005.10.048 BindingDB Entry DOI: 10.7270/Q2251HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50176510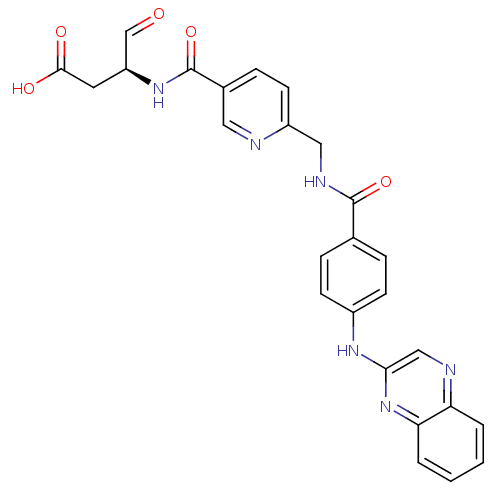 ((S)-4-oxo-3-(2-((4-(quinoxalin-2-ylamino)benzamido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human Caspase 1 | Bioorg Med Chem Lett 16: 559-62 (2005) Article DOI: 10.1016/j.bmcl.2005.10.048 BindingDB Entry DOI: 10.7270/Q2251HRD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003701 (CHEMBL3234954) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50432672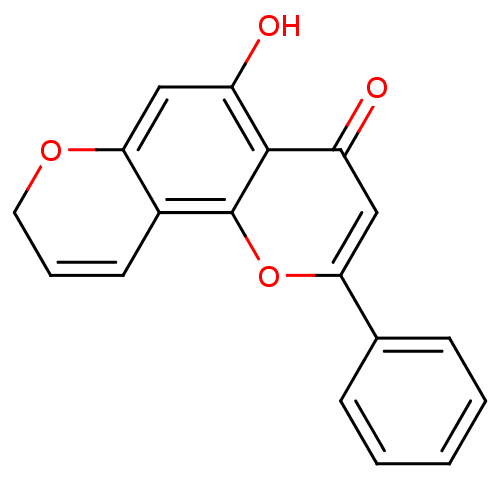 (CHEMBL2347912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1B1-dependent ethoxyresorufin-O-deethylase activity by spectrofluorimetric analysis in presence of NADPH regenerati... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003709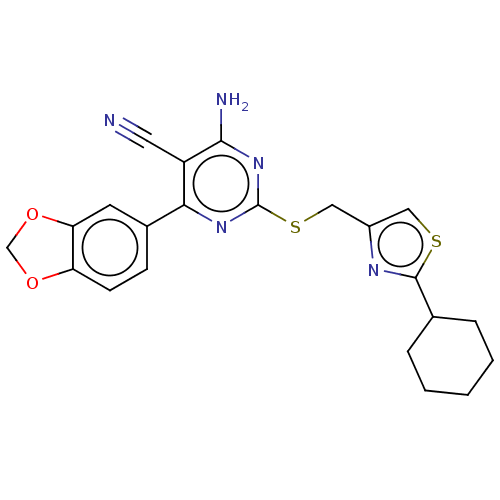 (CHEMBL3234963) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003696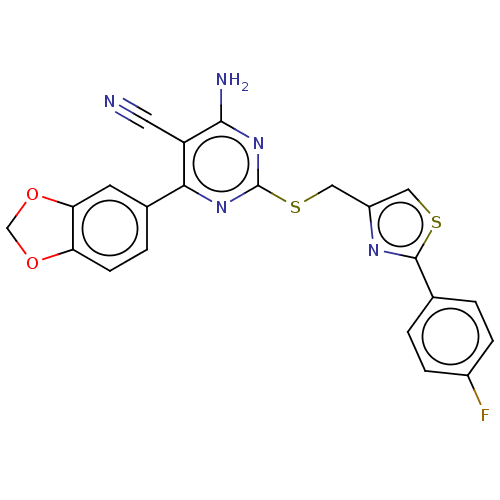 (CHEMBL3234949) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50176500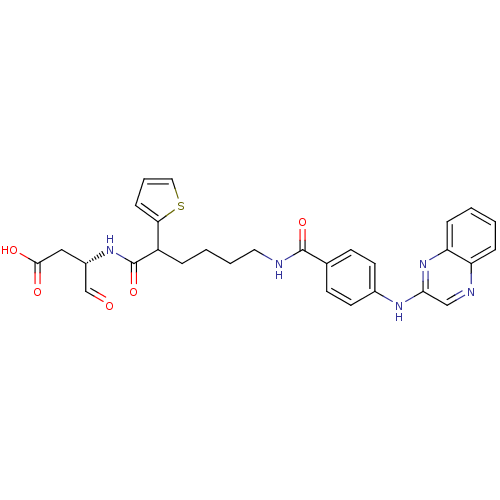 ((3S)-4-oxo-3-(6-(4-(quinoxalin-2-ylamino)benzamido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human