 Found 342 hits with Last Name = 'radika' and Initial = 'k'
Found 342 hits with Last Name = 'radika' and Initial = 'k' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102780

(2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H17N3O/c22-21(23)14-9-10-18-15(11-14)12-19(24-18)17-8-4-7-16(20(17)25)13-5-2-1-3-6-13/h1-12,24-25H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115874
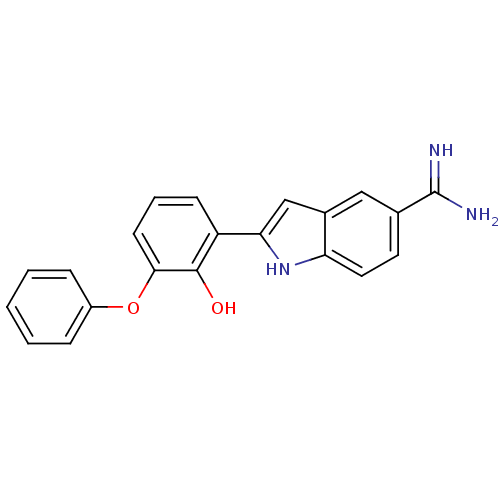
(2-(2-Hydroxy-3-phenoxy-phenyl)-1H-indole-5-carboxa...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(Oc2ccccc2)c1O Show InChI InChI=1S/C21H17N3O2/c22-21(23)13-9-10-17-14(11-13)12-18(24-17)16-7-4-8-19(20(16)25)26-15-5-2-1-3-6-15/h1-12,24-25H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115868
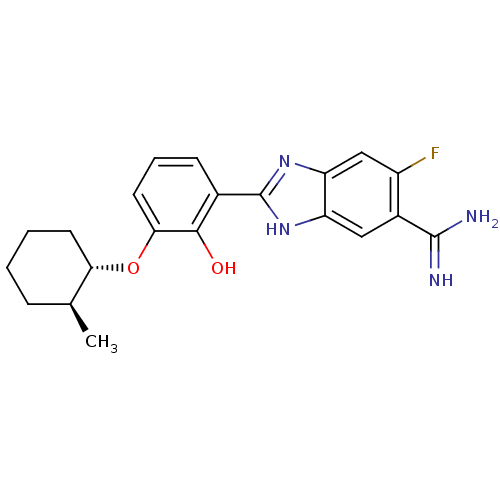
(2-{5-[AMINO(IMINIO)METHYL]-6-FLUORO-1H-BENZIMIDAZO...)Show SMILES C[C@H]1CCCC[C@@H]1Oc1cccc(-c2nc3cc(F)c(cc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C21H23FN4O2/c1-11-5-2-3-7-17(11)28-18-8-4-6-12(19(18)27)21-25-15-9-13(20(23)24)14(22)10-16(15)26-21/h4,6,8-11,17,27H,2-3,5,7H2,1H3,(H3,23,24)(H,25,26)/t11-,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50102790

(2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(cc(c1O)-c1ccccc1)[N+]([O-])=O Show InChI InChI=1S/C21H16N4O3/c22-21(23)13-6-7-18-14(8-13)9-19(24-18)17-11-15(25(27)28)10-16(20(17)26)12-4-2-1-3-5-12/h1-11,24,26H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102790

(2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(cc(c1O)-c1ccccc1)[N+]([O-])=O Show InChI InChI=1S/C21H16N4O3/c22-21(23)13-6-7-18-14(8-13)9-19(24-18)17-11-15(25(27)28)10-16(20(17)26)12-4-2-1-3-5-12/h1-11,24,26H,(H3,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Human Serine Protease tissue type Plasminogen Activator (t-PA). |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102790

(2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(cc(c1O)-c1ccccc1)[N+]([O-])=O Show InChI InChI=1S/C21H16N4O3/c22-21(23)13-6-7-18-14(8-13)9-19(24-18)17-11-15(25(27)28)10-16(20(17)26)12-4-2-1-3-5-12/h1-11,24,26H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
ComInhibition of Human Serine Protease Urokinase Plasminogen Activator (u-PA). |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115869

