 Found 271 hits with Last Name = 'raimbaud' and Initial = 'e'
Found 271 hits with Last Name = 'raimbaud' and Initial = 'e' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575780
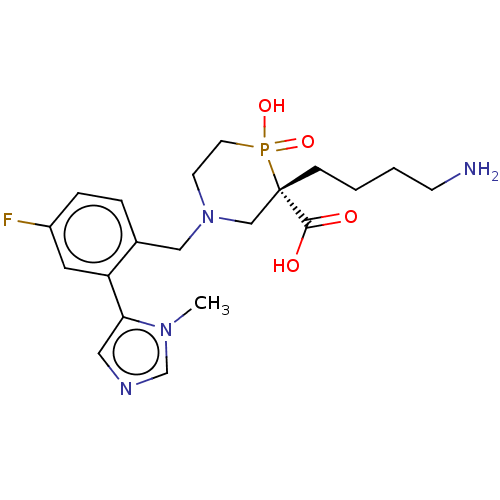
(CHEMBL4878039)Show SMILES Cn1cncc1-c1cc(F)ccc1CN1CCP(O)(=O)[C@@](CCCCN)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B
(Homo sapiens (Human)) | BDBM50575780

(CHEMBL4878039)Show SMILES Cn1cncc1-c1cc(F)ccc1CN1CCP(O)(=O)[C@@](CCCCN)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human pancreatic CPB incubated for 25 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM30344
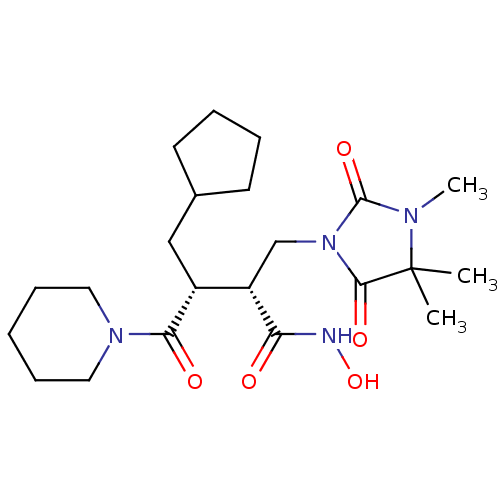
(Cipemastat | Trocade)Show SMILES CN1C(=O)N(C[C@@H]([C@@H](CC2CCCC2)C(=O)N2CCCCC2)C(=O)NO)C(=O)C1(C)C |r| Show InChI InChI=1S/C22H36N4O5/c1-22(2)20(29)26(21(30)24(22)3)14-17(18(27)23-31)16(13-15-9-5-6-10-15)19(28)25-11-7-4-8-12-25/h15-17,31H,4-14H2,1-3H3,(H,23,27)/t16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of Matrix metalloprotease-1 (MMP1) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Carboxypeptidase N catalytic chain
(Homo sapiens (Human)) | BDBM50575780

(CHEMBL4878039)Show SMILES Cn1cncc1-c1cc(F)ccc1CN1CCP(O)(=O)[C@@](CCCCN)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 2.82E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human plasma CPN incubated for 25 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50131941
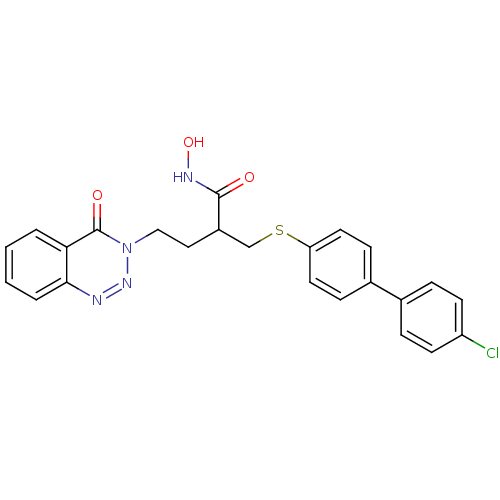
(2-(4'-Chloro-biphenyl-4-ylsulfanylmethyl)-N-hydrox...)Show SMILES ONC(=O)C(CCn1nnc2ccccc2c1=O)CSc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H21ClN4O3S/c25-19-9-5-16(6-10-19)17-7-11-20(12-8-17)33-15-18(23(30)27-32)13-14-29-24(31)21-3-1-2-4-22(21)26-28-29/h1-12,18,32H,13-15H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-2 (MMP2) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP9) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-2 (MMP2) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50131941