Caspase 1 | Bioorg Med Chem Lett 16: 559-62 (2005) Article DOI: 10.1016/j.bmcl.2005.10.048 BindingDB Entry DOI: 10.7270/Q2251HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50176519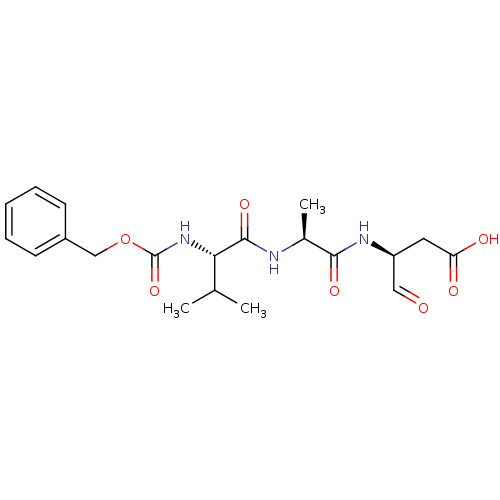 ((S)-3-[(S)-2-((S)-2-Benzyloxycarbonylamino-3-methy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human Caspase 1 | Bioorg Med Chem Lett 16: 559-62 (2005) Article DOI: 10.1016/j.bmcl.2005.10.048 BindingDB Entry DOI: 10.7270/Q2251HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50176509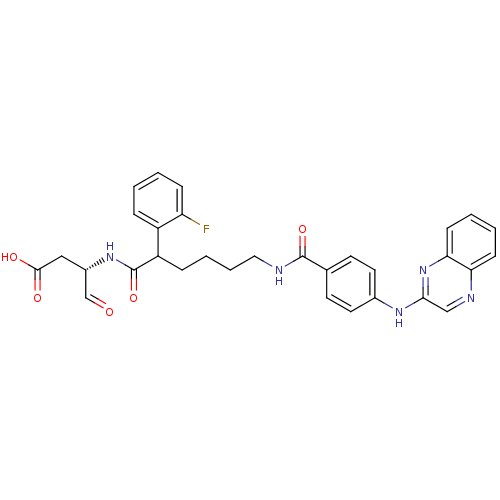 ((3S)-3-(2-(2-fluorophenyl)-6-(4-(quinoxalin-2-ylam...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human Caspase 1 | Bioorg Med Chem Lett 16: 559-62 (2005) Article DOI: 10.1016/j.bmcl.2005.10.048 BindingDB Entry DOI: 10.7270/Q2251HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003650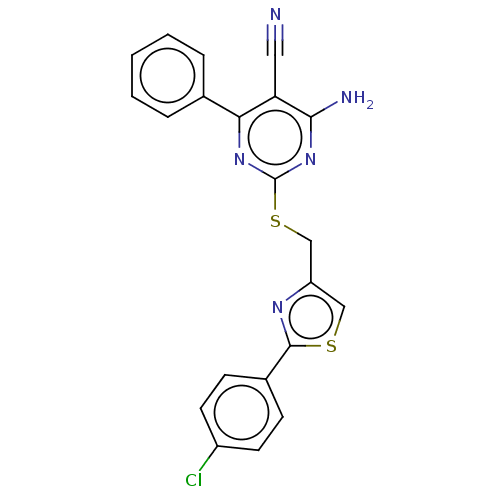 (CHEMBL3234938) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003621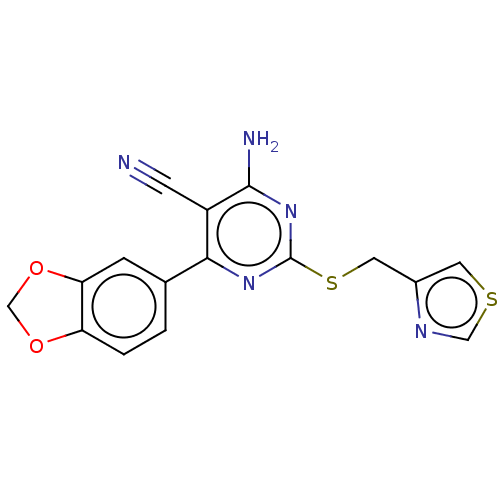 (CHEMBL3234965) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003702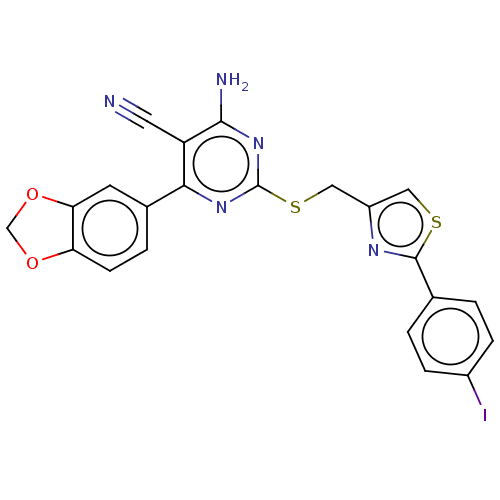 (CHEMBL3234955) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003707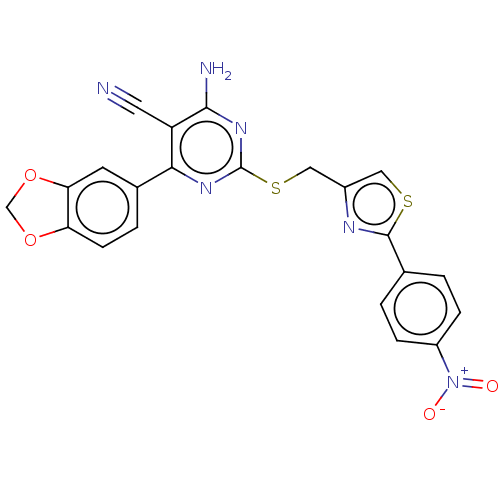 (CHEMBL3234961) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003705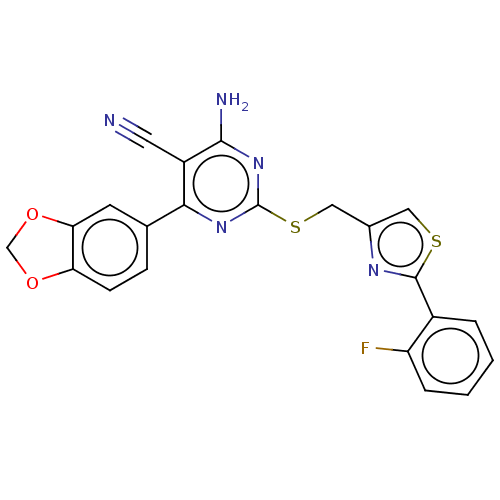 (CHEMBL3234959) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003697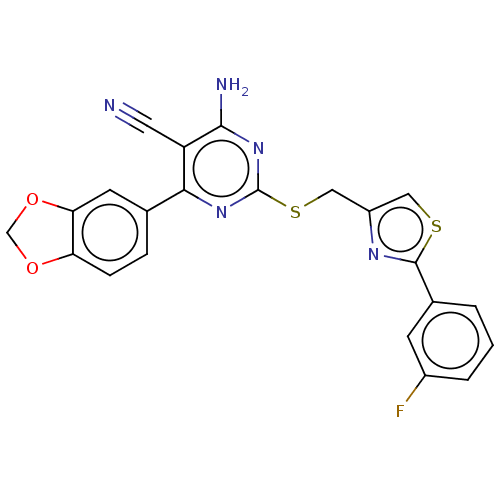 (CHEMBL3234950) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003649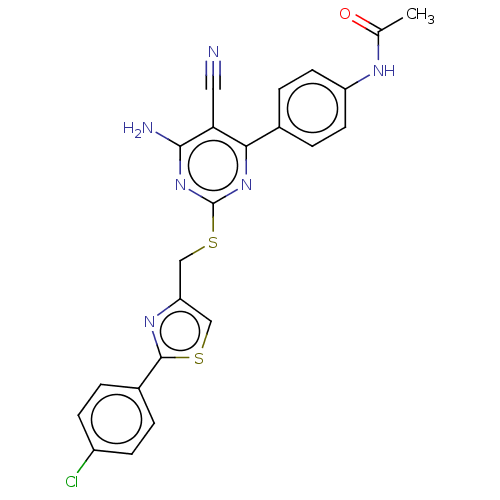 (CHEMBL3234937) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003692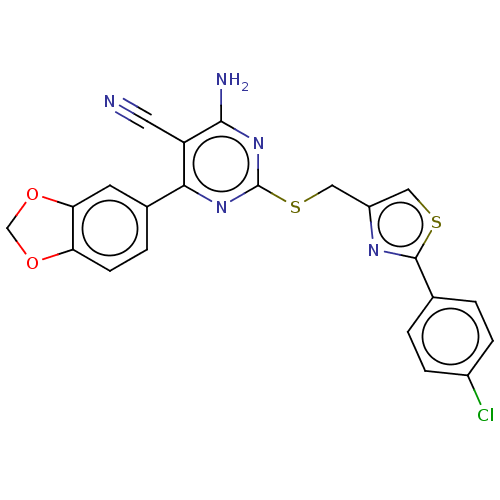 (CHEMBL3234945) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50432672 (CHEMBL2347912) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1A2-dependent methoxyresorufin-O-demethylase activity by spectrofluorimetric analysis in presence of NADPH regenera... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003689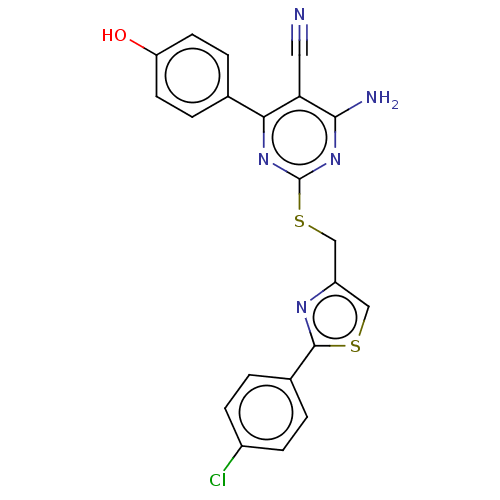 (CHEMBL3234940) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003695 (CHEMBL3234948) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003693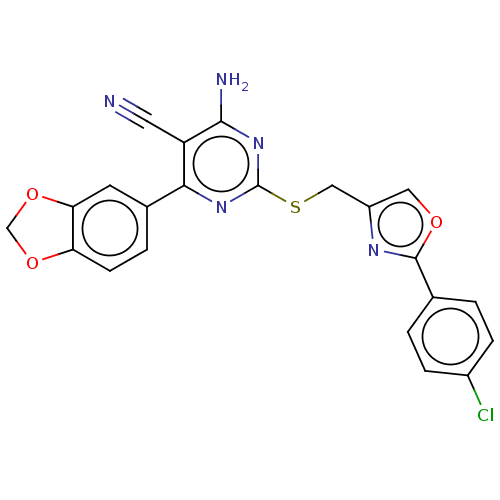 (CHEMBL3234946) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1B1 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1B1-dependent ethoxyresorufin-O-deethylase activity by spectrofluorimetric analysis in presence of NADPH regenerati... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50176505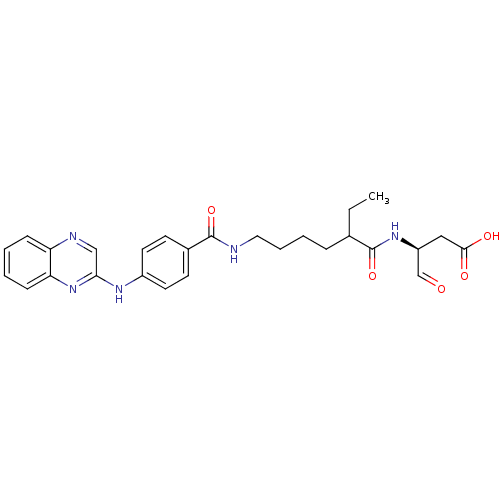 ((3S)-3-(2-ethyl-6-(4-(quinoxalin-2-ylamino)benzami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human Caspase 1 | Bioorg Med Chem Lett 16: 559-62 (2005) Article DOI: 10.1016/j.bmcl.2005.10.048 BindingDB Entry DOI: 10.7270/Q2251HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003706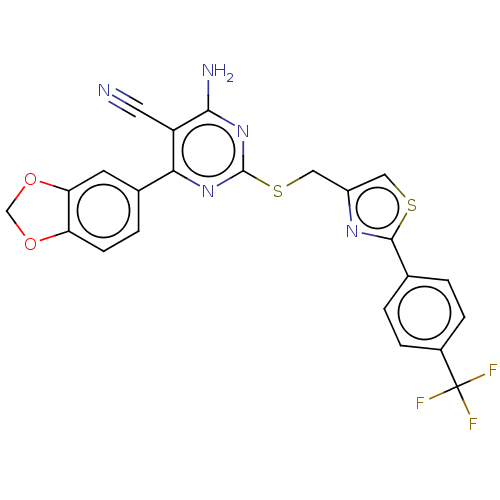 (CHEMBL3234960) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM263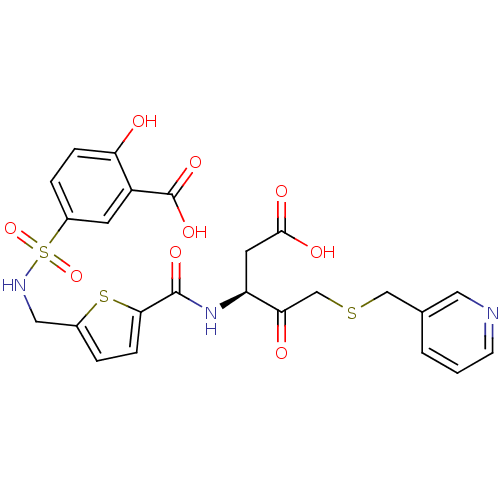 ((S)-5-({5-[1-Carboxymethyl-2-oxo-3-(pyridin-3-ylme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM226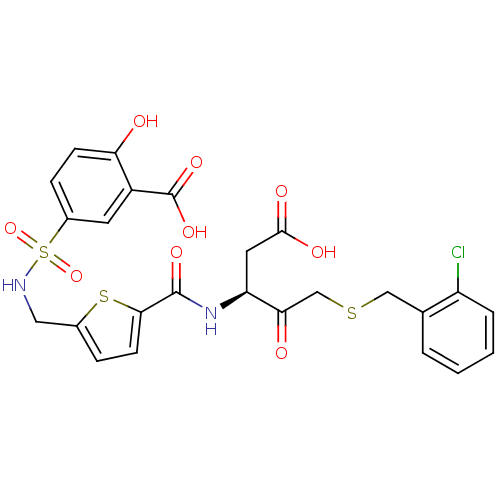 ((S)-5-({5-[1-Carboxymethyl-3-(2-chloro-benzylsulfa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM223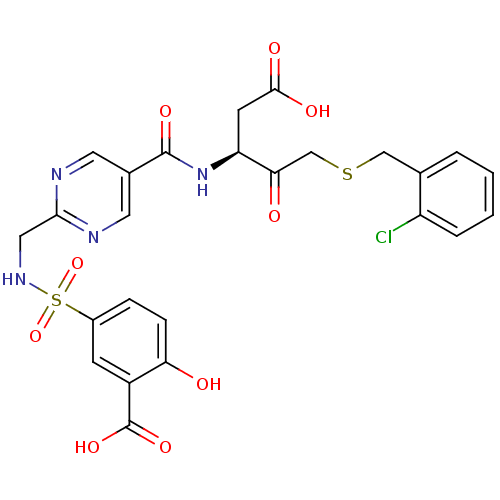 ((S)-5-({5-[1-Carboxymethyl-3-(2-chloro-benzylsulfa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM280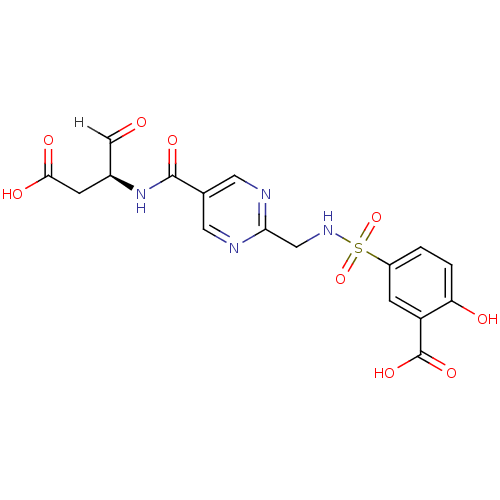 ((S)-5-{[5-(1-Carboxymethyl-2-oxo-ethylcarbamoyl)-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 20 | -43.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003694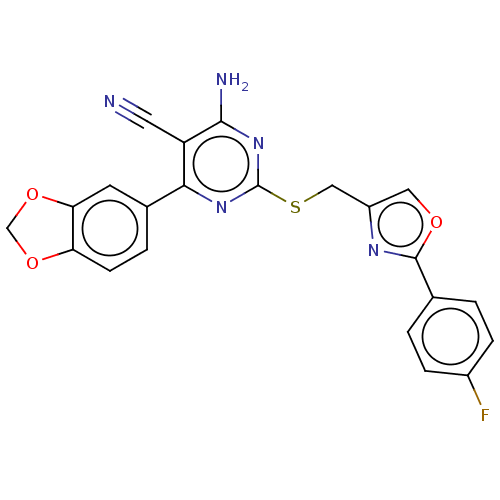 (CHEMBL3234947) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50014323 (2-PHENYL-4H-BENZO[H]CHROMEN-4-ONE | 2-Phenyl-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1A2-dependent methoxyresorufin-O-demethylase activity by spectrofluorimetric analysis in presence of NADPH regenera... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50113259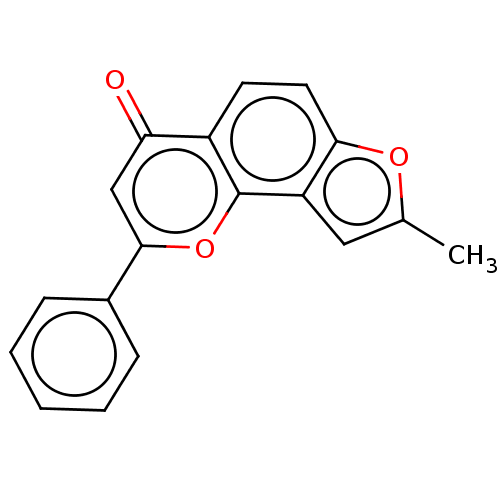 (CHEMBL3601433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1A2-dependent methoxyresorufin-O-demethylase activity by spectrofluorimetric analysis in presence of NADPH regenera... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM224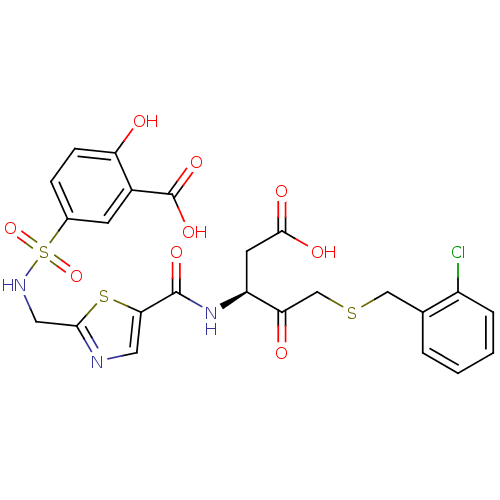 ((S)-5-({5-[1-Carboxymethyl-3-(2-chloro-benzylsulfa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM264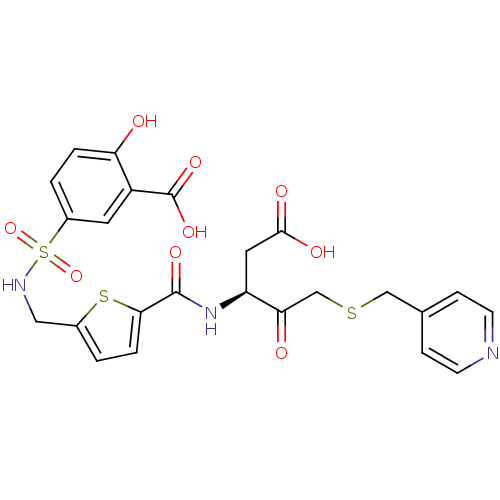 ((S)-5-({5-[1-Carboxymethyl-2-oxo-3-(pyridin-4-ylme...