(6-Fluoro-2-[2-hydroxy-3-((S)-2-methyl-cyclohexylox...)Show SMILES C[C@@H]1CCCC[C@@H]1Oc1cccc(-c2nc3cc(F)c(cc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C21H23FN4O2/c1-11-5-2-3-7-17(11)28-18-8-4-6-12(19(18)27)21-25-15-9-13(20(23)24)14(22)10-16(15)26-21/h4,6,8-11,17,27H,2-3,5,7H2,1H3,(H3,23,24)(H,25,26)/t11-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115871

(6-Fluoro-2-[2-hydroxy-3-((S)-2-methyl-cyclopentylo...)Show SMILES C[C@H]1CCC[C@@H]1Oc1cccc(-c2nc3cc(F)c(cc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C20H21FN4O2/c1-10-4-2-6-16(10)27-17-7-3-5-11(18(17)26)20-24-14-8-12(19(22)23)13(21)9-15(14)25-20/h3,5,7-10,16,26H,2,4,6H2,1H3,(H3,22,23)(H,24,25)/t10-,16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 32 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102780

(2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H17N3O/c22-21(23)14-9-10-18-15(11-14)12-19(24-18)17-8-4-7-16(20(17)25)13-5-2-1-3-6-13/h1-12,24-25H,(H3,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inihibtion of Human Serine Protease tissue type Plasminogen Activator |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102767

(2-(3-Bromo-2-hydroxy-phenyl)-1H-indole-5-carboxami...)Show InChI InChI=1S/C15H12BrN3O/c16-11-3-1-2-10(14(11)20)13-7-9-6-8(15(17)18)4-5-12(9)19-13/h1-7,19-20H,(H3,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115874

(2-(2-Hydroxy-3-phenoxy-phenyl)-1H-indole-5-carboxa...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(Oc2ccccc2)c1O Show InChI InChI=1S/C21H17N3O2/c22-21(23)13-9-10-17-14(11-13)12-18(24-17)16-7-4-8-19(20(16)25)26-15-5-2-1-3-6-15/h1-12,24-25H,(H3,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of tissue-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50101866
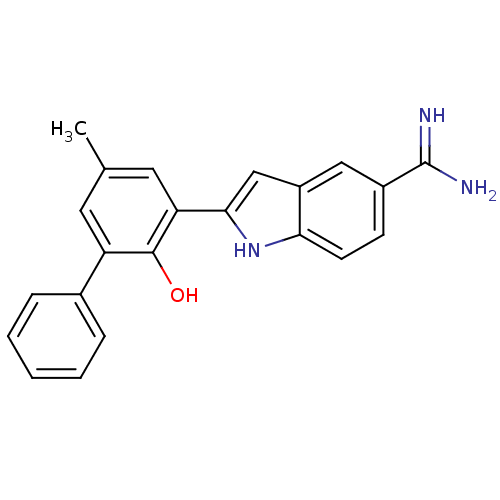
(2-(2-Hydroxy-5-methyl-biphenyl-3-yl)-1H-indole-5-c...)Show SMILES Cc1cc(-c2cc3cc(ccc3[nH]2)C(N)=N)c(O)c(c1)-c1ccccc1 Show InChI InChI=1S/C22H19N3O/c1-13-9-17(14-5-3-2-4-6-14)21(26)18(10-13)20-12-16-11-15(22(23)24)7-8-19(16)25-20/h2-12,25-26H,1H3,(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50102767