(2-(4'-Chloro-biphenyl-4-ylsulfanylmethyl)-N-hydrox...)Show SMILES ONC(=O)C(CCn1nnc2ccccc2c1=O)CSc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H21ClN4O3S/c25-19-9-5-16(6-10-19)17-7-11-20(12-8-17)33-15-18(23(30)27-32)13-14-29-24(31)21-3-1-2-4-22(21)26-28-29/h1-12,18,32H,13-15H2,(H,27,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP9) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575776
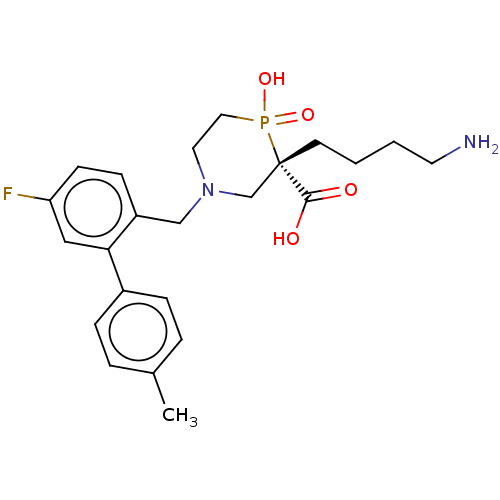
(CHEMBL4868605)Show SMILES Cc1ccc(cc1)-c1cc(F)ccc1CN1CCP(O)(=O)[C@@](CCCCN)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50452012
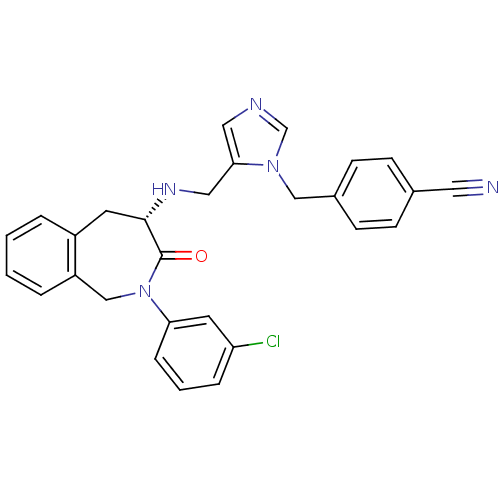
(CHEMBL2110187)Show SMILES Clc1cccc(c1)N1Cc2ccccc2C[C@H](NCc2cncn2Cc2ccc(cc2)C#N)C1=O |r| Show InChI InChI=1S/C28H24ClN5O/c29-24-6-3-7-25(13-24)34-18-23-5-2-1-4-22(23)12-27(28(34)35)32-16-26-15-31-19-33(26)17-21-10-8-20(14-30)9-11-21/h1-11,13,15,19,27,32H,12,16-18H2/t27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP3) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50131941