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 30 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM222 ((S)-5-({5-[1-Carboxymethyl-3-(2-chloro-benzylsulfa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 30 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50176508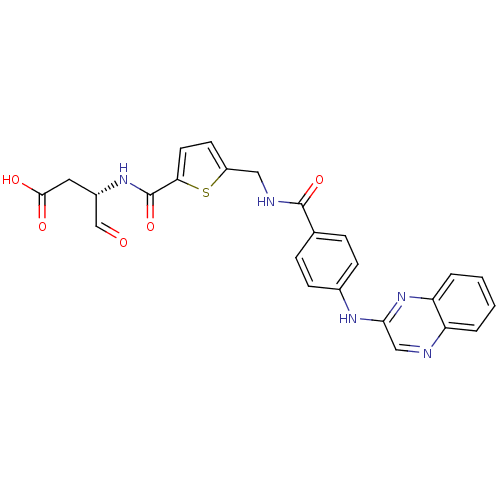 ((S)-4-oxo-3-(2-((4-(quinoxalin-2-ylamino)benzamido...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human Caspase 1 | Bioorg Med Chem Lett 16: 559-62 (2005) Article DOI: 10.1016/j.bmcl.2005.10.048 BindingDB Entry DOI: 10.7270/Q2251HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-3 (Homo sapiens (Human)) | BDBM227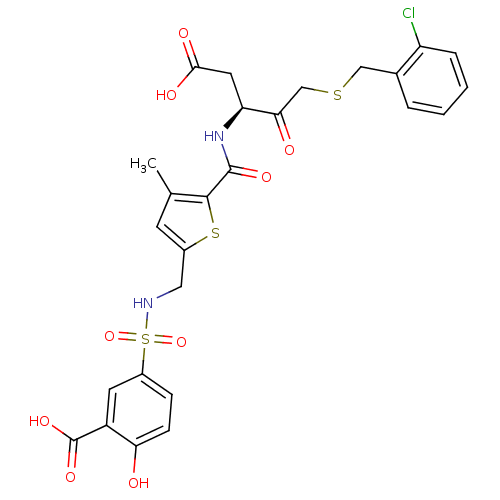 ((S)-5-({5-[1-Carboxymethyl-3-(2-chloro-benzylsulfa...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | 40 | -42.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Sunesis Pharmaceuticals | Assay Description The effectiveness of compounds against the activity of human recombinant caspase-1-8 was measured using fluorometric assays. Assays were carried out ... | J