(2-(3-Bromo-2-hydroxy-phenyl)-1H-indole-5-carboxami...)Show InChI InChI=1S/C15H12BrN3O/c16-11-3-1-2-10(14(11)20)13-7-9-6-8(15(17)18)4-5-12(9)19-13/h1-7,19-20H,(H3,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102786
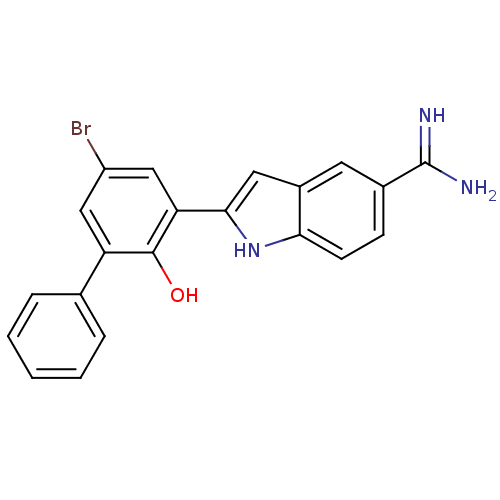
(2-(5-Bromo-2-hydroxy-biphenyl-3-yl)-1H-indole-5-ca...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(Br)cc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16BrN3O/c22-15-10-16(12-4-2-1-3-5-12)20(26)17(11-15)19-9-14-8-13(21(23)24)6-7-18(14)25-19/h1-11,25-26H,(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101873
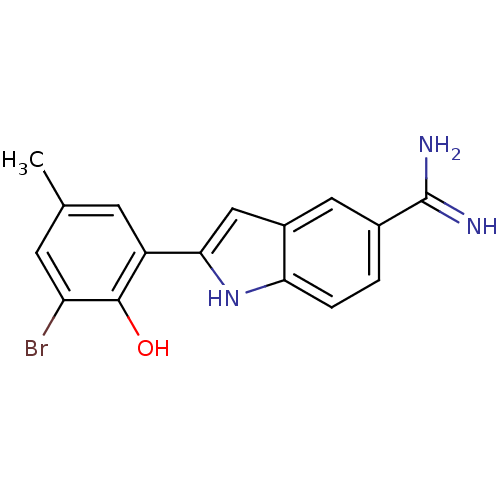
(2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-1H-indole-5-...)Show SMILES Cc1cc(Br)c(O)c(c1)-c1cc2cc(ccc2[nH]1)C(N)=N Show InChI InChI=1S/C16H14BrN3O/c1-8-4-11(15(21)12(17)5-8)14-7-10-6-9(16(18)19)2-3-13(10)20-14/h2-7,20-21H,1H3,(H3,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102778
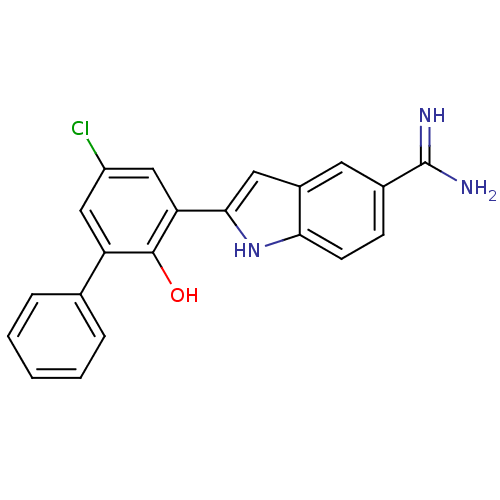
(2-(5-Chloro-2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(Cl)cc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16ClN3O/c22-15-10-16(12-4-2-1-3-5-12)20(26)17(11-15)19-9-14-8-13(21(23)24)6-7-18(14)25-19/h1-11,25-26H,(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50101873

(2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-1H-indole-5-...)Show SMILES Cc1cc(Br)c(O)c(c1)-c1cc2cc(ccc2[nH]1)C(N)=N Show InChI InChI=1S/C16H14BrN3O/c1-8-4-11(15(21)12(17)5-8)14-7-10-6-9(16(18)19)2-3-13(10)20-14/h2-7,20-21H,1H3,(H3,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
ComInhibition of Human Serine Protease Urokinase Plasminogen Activator (u-PA). |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50102790

(2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(cc(c1O)-c1ccccc1)[N+]([O-])=O Show InChI InChI=1S/C21H16N4O3/c22-21(23)13-6-7-18-14(8-13)9-19(24-18)17-11-15(25(27)28)10-16(20(17)26)12-4-2-1-3-5-12/h1-11,24,26H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Human Serine Protease Trypsin. |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50102780