(2-(4'-Chloro-biphenyl-4-ylsulfanylmethyl)-N-hydrox...)Show SMILES ONC(=O)C(CCn1nnc2ccccc2c1=O)CSc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C24H21ClN4O3S/c25-19-9-5-16(6-10-19)17-7-11-20(12-8-17)33-15-18(23(30)27-32)13-14-29-24(31)21-3-1-2-4-22(21)26-28-29/h1-12,18,32H,13-15H2,(H,27,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-13 (MMP13) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50131944
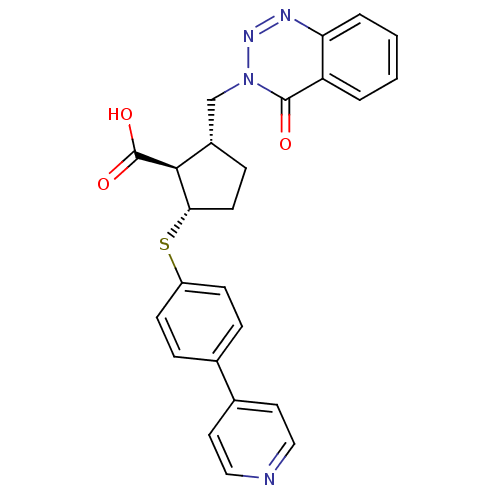
(2-(4-Oxo-4H-benzo[d][1,2,3]triazin-3-ylmethyl)-5-(...)Show SMILES OC(=O)[C@H]1[C@H](Cn2nnc3ccccc3c2=O)CC[C@@H]1Sc1ccc(cc1)-c1ccncc1 Show InChI InChI=1S/C25H22N4O3S/c30-24-20-3-1-2-4-21(20)27-28-29(24)15-18-7-10-22(23(18)25(31)32)33-19-8-5-16(6-9-19)17-11-13-26-14-12-17/h1-6,8-9,11-14,18,22-23H,7,10,15H2,(H,31,32)/t18-,22-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP3) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50092365
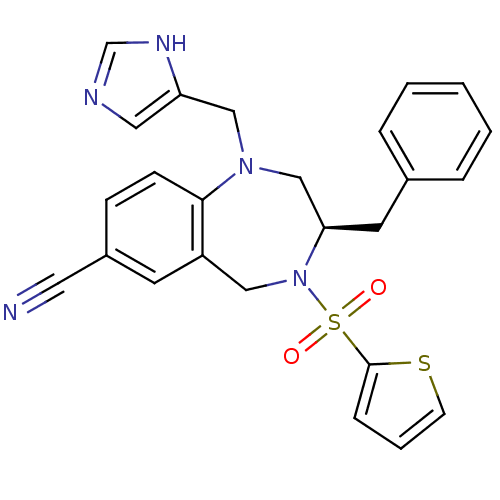
((R)-1-((1H-imidazol-5-yl)methyl)-3-benzyl-4-(thiop...)Show SMILES O=S(=O)(N1Cc2cc(ccc2N(Cc2cnc[nH]2)C[C@H]1Cc1ccccc1)C#N)c1cccs1 Show InChI InChI=1S/C25H23N5O2S2/c26-13-20-8-9-24-21(11-20)15-30(34(31,32)25-7-4-10-33-25)23(12-19-5-2-1-3-6-19)17-29(24)16-22-14-27-18-28-22/h1-11,14,18,23H,12,15-17H2,(H,27,28)/t23-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
UniChem
Patents
Similars
| MMDB
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-1 (MMP1) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50082556

((S)-2,2-Dimethyl-4-[4-(pyridin-4-yloxy)-benzenesul...)Show SMILES CC1(C)SCCN([C@H]1C(=O)NO)S(=O)(=O)c1ccc(Oc2ccncc2)cc1 Show InChI InChI=1S/C18H21N3O5S2/c1-18(2)16(17(22)20-23)21(11-12-27-18)28(24,25)15-5-3-13(4-6-15)26-14-7-9-19-10-8-14/h3-10,16,23H,11-12H2,1-2H3,(H,20,22)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-13 (MMP13) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP9) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50131964
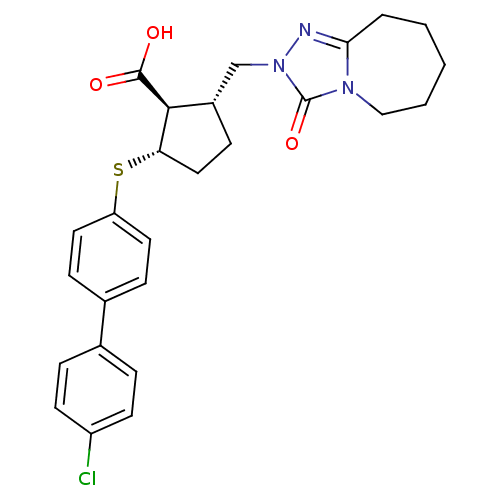
(2-(4'-Chloro-biphenyl-4-ylsulfanyl)-5-(3-oxo-6,7,8...)Show SMILES OC(=O)[C@H]1[C@H](Cn2nc3CCCCCn3c2=O)CC[C@@H]1Sc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H28ClN3O3S/c27-20-10-5-17(6-11-20)18-7-12-21(13-8-18)34-22-14-9-19(24(22)25(31)32)16-30-26(33)29-15-3-1-2-4-23(29)28-30/h5-8,10-13,19,22,24H,1-4,9,14-16H2,(H,31,32)/t19-,22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-2 (MMP2) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-2 (MMP2) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50131962
(2-(4'-Chloro-biphenyl-4-sulfonyl)-5-(4-oxo-4H-benz...)Show SMILES OC(=O)[C@H]1[C@H](Cn2nnc3ccccc3c2=O)CC[C@@H]1S(=O)(=O)c1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H22ClN3O5S/c27-19-10-5-16(6-11-19)17-7-12-20(13-8-17)36(34,35)23-14-9-18(24(23)26(32)33)15-30-25(31)21-3-1-2-4-22(21)28-29-30/h1-8,10-13,18,23-24H,9,14-15H2,(H,32,33)/t18-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-13 (MMP13) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50131953
(2-(4'-Chloro-biphenyl-4-ylsulfanyl)-5-(4-oxo-4H-be...)Show SMILES OC(=O)[C@@H]1[C@@H](Cn2nnc3ccccc3c2=O)CC[C@H]1Sc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H22ClN3O3S/c27-19-10-5-16(6-11-19)17-7-12-20(13-8-17)34-23-14-9-18(24(23)26(32)33)15-30-25(31)21-3-1-2-4-22(21)28-29-30/h1-8,10-13,18,23-24H,9,14-15H2,(H,32,33)/t18-,23-,24-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-13 (MMP13) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575768
(CHEMBL4846664)Show SMILES NCCCCC1(CN(Cc2ccccc2-c2cccnc2)CCP1(O)=O)C(O)=O | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50131958

