

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 9.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of 20S proteasome in human erythrocytes using Suc-Leu-Leu-Val-Tyr-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari Aldo Moro Curated by ChEMBL | Assay Description Mixed type inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's method | ACS Med Chem Lett 11: 869-876 (2020) Article DOI: 10.1021/acsmedchemlett.9b00656 BindingDB Entry DOI: 10.7270/Q2WS8XS4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50540764 (CHEMBL4648322) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari Aldo Moro Curated by ChEMBL | Assay Description Mixed type inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's method | ACS Med Chem Lett 11: 869-876 (2020) Article DOI: 10.1021/acsmedchemlett.9b00656 BindingDB Entry DOI: 10.7270/Q2WS8XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Bifunctional methylenetetrahydrofolate dehydrogenase/cyclohydrolase, mitochondrial (Homo sapiens (Human)) | BDBM50123588 (CHEMBL297258) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Dundee Curated by ChEMBL | Assay Description Competitive inhibition of human FolD dehydrogenase activity | J Med Chem 58: 7938-48 (2015) Article DOI: 10.1021/acs.jmedchem.5b00687 BindingDB Entry DOI: 10.7270/Q2SN0BSR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50021350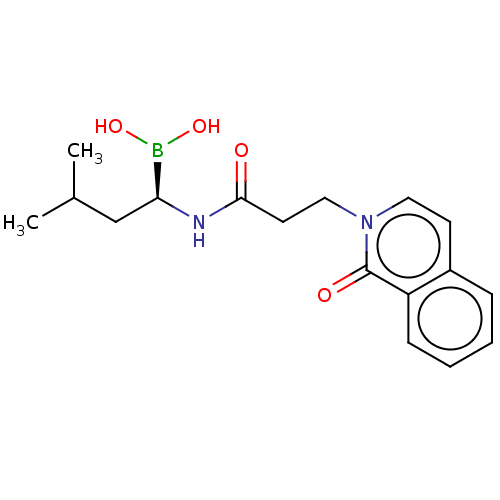 (CHEMBL3287940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 51 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of 20S proteasome in human erythrocytes using Suc-Leu-Leu-Val-Tyr-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50021352 (CHEMBL3287943) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 53 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of 20S proteasome in human erythrocytes using Suc-Leu-Leu-Val-Tyr-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50021348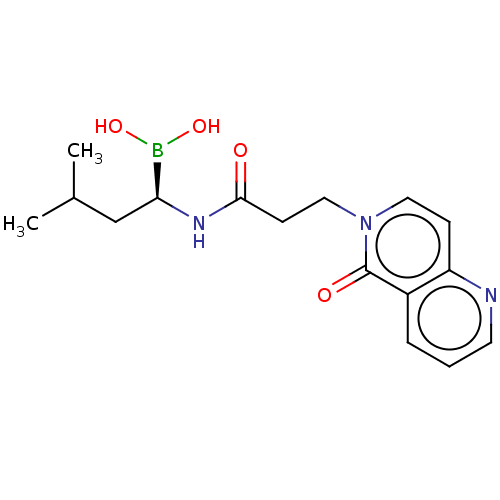 (CHEMBL3287937) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of 20S proteasome in human erythrocytes using Suc-Leu-Leu-Val-Tyr-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50021353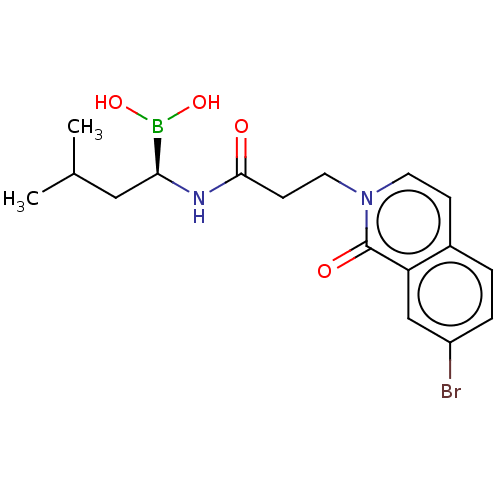 (CHEMBL3287944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of 20S proteasome in human erythrocytes using Suc-Leu-Leu-Val-Tyr-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50021355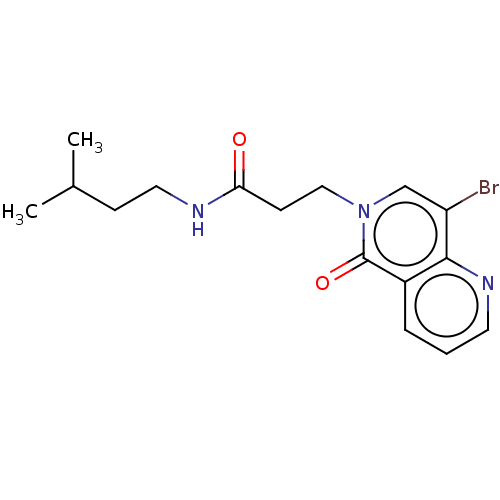 (CHEMBL3287946) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of 20S proteasome in human erythrocytes using Suc-Leu-Leu-Val-Tyr-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50021351 (CHEMBL3287942) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 85 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of 20S proteasome in human erythrocytes using Suc-Leu-Leu-Val-Tyr-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50424045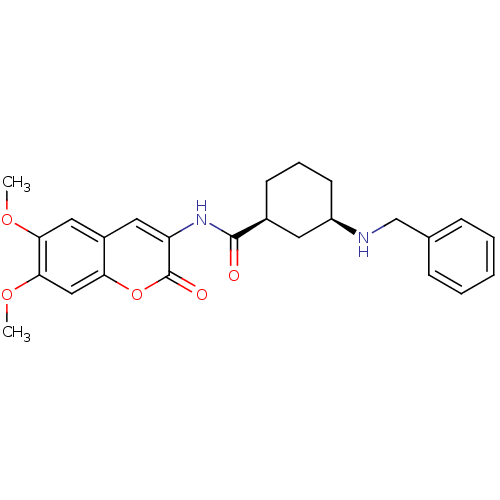 (CHEMBL2314726) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 93 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari Aldo Moro Curated by ChEMBL | Assay Description Mixed type inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's method | ACS Med Chem Lett 11: 869-876 (2020) Article DOI: 10.1021/acsmedchemlett.9b00656 BindingDB Entry DOI: 10.7270/Q2WS8XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50424045 (CHEMBL2314726) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 93.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bari Aldo Moro Curated by ChEMBL | Assay Description Mixed type inhibition of human AChE using acetylthiocholineiodide as substrate measured for every 30 sec for 5 mins by Ellman's method | ACS Med Chem Lett 11: 869-876 (2020) Article DOI: 10.1021/acsmedchemlett.9b00656 BindingDB Entry DOI: 10.7270/Q2WS8XS4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50434761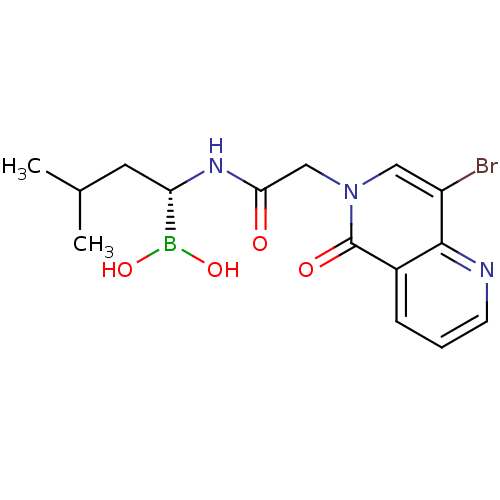 (CHEMBL2385819) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of 20S proteasome in human erythrocytes using Suc-Leu-Leu-Val-Tyr-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50021350 (CHEMBL3287940) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of 20S proteasome in human erythrocytes using Z-Leu-Leu-Glu-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50021352 (CHEMBL3287943) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of bovine pancreatic alpha-chymotrypsin using Suc-Leu-Leu-Val-Tyr-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50021352 (CHEMBL3287943) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of 20S proteasome in human erythrocytes using Z-Leu-Leu-Glu-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50434763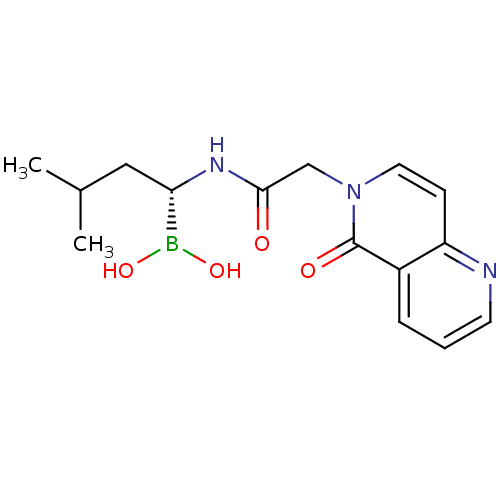 (CHEMBL2385817) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of 20S proteasome in human erythrocytes using Suc-Leu-Leu-Val-Tyr-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50021349 (CHEMBL3287939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of 20S proteasome in human erythrocytes using Suc-Leu-Leu-Val-Tyr-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50021348 (CHEMBL3287937) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 690 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of 20S proteasome in human erythrocytes using Z-Leu-Leu-Glu-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50021349 (CHEMBL3287939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 990 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of 20S proteasome in human erythrocytes using Z-Leu-Leu-Glu-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50021351 (CHEMBL3287942) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of 20S proteasome in human erythrocytes using Z-Leu-Leu-Glu-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50434763 (CHEMBL2385817) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of 20S proteasome in human erythrocytes using Z-Leu-Leu-Glu-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50021353 (CHEMBL3287944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of 20S proteasome in human erythrocytes using Z-Leu-Leu-Glu-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50021356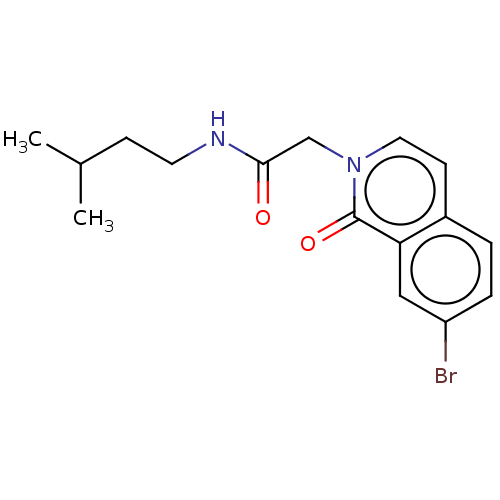 (CHEMBL3287947) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of 20S proteasome in human erythrocytes using Suc-Leu-Leu-Val-Tyr-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50069989 ((R)-3-methyl-1-((S)-3-phenyl-2-(pyrazine-2-carboxa...