(2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H17N3O/c22-21(23)14-9-10-18-15(11-14)12-19(24-18)17-8-4-7-16(20(17)25)13-5-2-1-3-6-13/h1-12,24-25H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50102792
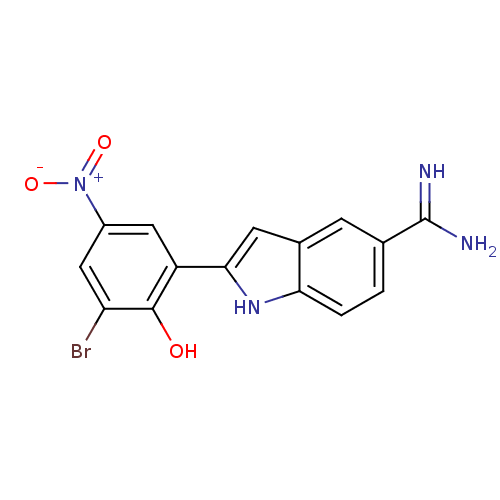
(2-(2-Hydroxy-3-bromo-5-nitro-phenyl)-1H-indole-5-c...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(cc(Br)c1O)[N+]([O-])=O Show InChI InChI=1S/C15H11BrN4O3/c16-11-6-9(20(22)23)5-10(14(11)21)13-4-8-3-7(15(17)18)1-2-12(8)19-13/h1-6,19,21H,(H3,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50115874

(2-(2-Hydroxy-3-phenoxy-phenyl)-1H-indole-5-carboxa...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(Oc2ccccc2)c1O Show InChI InChI=1S/C21H17N3O2/c22-21(23)13-9-10-17-14(11-13)12-18(24-17)16-7-4-8-19(20(16)25)26-15-5-2-1-3-6-15/h1-12,24-25H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 78 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of Coagulation factor X |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115860

(6-Fluoro-2-[2-hydroxy-3-(tetrahydro-furan-2-ylmeth...)Show SMILES NC(=N)c1cc2[nH]c(nc2cc1F)-c1cccc(OCC2CCCO2)c1O Show InChI InChI=1S/C19H19FN4O3/c20-13-8-15-14(7-12(13)18(21)22)23-19(24-15)11-4-1-5-16(17(11)25)27-9-10-3-2-6-26-10/h1,4-5,7-8,10,25H,2-3,6,9H2,(H3,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50102784
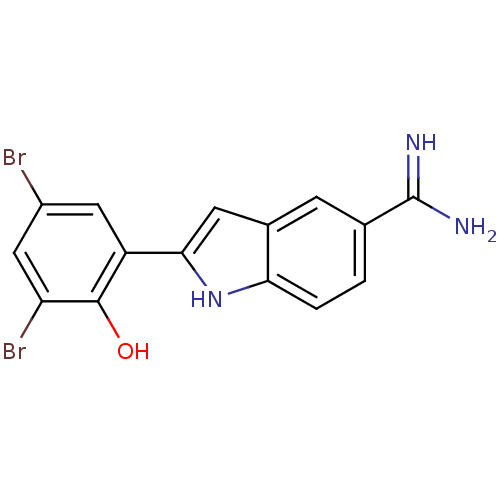
(2-(3,5-Dibromo-2-hydroxy-phenyl)-1H-indole-5-carbo...)Show InChI InChI=1S/C15H11Br2N3O/c16-9-5-10(14(21)11(17)6-9)13-4-8-3-7(15(18)19)1-2-12(8)20-13/h1-6,20-21H,(H3,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 89 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102786

(2-(5-Bromo-2-hydroxy-biphenyl-3-yl)-1H-indole-5-ca...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(Br)cc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16BrN3O/c22-15-10-16(12-4-2-1-3-5-12)20(26)17(11-15)19-9-14-8-13(21(23)24)6-7-18(14)25-19/h1-11,25-26H,(H3,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inihibtion of Human Serine Protease tissue type Plasminogen Activator |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50102780

(2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H17N3O/c22-21(23)14-9-10-18-15(11-14)12-19(24-18)17-8-4-7-16(20(17)25)13-5-2-1-3-6-13/h1-12,24-25H,(H3,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Human Serine Protease Plasmin |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102784

(2-(3,5-Dibromo-2-hydroxy-phenyl)-1H-indole-5-carbo...)Show InChI InChI=1S/C15H11Br2N3O/c16-9-5-10(14(21)11(17)6-9)13-4-8-3-7(15(18)19)1-2-12(8)20-13/h1-6,20-21H,(H3,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibitory concentration against Human Serine Protease Urokinase Plasminogen Activator |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50115874