(2-[2-(4'-Chloro-biphenyl-4-yl)-ethanesulfonyl]-5-(...)Show SMILES OC(=O)[C@H]1[C@H](Cn2nnc3ccccc3c2=O)CC[C@@H]1S(=O)(=O)CCc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C28H26ClN3O5S/c29-22-12-9-20(10-13-22)19-7-5-18(6-8-19)15-16-38(36,37)25-14-11-21(26(25)28(34)35)17-32-27(33)23-3-1-2-4-24(23)30-31-32/h1-10,12-13,21,25-26H,11,14-17H2,(H,34,35)/t21-,25-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-13 (MMP13) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50131952
(2-(Biphenyl-4-ylmethylsulfanyl)-5-(1,3-dioxo-1,3-d...)Show SMILES OC(=O)[C@H]1[C@H](CN2C(=O)c3ccccc3C2=O)CC[C@@H]1SCc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C28H25NO4S/c30-26-22-8-4-5-9-23(22)27(31)29(26)16-21-14-15-24(25(21)28(32)33)34-17-18-10-12-20(13-11-18)19-6-2-1-3-7-19/h1-13,21,24-25H,14-17H2,(H,32,33)/t21-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-2 (MMP2) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50131937
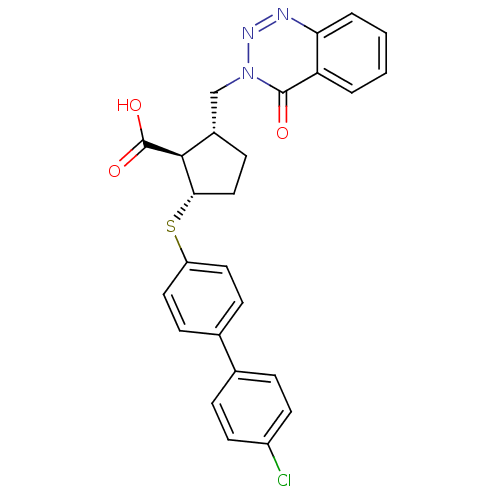
(2-(4'-Chloro-biphenyl-4-ylsulfanyl)-5-(4-oxo-4H-be...)Show SMILES OC(=O)[C@H]1[C@H](Cn2nnc3ccccc3c2=O)CC[C@@H]1Sc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H22ClN3O3S/c27-19-10-5-16(6-11-19)17-7-12-20(13-8-17)34-23-14-9-18(24(23)26(32)33)15-30-25(31)21-3-1-2-4-22(21)28-29-30/h1-8,10-13,18,23-24H,9,14-15H2,(H,32,33)/t18-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP9) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50131937