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of bovine pancreatic alpha-chymotrypsin using Suc-Leu-Leu-Val-Tyr-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50128352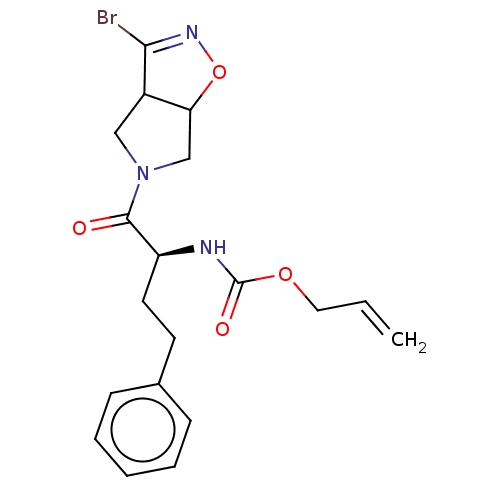 (CHEMBL3629184) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-7-amino-4-methylcoumarin assessed as substrate hydrolysis measured by fluore... | Bioorg Med Chem 23: 7053-60 (2015) Article DOI: 10.1016/j.bmc.2015.09.029 BindingDB Entry DOI: 10.7270/Q2ZW1NQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50128350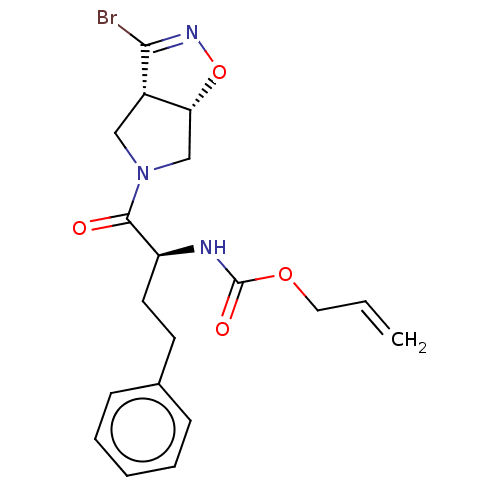 (CHEMBL3629186) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.73E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-7-amino-4-methylcoumarin assessed as substrate hydrolysis measured by fluore... | Bioorg Med Chem 23: 7053-60 (2015) Article DOI: 10.1016/j.bmc.2015.09.029 BindingDB Entry DOI: 10.7270/Q2ZW1NQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50128351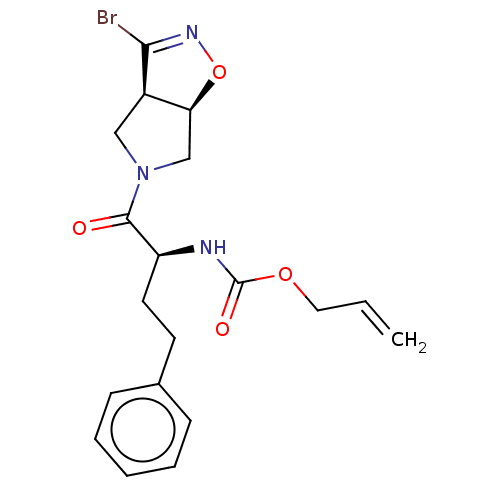 (CHEMBL3629185) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-7-amino-4-methylcoumarin assessed as substrate hydrolysis measured by fluore... | Bioorg Med Chem 23: 7053-60 (2015) Article DOI: 10.1016/j.bmc.2015.09.029 BindingDB Entry DOI: 10.7270/Q2ZW1NQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50021354 (CHEMBL3287945) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.57E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like activity of 20S proteasome in human erythrocytes using Suc-Leu-Leu-Val-Tyr-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551183 (CHEMBL4747846) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50128345 (CHEMBL3629346) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-7-amino-4-methylcoumarin assessed as substrate hydrolysis measured by fluore... | Bioorg Med Chem 23: 7053-60 (2015) Article DOI: 10.1016/j.bmc.2015.09.029 BindingDB Entry DOI: 10.7270/Q2ZW1NQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50128349 (CHEMBL3629339) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-7-amino-4-methylcoumarin assessed as substrate hydrolysis measured by fluore... | Bioorg Med Chem 23: 7053-60 (2015) Article DOI: 10.1016/j.bmc.2015.09.029 BindingDB Entry DOI: 10.7270/Q2ZW1NQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50128354 (CHEMBL3629181) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-7-amino-4-methylcoumarin assessed as substrate hydrolysis measured by fluore... | Bioorg