

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50250852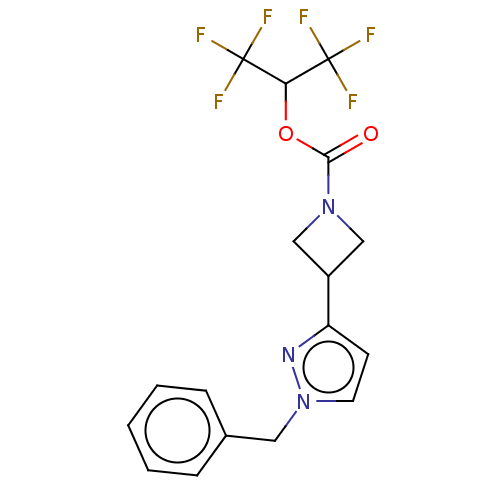 (CHEMBL4078217) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250848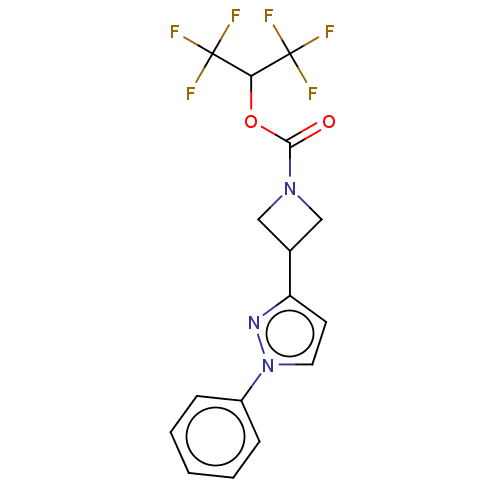 (CHEMBL4059676) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250859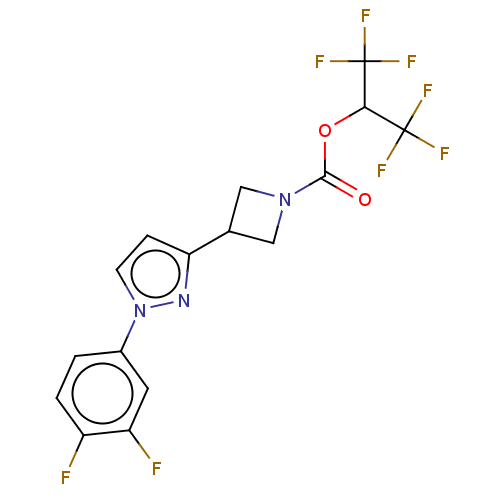 (CHEMBL4097203) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250855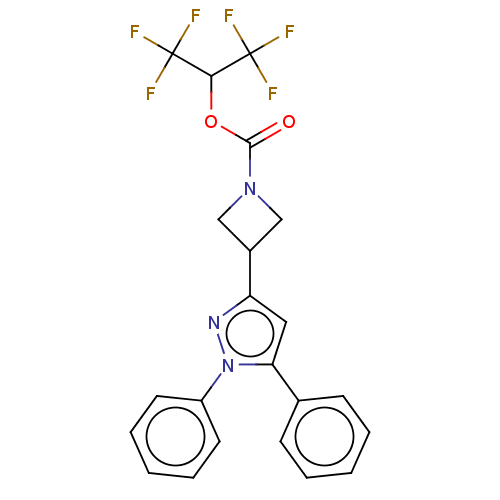 (CHEMBL4077745) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250850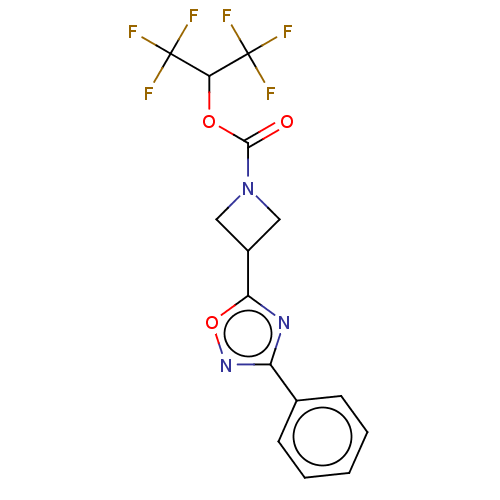 (CHEMBL4089505) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250852 (CHEMBL4078217) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50250855 (CHEMBL4077745) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50250859 (CHEMBL4097203) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.670 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250854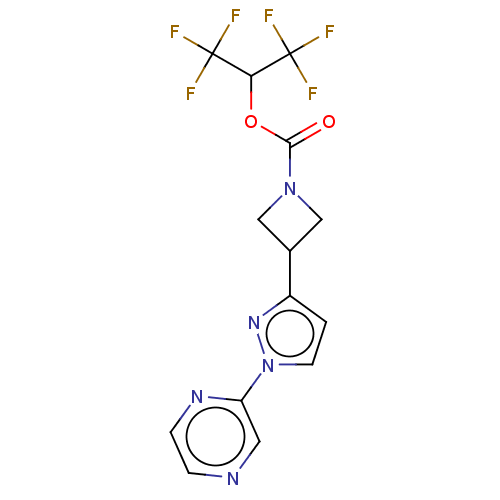 (CHEMBL4079190) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50250854 (CHEMBL4079190) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50086083 (1-[2-(3,4,5-Trimethoxy-phenyl)-pent-4-enoyl]-piper...