(2-(4'-Chloro-biphenyl-4-ylsulfanyl)-5-(4-oxo-4H-be...)Show SMILES OC(=O)[C@H]1[C@H](Cn2nnc3ccccc3c2=O)CC[C@@H]1Sc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H22ClN3O3S/c27-19-10-5-16(6-11-19)17-7-12-20(13-8-17)34-23-14-9-18(24(23)26(32)33)15-30-25(31)21-3-1-2-4-22(21)28-29-30/h1-8,10-13,18,23-24H,9,14-15H2,(H,32,33)/t18-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP9) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50131953

(2-(4'-Chloro-biphenyl-4-ylsulfanyl)-5-(4-oxo-4H-be...)Show SMILES OC(=O)[C@@H]1[C@@H](Cn2nnc3ccccc3c2=O)CC[C@H]1Sc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H22ClN3O3S/c27-19-10-5-16(6-11-19)17-7-12-20(13-8-17)34-23-14-9-18(24(23)26(32)33)15-30-25(31)21-3-1-2-4-22(21)28-29-30/h1-8,10-13,18,23-24H,9,14-15H2,(H,32,33)/t18-,23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP9) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139576
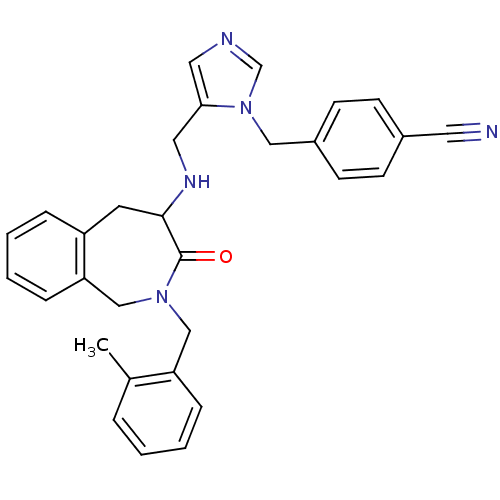
(4-(5-{[2-(2-Methyl-benzyl)-3-oxo-2,3,4,5-tetrahydr...)Show SMILES Cc1ccccc1CN1Cc2ccccc2CC(NCc2cncn2Cc2ccc(cc2)C#N)C1=O Show InChI InChI=1S/C30H29N5O/c1-22-6-2-3-8-26(22)19-34-20-27-9-5-4-7-25(27)14-29(30(34)36)33-17-28-16-32-21-35(28)18-24-12-10-23(15-31)11-13-24/h2-13,16,21,29,33H,14,17-20H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50131952

(2-(Biphenyl-4-ylmethylsulfanyl)-5-(1,3-dioxo-1,3-d...)Show SMILES OC(=O)[C@H]1[C@H](CN2C(=O)c3ccccc3C2=O)CC[C@@H]1SCc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C28H25NO4S/c30-26-22-8-4-5-9-23(22)27(31)29(26)16-21-14-15-24(25(21)28(32)33)34-17-18-10-12-20(13-11-18)19-6-2-1-3-7-19/h1-13,21,24-25H,14-17H2,(H,32,33)/t21-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP3) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50131949
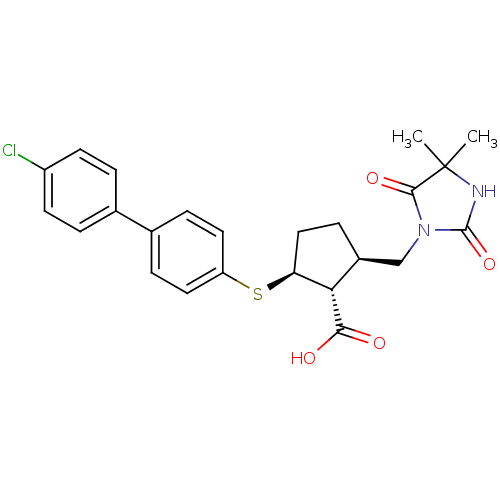
(2-(4'-Chloro-biphenyl-4-ylsulfanyl)-5-(4,4-dimethy...)Show SMILES CC1(C)NC(=O)N(C[C@@H]2CC[C@H](Sc3ccc(cc3)-c3ccc(Cl)cc3)[C@H]2C(O)=O)C1=O Show InChI InChI=1S/C24H25ClN2O4S/c1-24(2)22(30)27(23(31)26-24)13-16-7-12-19(20(16)21(28)29)32-18-10-5-15(6-11-18)14-3-8-17(25)9-4-14/h3-6,8-11,16,19-20H,7,12-13H2,1-2H3,(H,26,31)(H,28,29)/t16-,19-,20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-13 (MMP13) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50131949