(2-(2-Hydroxy-3-phenoxy-phenyl)-1H-indole-5-carboxa...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(Oc2ccccc2)c1O Show InChI InChI=1S/C21H17N3O2/c22-21(23)13-9-10-17-14(11-13)12-18(24-17)16-7-4-8-19(20(16)25)26-15-5-2-1-3-6-15/h1-12,24-25H,(H3,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of plasmin |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115875

(6-Fluoro-2-[2-hydroxy-3-(4-methyl-cyclohexyloxy)-p...)Show SMILES C[C@H]1CC[C@H](CC1)Oc1cccc(-c2nc3cc(F)c(cc3[nH]2)C(N)=N)c1O |wD:4.7,1.0,(9.17,-1.34,;7.63,-1.36,;6.84,-.03,;5.3,-.05,;4.56,-1.4,;5.34,-2.73,;6.87,-2.71,;3.02,-1.43,;2.22,-.1,;2.97,1.25,;2.17,2.57,;.63,2.54,;-.11,1.19,;-1.65,1.17,;-2.54,-.09,;-4.01,.37,;-5.32,-.44,;-6.66,.32,;-7.99,-.47,;-6.69,1.86,;-5.37,2.64,;-4.03,1.91,;-2.58,2.4,;-8.04,2.61,;-9.37,1.81,;-8.06,4.14,;.68,-.12,;-.07,-1.48,)| Show InChI InChI=1S/C21H23FN4O2/c1-11-5-7-12(8-6-11)28-18-4-2-3-13(19(18)27)21-25-16-9-14(20(23)24)15(22)10-17(16)26-21/h2-4,9-12,27H,5-8H2,1H3,(H3,23,24)(H,25,26)/t11-,12+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50102790

(2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(cc(c1O)-c1ccccc1)[N+]([O-])=O Show InChI InChI=1S/C21H16N4O3/c22-21(23)13-6-7-18-14(8-13)9-19(24-18)17-11-15(25(27)28)10-16(20(17)26)12-4-2-1-3-5-12/h1-11,24,26H,(H3,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Human Serine Protease Thrombin. |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50101866

(2-(2-Hydroxy-5-methyl-biphenyl-3-yl)-1H-indole-5-c...)Show SMILES Cc1cc(-c2cc3cc(ccc3[nH]2)C(N)=N)c(O)c(c1)-c1ccccc1 Show InChI InChI=1S/C22H19N3O/c1-13-9-17(14-5-3-2-4-6-14)21(26)18(10-13)20-12-16-11-15(22(23)24)7-8-19(16)25-20/h2-12,25-26H,1H3,(H3,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inihibtion of Human Serine Protease tissue type Plasminogen Activator |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115872

(6-Fluoro-2-[2-hydroxy-3-((S)-2-methyl-4,4-dimethyl...)Show SMILES C[C@H]1CC(C)(C)C[C@@H]1Oc1cccc(-c2nc3cc(F)c(cc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C22H25FN4O2/c1-11-9-22(2,3)10-18(11)29-17-6-4-5-12(19(17)28)21-26-15-7-13(20(24)25)14(23)8-16(15)27-21/h4-8,11,18,28H,9-10H2,1-3H3,(H3,24,25)(H,26,27)/t11-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50102767

(2-(3-Bromo-2-hydroxy-phenyl)-1H-indole-5-carboxami...)Show InChI InChI=1S/C15H12BrN3O/c16-11-3-1-2-10(14(11)20)13-7-9-6-8(15(17)18)4-5-12(9)19-13/h1-7,19-20H,(H3,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Activity against Human Serine Protease Thrombin |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50102778

(2-(5-Chloro-2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(Cl)cc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16ClN3O/c22-15-10-16(12-4-2-1-3-5-12)20(26)17(11-15)19-9-14-8-13(21(23)24)6-7-18(14)25-19/h1-11,25-26H,(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Activity against Human Serine Protease Trypsin |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50101866