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50086090 (1-[2-(3,4,5-Trimethoxy-phenyl)-butyryl]-piperidine...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250849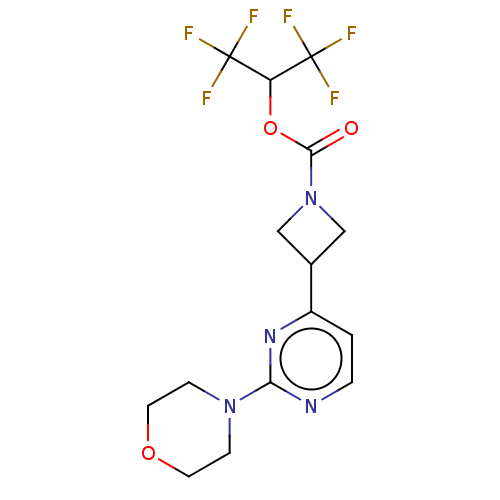 (CHEMBL4068332) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250853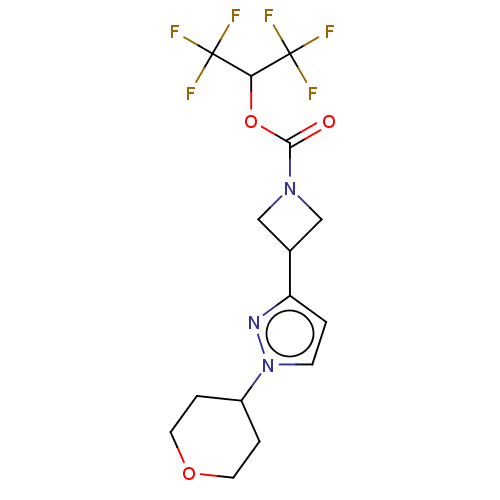 (CHEMBL4102496) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250851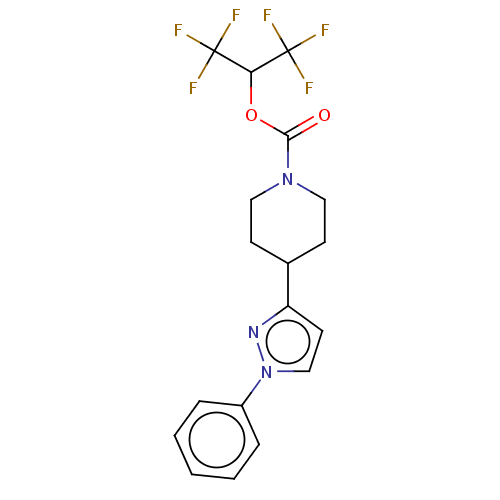 (CHEMBL4096459) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250858 (CHEMBL4078417) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250857 (CHEMBL4081625) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50086094 (1-[2-(3,4,5-Trimethoxy-phenyl)-pentanoyl]-piperidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50086077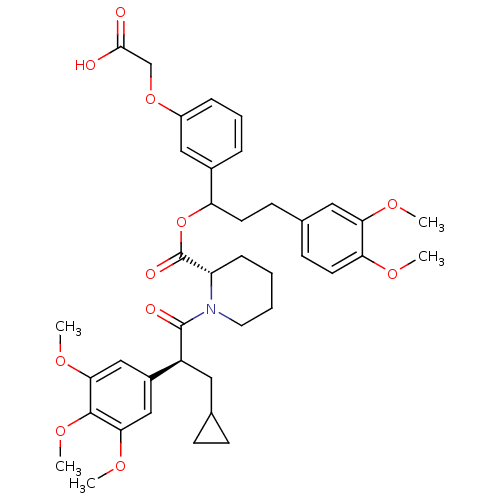 (1-[3-Cyclopropyl-2-(3,4,5-trimethoxy-phenyl)-propi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50086092 (1-[3-Methyl-2-(3,4,5-trimethoxy-phenyl)-butyryl]-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50250853 (CHEMBL4102496) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50086078 (1-(2-Cyclohexyl-2-phenyl-acetyl)-piperidine-2-carb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50086087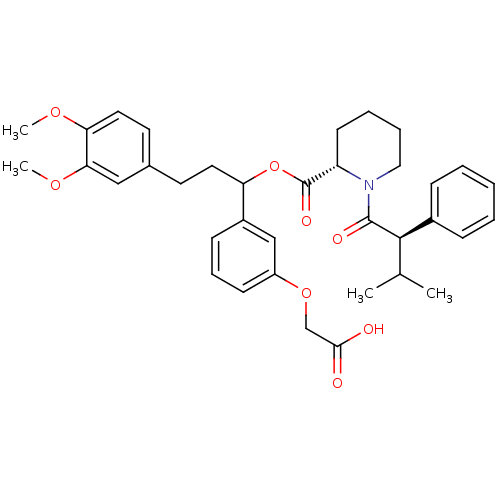 (1-(3-Methyl-2-phenyl-butyryl)-piperidine-2-carboxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50086082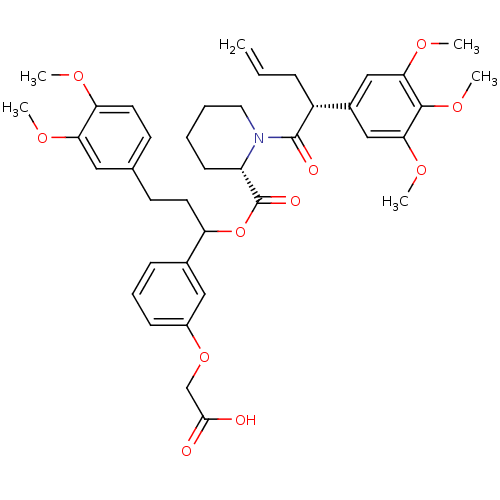 (1-[2-(3,4,5-Trimethoxy-phenyl)-pent-4-enoyl]-piper...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50250856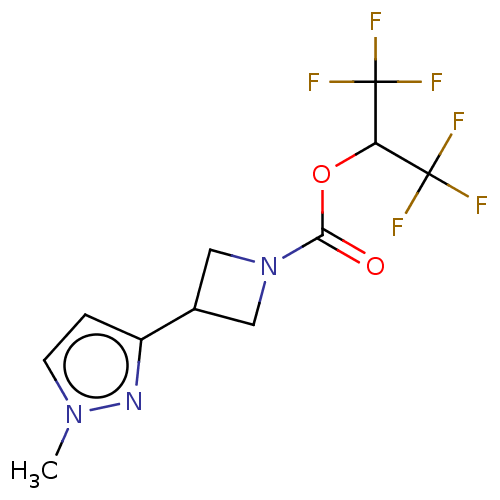 (CHEMBL4104911) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | >20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Monoglyceride lipase (Homo sapiens (Human)) | BDBM50250856 (CHEMBL4104911) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of MAGL in human brain vascular pericytes after 1 hr by TAMRA azide-based In-gel fluorescence method | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50086085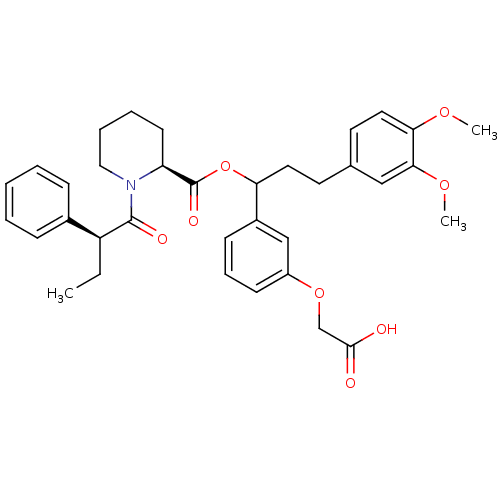 (1-(2-Phenyl-butyryl)-piperidine-2-carboxylic acid ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50086075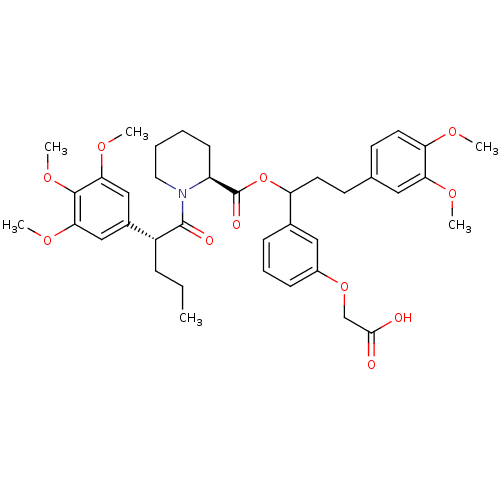 (1-[2-(3,4,5-Trimethoxy-phenyl)-pentanoyl]-piperidi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50086086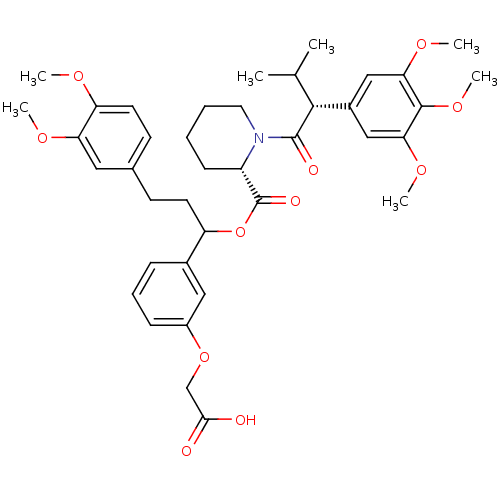 (1-[3-Methyl-2-(3,4,5-trimethoxy-phenyl)-butyryl]-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50086079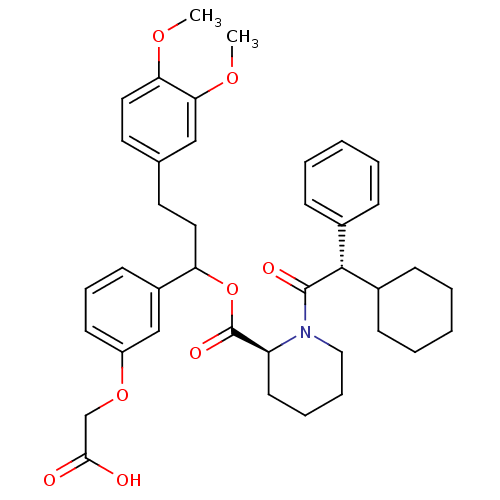 (1-(2-Cyclohexyl-2-phenyl-acetyl)-piperidine-2-carb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 66 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50086080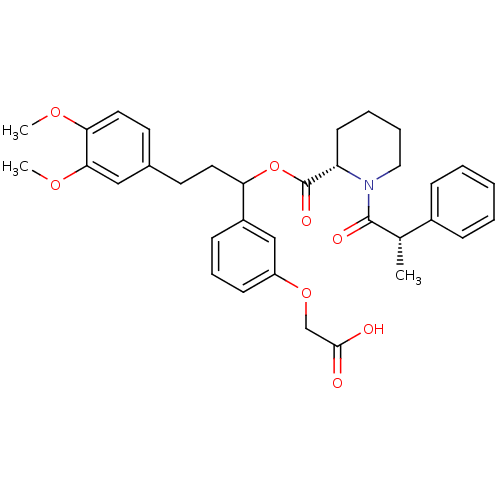 (1-(2-Phenyl-propionyl)-piperidine-2-carboxylic aci...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 94 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50086089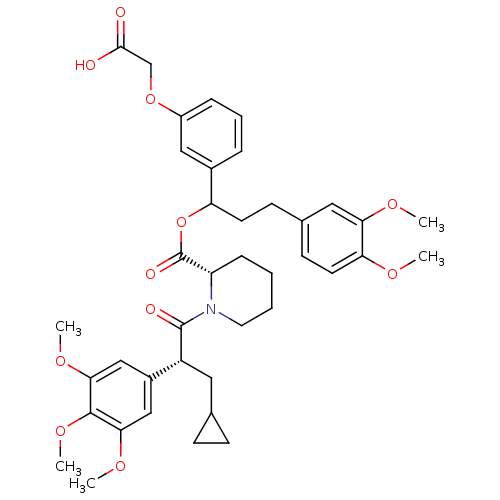 (1-[3-Cyclopropyl-2-(3,4,5-trimethoxy-phenyl)-propi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 126 