Med Chem 23: 7053-60 (2015) Article DOI: 10.1016/j.bmc.2015.09.029 BindingDB Entry DOI: 10.7270/Q2ZW1NQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50128347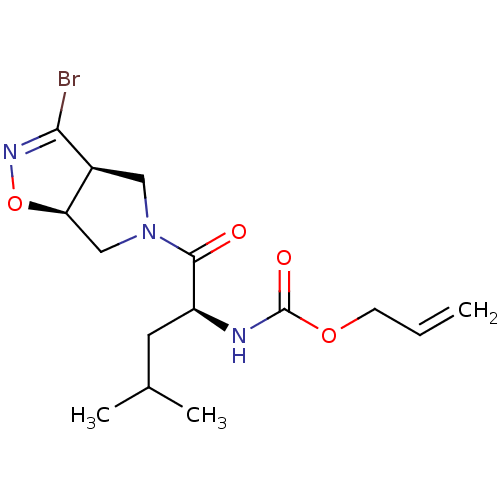 (CHEMBL3629341) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.52E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-7-amino-4-methylcoumarin assessed as substrate hydrolysis measured by fluore... | Bioorg Med Chem 23: 7053-60 (2015) Article DOI: 10.1016/j.bmc.2015.09.029 BindingDB Entry DOI: 10.7270/Q2ZW1NQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50128348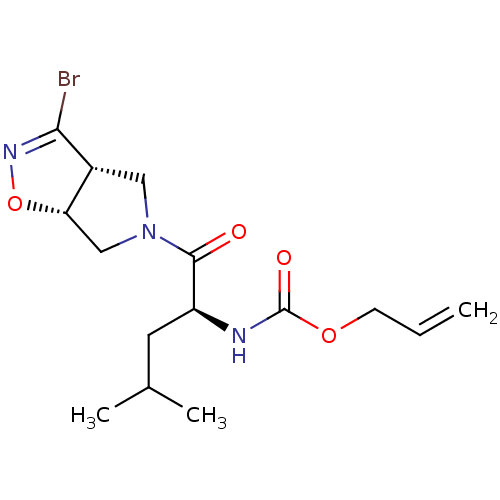 (CHEMBL3629340) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-7-amino-4-methylcoumarin assessed as substrate hydrolysis measured by fluore... | Bioorg Med Chem 23: 7053-60 (2015) Article DOI: 10.1016/j.bmc.2015.09.029 BindingDB Entry DOI: 10.7270/Q2ZW1NQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50021349 (CHEMBL3287939) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.11E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of bovine pancreatic alpha-chymotrypsin using Suc-Leu-Leu-Val-Tyr-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551184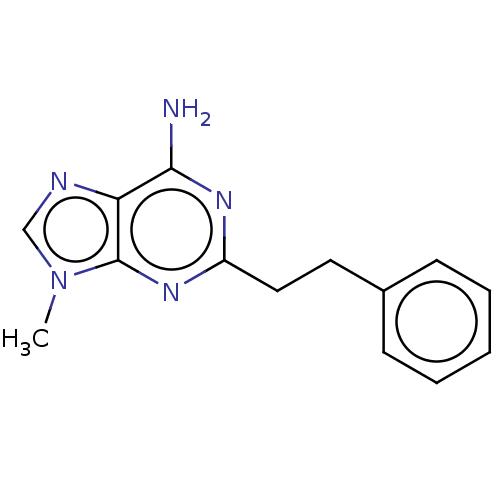 (CHEMBL4797185) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM108063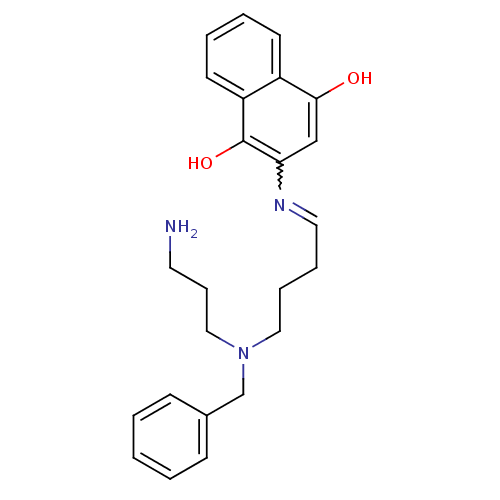 (2‐({4‐[(3‐ aminopropyl)(benzyl)a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+4 | -29.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Institute of Chemistry, Federal University of Rio de Janeiro, Rio de Janeiro, 21941-909, Brazil. | Assay Description The reaction mixture (final volume 200 µL) contained 5 µg/mL MAO-A or MAO-B in a 50 mM sodium phosphate buffer pH 7.4, 1 mM p-tyramine (sub... | Chem Biol Drug Des 83: 401-10 (2014) Article DOI: 10.1111/cbdd.12255 BindingDB Entry DOI: 10.7270/Q2K072W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50128346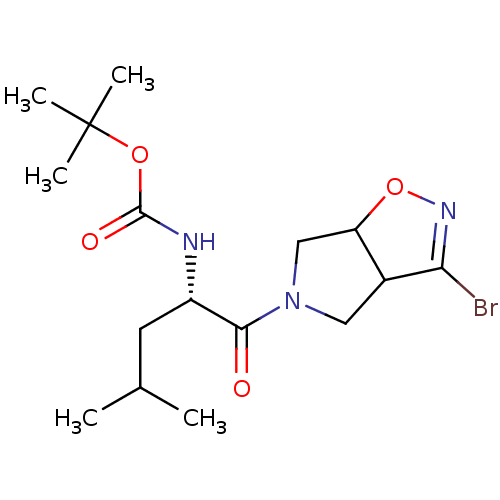 (CHEMBL3629344) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-7-amino-4-methylcoumarin assessed as substrate hydrolysis measured by fluore... | Bioorg Med Chem 23: 7053-60 (2015) Article DOI: 10.1016/j.bmc.2015.09.029 BindingDB Entry DOI: 