(2-(4'-Chloro-biphenyl-4-ylsulfanyl)-5-(4,4-dimethy...)Show SMILES CC1(C)NC(=O)N(C[C@@H]2CC[C@H](Sc3ccc(cc3)-c3ccc(Cl)cc3)[C@H]2C(O)=O)C1=O Show InChI InChI=1S/C24H25ClN2O4S/c1-24(2)22(30)27(23(31)26-24)13-16-7-12-19(20(16)21(28)29)32-18-10-5-15(6-11-18)14-3-8-17(25)9-4-14/h3-6,8-11,16,19-20H,7,12-13H2,1-2H3,(H,26,31)(H,28,29)/t16-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP9) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50131937

(2-(4'-Chloro-biphenyl-4-ylsulfanyl)-5-(4-oxo-4H-be...)Show SMILES OC(=O)[C@H]1[C@H](Cn2nnc3ccccc3c2=O)CC[C@@H]1Sc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H22ClN3O3S/c27-19-10-5-16(6-11-19)17-7-12-20(13-8-17)34-23-14-9-18(24(23)26(32)33)15-30-25(31)21-3-1-2-4-22(21)28-29-30/h1-8,10-13,18,23-24H,9,14-15H2,(H,32,33)/t18-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP3) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50131937

(2-(4'-Chloro-biphenyl-4-ylsulfanyl)-5-(4-oxo-4H-be...)Show SMILES OC(=O)[C@H]1[C@H](Cn2nnc3ccccc3c2=O)CC[C@@H]1Sc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H22ClN3O3S/c27-19-10-5-16(6-11-19)17-7-12-20(13-8-17)34-23-14-9-18(24(23)26(32)33)15-30-25(31)21-3-1-2-4-22(21)28-29-30/h1-8,10-13,18,23-24H,9,14-15H2,(H,32,33)/t18-,23-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP3) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM30344

(Cipemastat | Trocade)Show SMILES CN1C(=O)N(C[C@@H]([C@@H](CC2CCCC2)C(=O)N2CCCCC2)C(=O)NO)C(=O)C1(C)C |r| Show InChI InChI=1S/C22H36N4O5/c1-22(2)20(29)26(21(30)24(22)3)14-17(18(27)23-31)16(13-15-9-5-6-10-15)19(28)25-11-7-4-8-12-25/h15-17,31H,4-14H2,1-3H3,(H,23,27)/t16-,17+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-13 (MMP13) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50063917

((2S,3R)-N(4)-[(2S)-3,3-dimethyl-1-(methylamino)-1-...)Show SMILES CNC(=O)[C@@H](NC(=O)[C@H](CC(C)C)[C@H](O)C(=O)NO)C(C)(C)C |r| Show InChI InChI=1S/C15H29N3O5/c1-8(2)7-9(10(19)13(21)18-23)12(20)17-11(14(22)16-6)15(3,4)5/h8-11,19,23H,7H2,1-6H3,(H,16,22)(H,17,20)(H,18,21)/t9-,10+,11-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-13 (MMP13) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50131952

(2-(Biphenyl-4-ylmethylsulfanyl)-5-(1,3-dioxo-1,3-d...)Show SMILES OC(=O)[C@H]1[C@H](CN2C(=O)c3ccccc3C2=O)CC[C@@H]1SCc1ccc(cc1)-c1ccccc1 Show InChI InChI=1S/C28H25NO4S/c30-26-22-8-4-5-9-23(22)27(31)29(26)16-21-14-15-24(25(21)28(32)33)34-17-18-10-12-20(13-11-18)19-6-2-1-3-7-19/h1-13,21,24-25H,14-17H2,(H,32,33)/t21-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP9) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50131964