Med Chem 45: 5005-22 (2002) Article DOI: 10.1021/jm020230j BindingDB Entry DOI: 10.7270/Q2B56GW5 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A1 (Homo sapiens (Human)) | BDBM50113261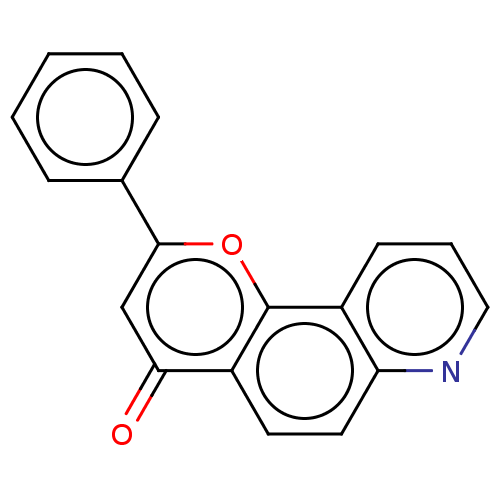 (CHEMBL3601435) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1A1-dependent ethoxyresorufin-O-deethylase activity by spectrofluorimetric analysis in presence of NADPH regenerati... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A2b (Homo sapiens (Human)) | BDBM50003621 (CHEMBL3234965) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]PSB603 from human adenosine A2B receptor expressed in CHO cell membranes after 2 hrs by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Adenosine receptor A1 (Homo sapiens (Human)) | BDBM50003703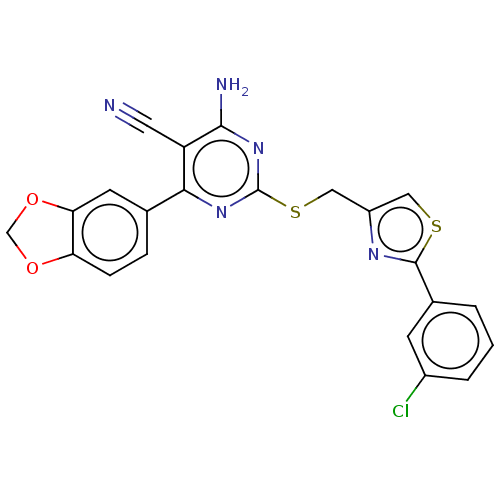 (CHEMBL3234956) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University Curated by ChEMBL | Assay Description Displacement of [3H]DPCPX from human adenosine A1 receptor expressed in CHO cell membranes after 1 hr by beta scintillation counting analysis | J Med Chem 57: 3213-22 (2014) Article DOI: 10.1021/jm401643m