(2-(2-Hydroxy-5-methyl-biphenyl-3-yl)-1H-indole-5-c...)Show SMILES Cc1cc(-c2cc3cc(ccc3[nH]2)C(N)=N)c(O)c(c1)-c1ccccc1 Show InChI InChI=1S/C22H19N3O/c1-13-9-17(14-5-3-2-4-6-14)21(26)18(10-13)20-12-16-11-15(22(23)24)7-8-19(16)25-20/h2-12,25-26H,1H3,(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Activity against Human Serine Protease Trypsin |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50102780

(2-(2-Hydroxy-3-phenyl-phenyl)-1H-indole-5-carboxam...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(-c2ccccc2)c1O Show InChI InChI=1S/C21H17N3O/c22-21(23)14-9-10-18-15(11-14)12-19(24-18)17-8-4-7-16(20(17)25)13-5-2-1-3-6-13/h1-12,24-25H,(H3,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Activity against Human Serine Protease Trypsin |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Tissue-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102778

(2-(5-Chloro-2-hydroxy-biphenyl-3-yl)-1H-indole-5-c...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(Cl)cc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16ClN3O/c22-15-10-16(12-4-2-1-3-5-12)20(26)17(11-15)19-9-14-8-13(21(23)24)6-7-18(14)25-19/h1-11,25-26H,(H3,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inihibtion of Human Serine Protease tissue type Plasminogen Activator |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115873
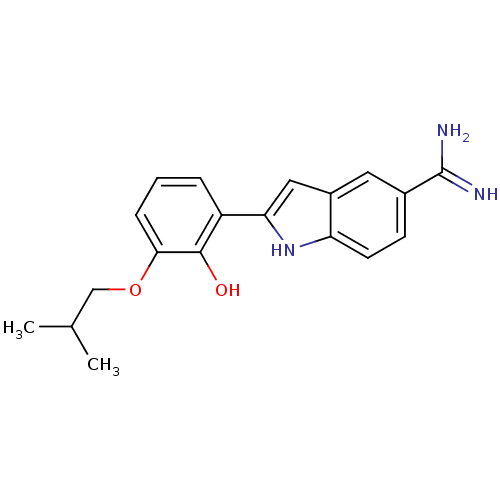
(2-(2-Hydroxy-3-isobutoxy-phenyl)-1H-indole-5-carbo...)Show SMILES CC(C)COc1cccc(-c2cc3cc(ccc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C19H21N3O2/c1-11(2)10-24-17-5-3-4-14(18(17)23)16-9-13-8-12(19(20)21)6-7-15(13)22-16/h3-9,11,22-23H,10H2,1-2H3,(H3,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50101873

(2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-1H-indole-5-...)Show SMILES Cc1cc(Br)c(O)c(c1)-c1cc2cc(ccc2[nH]1)C(N)=N Show InChI InChI=1S/C16H14BrN3O/c1-8-4-11(15(21)12(17)5-8)14-7-10-6-9(16(18)19)2-3-13(10)20-14/h2-7,20-21H,1H3,(H3,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Human Serine Protease Thrombin. |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115866

(6-Fluoro-2-[2-hydroxy-3-((S)-3-methyl-cyclohexylox...)Show SMILES C[C@H]1CCC[C@@H](C1)Oc1cccc(-c2nc3cc(F)c(cc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C21H23FN4O2/c1-11-4-2-5-12(8-11)28-18-7-3-6-13(19(18)27)21-25-16-9-14(20(23)24)15(22)10-17(16)26-21/h3,6-7,9-12,27H,2,4-5,8H2,1H3,(H3,23,24)(H,25,26)/t11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115865

(2-{5-[AMINO(IMINIO)METHYL]-6-FLUORO-1H-BENZIMIDAZO...)Show SMILES CC(C)COc1cccc(-c2nc3cc(F)c(cc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C18H19FN4O2/c1-9(2)8-25-15-5-3-4-10(16(15)24)18-22-13-6-11(17(20)21)12(19)7-14(13)23-18/h3-7,9,24H,8H2,1-2H3,(H3,20,21)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50102790