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50086084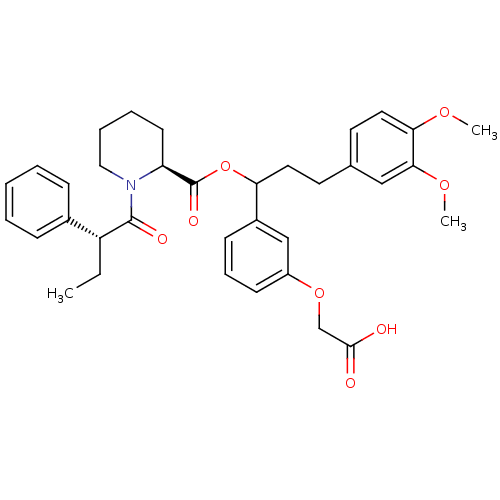 (1-(2-Phenyl-butyryl)-piperidine-2-carboxylic acid ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 133 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50086081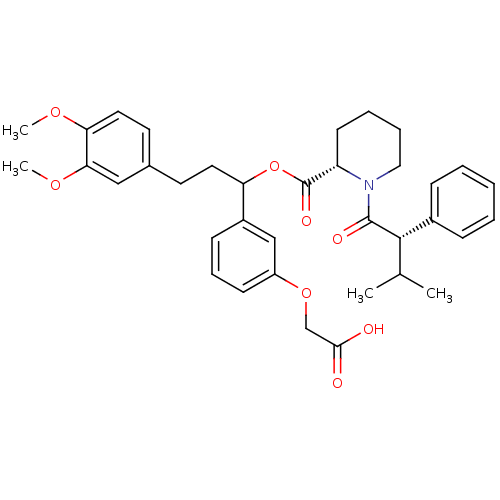 (1-(3-Methyl-2-phenyl-butyryl)-piperidine-2-carboxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50086093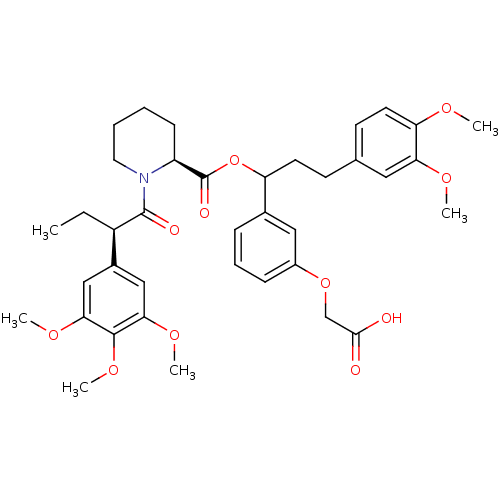 (1-[2-(3,4,5-Trimethoxy-phenyl)-butyryl]-piperidine...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 187 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50250848 (CHEMBL4059676) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50086076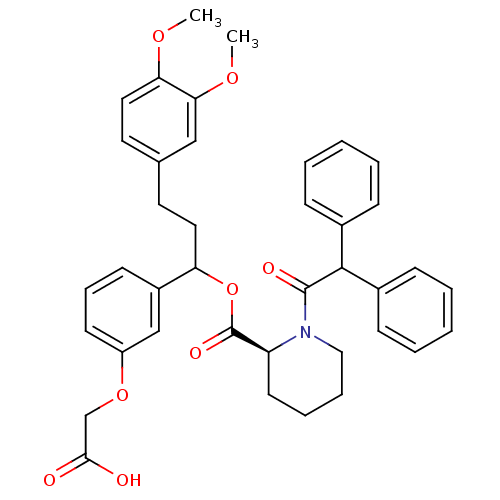 (1-Diphenylacetyl-piperidine-2-carboxylic acid 1-(3...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 273 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50250850 (CHEMBL4089505) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50086091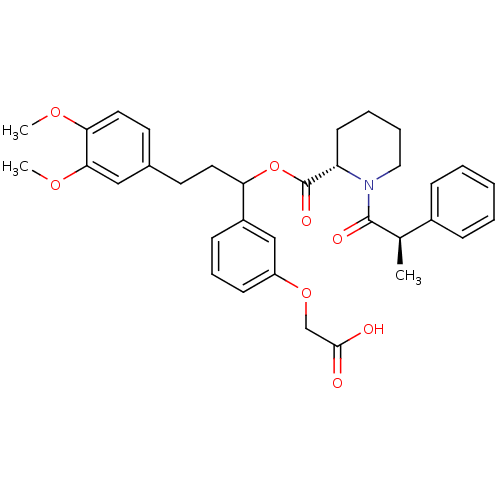 (1-(2-Phenyl-propionyl)-piperidine-2-carboxylic aci...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peptidyl-prolyl cis-trans isomerase FKBP1A (Homo sapiens (Human)) | BDBM50132547 (1-Phenylacetyl-piperidine-2-carboxylic acid 1-(3-c...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
ARIAD Gene Therapeutics Curated by ChEMBL | Assay Description Binding affinity for Mutant F36V-FK506 binding protein by competitive assay based on fluorescence polarization. | J Med Chem 43: 1135-42 (2000) BindingDB Entry DOI: 10.7270/Q2057F45 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50250857 (CHEMBL4081625) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Fatty-acid amide hydrolase 1 (Homo sapiens (Human)) | BDBM50250849 (CHEMBL4068332) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant FAAH using arachidonoyl-AMC as substrate preincubated for 30 mins followed by substrate addition measured after 60 mi... | J Med Chem 60: 9860-9873 (2017) Article DOI: 10.1021/acs.jmedchem.7b01531 BindingDB Entry DOI: 10.7270/Q2N300CG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||