10.7270/Q2ZW1NQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551180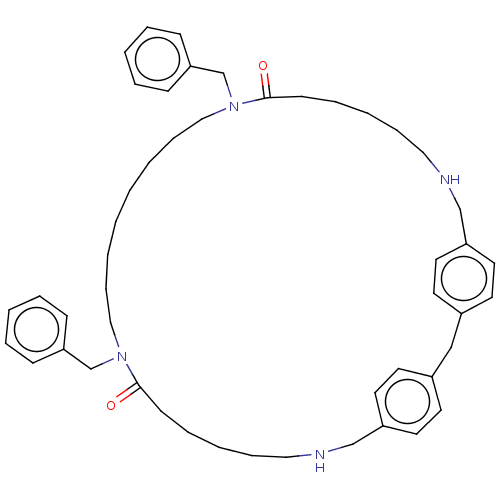 (CHEMBL4760251) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50128355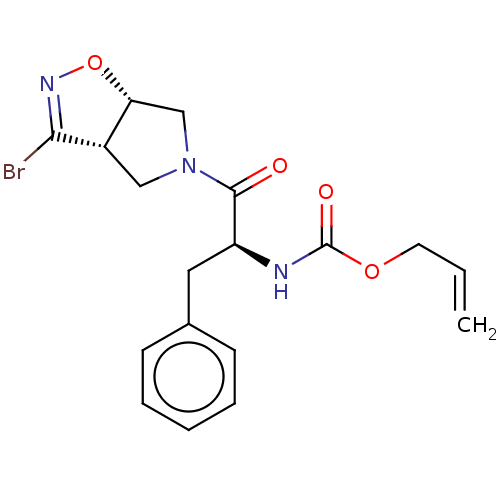 (CHEMBL3629180) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-7-amino-4-methylcoumarin assessed as substrate hydrolysis measured by fluore... | Bioorg Med Chem 23: 7053-60 (2015) Article DOI: 10.1016/j.bmc.2015.09.029 BindingDB Entry DOI: 10.7270/Q2ZW1NQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551177 (CHEMBL4762279) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50128356 (CHEMBL3629179) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-7-amino-4-methylcoumarin assessed as substrate hydrolysis measured by fluore... | Bioorg Med Chem 23: 7053-60 (2015) Article DOI: 10.1016/j.bmc.2015.09.029 BindingDB Entry DOI: 10.7270/Q2ZW1NQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50128357 (CHEMBL3629178) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.68E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-7-amino-4-methylcoumarin assessed as substrate hydrolysis measured by fluore... | Bioorg Med Chem 23: 7053-60 (2015) Article DOI: 10.1016/j.bmc.2015.09.029 BindingDB Entry DOI: 10.7270/Q2ZW1NQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-1 (Homo sapiens (Human)) | BDBM50434761 (CHEMBL2385819) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of PGPH-like activity of 20S proteasome in human erythrocytes using Z-Leu-Leu-Glu-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cysteine protease (Trypanosoma brucei rhodesiense) | BDBM50128353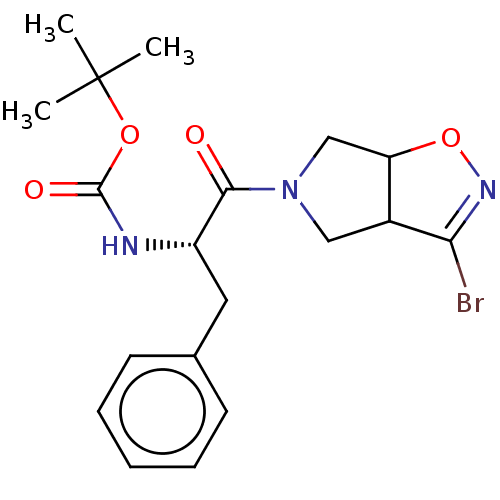 (CHEMBL3629183) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.77E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Messina Curated by ChEMBL | Assay Description Inhibition of Trypanosoma brucei rhodesiense rhodesain using Cbz-Phe-Arg-7-amino-4-methylcoumarin assessed as substrate hydrolysis measured by fluore... | Bioorg Med Chem 23: 7053-60 (2015) Article DOI: 10.1016/j.bmc.2015.09.029 BindingDB Entry DOI: 10.7270/Q2ZW1NQX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] B (Homo sapiens (Human)) | BDBM108061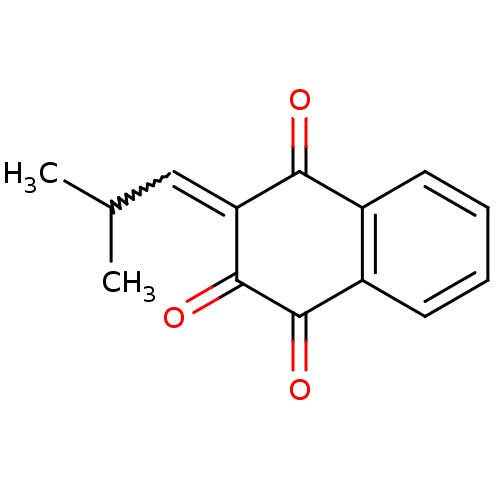 (Nor-lapachol) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.80E+4 | -28.