(2-(2-Hydroxy-5-nitro-biphenyl-3-yl)-1H-indole-5-ca...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(cc(c1O)-c1ccccc1)[N+]([O-])=O Show InChI InChI=1S/C21H16N4O3/c22-21(23)13-6-7-18-14(8-13)9-19(24-18)17-11-15(25(27)28)10-16(20(17)26)12-4-2-1-3-5-12/h1-11,24,26H,(H3,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Human Serine Protease Plasmin. |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115856
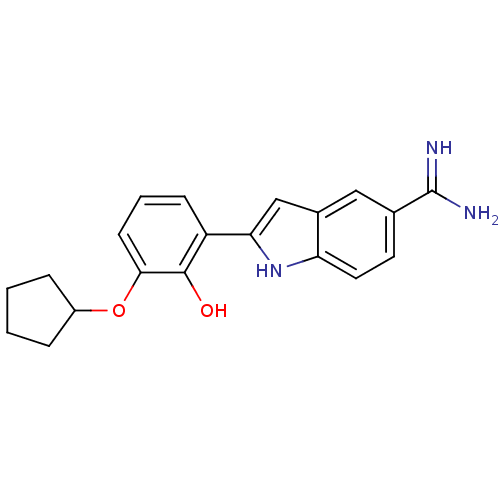
(2-(3-Cyclopentyloxy-2-hydroxy-phenyl)-1H-indole-5-...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cccc(OC2CCCC2)c1O Show InChI InChI=1S/C20H21N3O2/c21-20(22)12-8-9-16-13(10-12)11-17(23-16)15-6-3-7-18(19(15)24)25-14-4-1-2-5-14/h3,6-11,14,23-24H,1-2,4-5H2,(H3,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50102786

(2-(5-Bromo-2-hydroxy-biphenyl-3-yl)-1H-indole-5-ca...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(Br)cc(-c2ccccc2)c1O Show InChI InChI=1S/C21H16BrN3O/c22-15-10-16(12-4-2-1-3-5-12)20(26)17(11-15)19-9-14-8-13(21(23)24)6-7-18(14)25-19/h1-11,25-26H,(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM14169
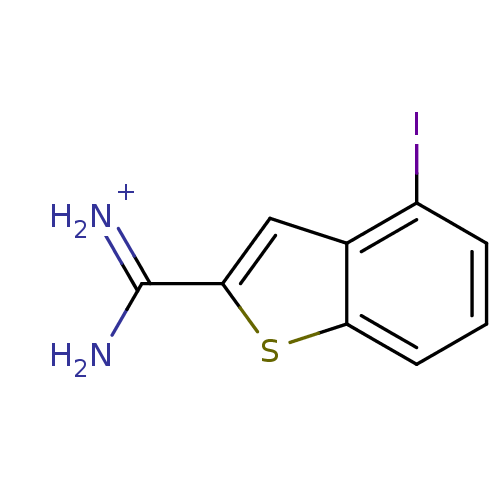
(4-Iodobenzo[b]thiophene-2-carboxamidine | APC-6860...)Show InChI InChI=1S/C9H7IN2S/c10-6-2-1-3-7-5(6)4-8(13-7)9(11)12/h1-4H,(H3,11,12)/p+1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| 210 | -37.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 22 |
Axys Pharmaceutical
| Assay Description
Each enzyme was assayed with a set of different concentrations of each inhibitor. After addition of the appropriate substrate, the rate of hydrolysis... |
Chem Biol 7: 299-312 (2000)
Article DOI: 10.1016/S1074-5521(00)00104-6
BindingDB Entry DOI: 10.7270/Q2NG4NWC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101866

(2-(2-Hydroxy-5-methyl-biphenyl-3-yl)-1H-indole-5-c...)Show SMILES Cc1cc(-c2cc3cc(ccc3[nH]2)C(N)=N)c(O)c(c1)-c1ccccc1 Show InChI InChI=1S/C22H19N3O/c1-13-9-17(14-5-3-2-4-6-14)21(26)18(10-13)20-12-16-11-15(22(23)24)7-8-19(16)25-20/h2-12,25-26H,1H3,(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Binding affinity against human coagulation factor X |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115876