(2-(4'-Chloro-biphenyl-4-ylsulfanyl)-5-(3-oxo-6,7,8...)Show SMILES OC(=O)[C@H]1[C@H](Cn2nc3CCCCCn3c2=O)CC[C@@H]1Sc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H28ClN3O3S/c27-20-10-5-17(6-11-20)18-7-12-21(13-8-18)34-22-14-9-19(24(22)25(31)32)16-30-26(33)29-15-3-1-2-4-23(29)28-30/h5-8,10-13,19,22,24H,1-4,9,14-16H2,(H,31,32)/t19-,22-,24-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP9) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50131960

(2-(4'-Methylsulfanyl-biphenyl-4-ylsulfanyl)-5-(4-o...)Show SMILES CSc1ccc(cc1)-c1ccc(S[C@H]2CC[C@@H](Cn3nnc4ccccc4c3=O)[C@@H]2C(O)=O)cc1 Show InChI InChI=1S/C27H25N3O3S2/c1-34-20-11-6-17(7-12-20)18-8-13-21(14-9-18)35-24-15-10-19(25(24)27(32)33)16-30-26(31)22-4-2-3-5-23(22)28-29-30/h2-9,11-14,19,24-25H,10,15-16H2,1H3,(H,32,33)/t19-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP9) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139567
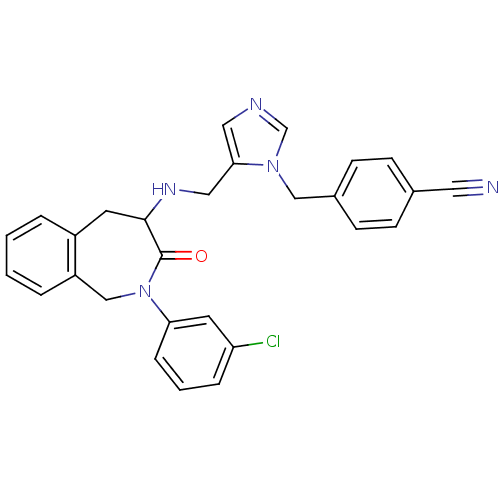
(4-(5-{[2-(3-Chloro-phenyl)-3-oxo-2,3,4,5-tetrahydr...)Show SMILES Clc1cccc(c1)N1Cc2ccccc2CC(NCc2cncn2Cc2ccc(cc2)C#N)C1=O Show InChI InChI=1S/C28H24ClN5O/c29-24-6-3-7-25(13-24)34-18-23-5-2-1-4-22(23)12-27(28(34)35)32-16-26-15-31-19-33(26)17-21-10-8-20(14-30)9-11-21/h1-11,13,15,19,27,32H,12,16-18H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575775
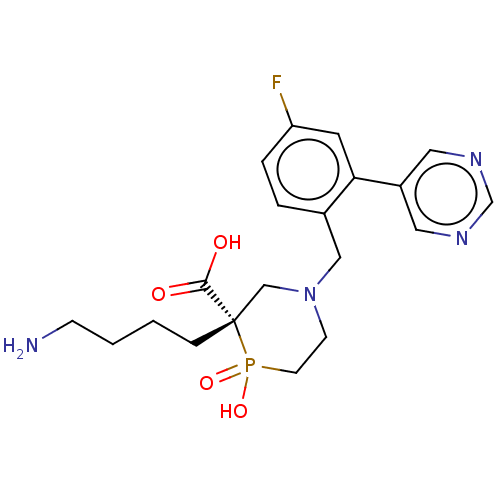
(CHEMBL4861532)Show SMILES NCCCC[C@]1(CN(Cc2ccc(F)cc2-c2cncnc2)CCP1(O)=O)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
Carboxypeptidase B2
(Homo sapiens (Human)) | BDBM50575770
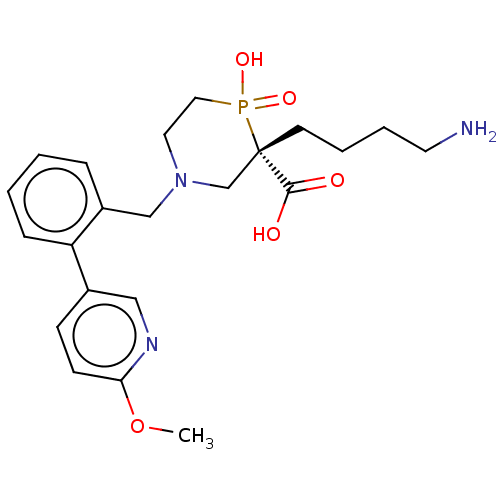
(CHEMBL4858095)Show SMILES COc1ccc(cn1)-c1ccccc1CN1CCP(O)(=O)[C@@](CCCCN)(C1)C(O)=O |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant human activated TAFI incubated for 45 mins using hippuryl-arginine as substrate by spectrophotometry |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c02072
BindingDB Entry DOI: 10.7270/Q2SB49JM |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM50131960