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Institute of Chemistry, Federal University of Rio de Janeiro, Rio de Janeiro, 21941-909, Brazil. | Assay Description The reaction mixture (final volume 200 µL) contained 5 µg/mL MAO-A or MAO-B in a 50 mM sodium phosphate buffer pH 7.4, 1 mM p-tyramine (sub... | Chem Biol Drug Des 83: 401-10 (2014) Article DOI: 10.1111/cbdd.12255 BindingDB Entry DOI: 10.7270/Q2K072W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Pteridine reductase 1 (Leishmania major) | BDBM50551178 (CHEMBL4785591) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.92E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of Leishmania major PTR1 using H2B as substrate preincubated for 10 mins followed by NADPH addition and measured up to 50 mins relative to... | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112047 BindingDB Entry DOI: 10.7270/Q2F47SSH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Amine oxidase [flavin-containing] A (Homo sapiens (Human)) | BDBM108062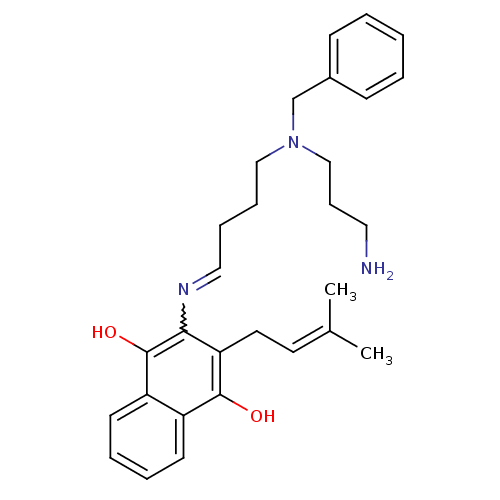 (2‐({4‐[(3‐aminopropyl)(benzyl)am...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.20E+4 | -27.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 37 |
Institute of Chemistry, Federal University of Rio de Janeiro, Rio de Janeiro, 21941-909, Brazil. | Assay Description The reaction mixture (final volume 200 µL) contained 5 µg/mL MAO-A or MAO-B in a 50 mM sodium phosphate buffer pH 7.4, 1 mM p-tyramine (sub... | Chem Biol Drug Des 83: 401-10 (2014) Article DOI: 10.1111/cbdd.12255 BindingDB Entry DOI: 10.7270/Q2K072W1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50021353 (CHEMBL3287944) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 2.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Messina Curated by ChEMBL | Assay Description Inhibition of bovine pancreatic alpha-chymotrypsin using Suc-Leu-Leu-Val-Tyr-AMC as substrate by fluorescence assay | Eur J Med Chem 83: 1-14 (2014) Article DOI: 10.1016/j.ejmech.2014.06.017 BindingDB Entry DOI: 10.7270/Q2DZ09WC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 1091 total ) | Next | Last >> |