(2-(3-Cyclopentyloxy-2-hydroxy-phenyl)-6-fluoro-1H-...)Show SMILES NC(=N)c1cc2[nH]c(nc2cc1F)-c1cccc(OC2CCCC2)c1O Show InChI InChI=1S/C19H19FN4O2/c20-13-9-15-14(8-12(13)18(21)22)23-19(24-15)11-6-3-7-16(17(11)25)26-10-4-1-2-5-10/h3,6-10,25H,1-2,4-5H2,(H3,21,22)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115861

(2-(3-Cyclopentyloxy-2-hydroxy-phenyl)-1H-benzoimid...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cccc(OC2CCCC2)c1O Show InChI InChI=1S/C19H20N4O2/c20-18(21)11-8-9-14-15(10-11)23-19(22-14)13-6-3-7-16(17(13)24)25-12-4-1-2-5-12/h3,6-10,12,24H,1-2,4-5H2,(H3,20,21)(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50102792

(2-(2-Hydroxy-3-bromo-5-nitro-phenyl)-1H-indole-5-c...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(cc(Br)c1O)[N+]([O-])=O Show InChI InChI=1S/C15H11BrN4O3/c16-11-6-9(20(22)23)5-10(14(11)21)13-4-8-3-7(15(17)18)1-2-12(8)19-13/h1-6,19,21H,(H3,17,18) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| PubMed
| 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
Inhibition of Human Serine Protease Thrombin. |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50115857
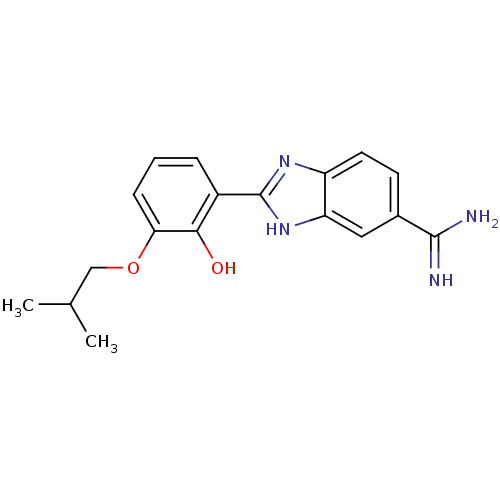
(2-(2-Hydroxy-3-isobutoxy-phenyl)-1H-benzoimidazole...)Show SMILES CC(C)COc1cccc(-c2nc3ccc(cc3[nH]2)C(N)=N)c1O Show InChI InChI=1S/C18H20N4O2/c1-10(2)9-24-15-5-3-4-12(16(15)23)18-21-13-7-6-11(17(19)20)8-14(13)22-18/h3-8,10,23H,9H2,1-2H3,(H3,19,20)(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Celera
Curated by ChEMBL
| Assay Description
Inhibition of urokinase-type plasminogen activator |
Bioorg Med Chem Lett 12: 2019-22 (2002)
BindingDB Entry DOI: 10.7270/Q237782T |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50102785
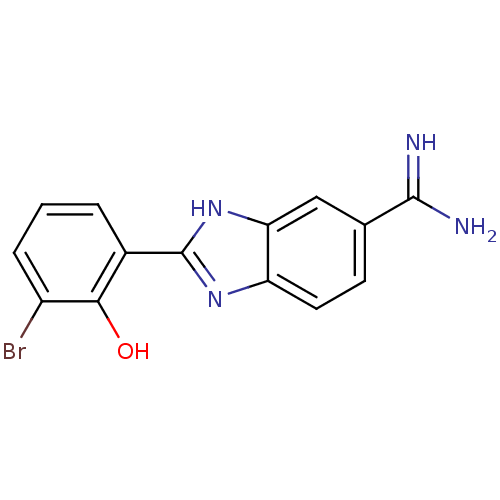
(2-(3-Bromo-2-hydroxy-phenyl)-1H-benzoimidazole-5-c...)Show InChI InChI=1S/C14H11BrN4O/c15-9-3-1-2-8(12(9)20)14-18-10-5-4-7(13(16)17)6-11(10)19-14/h1-6,20H,(H3,16,17)(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals Inc.
Curated by ChEMBL
| Assay Description
ComInhibition of Human Serine Protease Urokinase Plasminogen Activator (u-PA). |
J Med Chem 44: 2753-71 (2001)
BindingDB Entry DOI: 10.7270/Q2RX9BC7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data