BindingDB Entry DOI: 10.7270/Q27P90X6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Caspase-1 (Homo sapiens (Human)) | BDBM50176502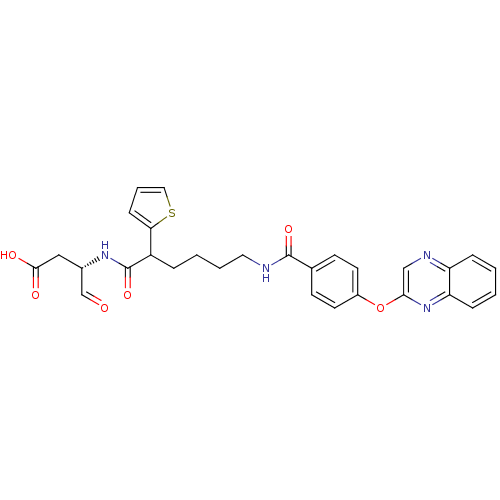 ((3S)-4-oxo-3-(6-(4-(quinoxalin-2-yloxy)benzamido)-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sunesis Pharmaceuticals Curated by ChEMBL | Assay Description Inhibitory activity against human Caspase 1 | Bioorg Med Chem Lett 16: 559-62 (2005) Article DOI: 10.1016/j.bmcl.2005.10.048 BindingDB Entry DOI: 10.7270/Q2251HRD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cytochrome P450 1A2 (Homo sapiens (Human)) | BDBM50113260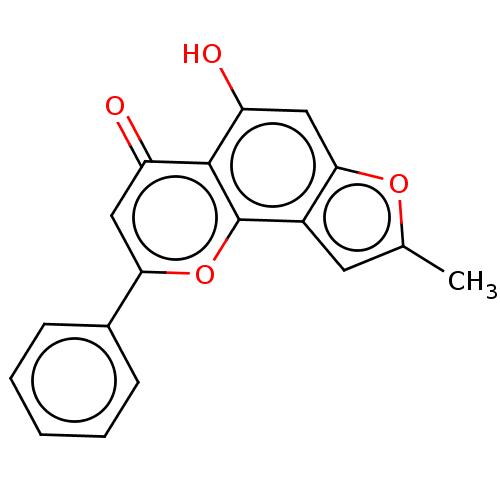 (CHEMBL3601434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Xavier University of Louisiana Curated by ChEMBL | Assay Description Inhibition of human microsomal CYP1A2-dependent methoxyresorufin-O-demethylase activity by spectrofluorimetric analysis in presence of NADPH regenera... | J Med Chem 58: 6481-93 (2015) Article DOI: 10.1021/acs.jmedchem.5b00494 BindingDB Entry DOI: 10.7270/Q2183895 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 3019 total ) | Next | Last >> |