(2-(4'-Methylsulfanyl-biphenyl-4-ylsulfanyl)-5-(4-o...)Show SMILES CSc1ccc(cc1)-c1ccc(S[C@H]2CC[C@@H](Cn3nnc4ccccc4c3=O)[C@@H]2C(O)=O)cc1 Show InChI InChI=1S/C27H25N3O3S2/c1-34-20-11-6-17(7-12-20)18-8-13-21(14-9-18)35-24-15-10-19(25(24)27(32)33)16-30-26(31)22-4-2-3-5-23(22)28-29-30/h2-9,11-14,19,24-25H,10,15-16H2,1H3,(H,32,33)/t19-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-2 (MMP2) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50131947
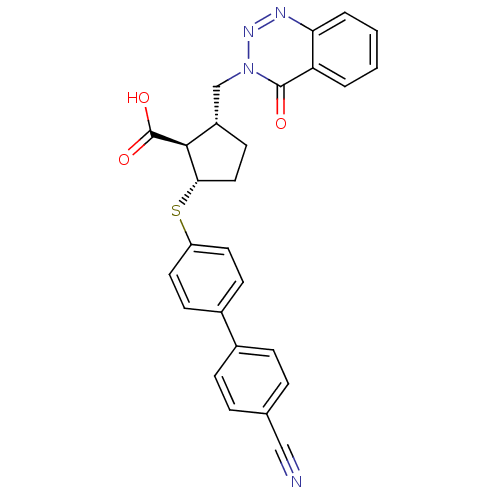
(2-(4'-Cyano-biphenyl-4-ylsulfanyl)-5-(4-oxo-4H-ben...)Show SMILES OC(=O)[C@H]1[C@H](Cn2nnc3ccccc3c2=O)CC[C@@H]1Sc1ccc(cc1)-c1ccc(cc1)C#N Show InChI InChI=1S/C27H22N4O3S/c28-15-17-5-7-18(8-6-17)19-9-12-21(13-10-19)35-24-14-11-20(25(24)27(33)34)16-31-26(32)22-3-1-2-4-23(22)29-30-31/h1-10,12-13,20,24-25H,11,14,16H2,(H,33,34)/t20-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP3) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50131937

(2-(4'-Chloro-biphenyl-4-ylsulfanyl)-5-(4-oxo-4H-be...)Show SMILES OC(=O)[C@H]1[C@H](Cn2nnc3ccccc3c2=O)CC[C@@H]1Sc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H22ClN3O3S/c27-19-10-5-16(6-11-19)17-7-12-20(13-8-17)34-23-14-9-18(24(23)26(32)33)15-30-25(31)21-3-1-2-4-22(21)28-29-30/h1-8,10-13,18,23-24H,9,14-15H2,(H,32,33)/t18-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-13 (MMP13) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50131937

(2-(4'-Chloro-biphenyl-4-ylsulfanyl)-5-(4-oxo-4H-be...)Show SMILES OC(=O)[C@H]1[C@H](Cn2nnc3ccccc3c2=O)CC[C@@H]1Sc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H22ClN3O3S/c27-19-10-5-16(6-11-19)17-7-12-20(13-8-17)34-23-14-9-18(24(23)26(32)33)15-30-25(31)21-3-1-2-4-22(21)28-29-30/h1-8,10-13,18,23-24H,9,14-15H2,(H,32,33)/t18-,23-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-13 (MMP13) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Collagenase 3
(Homo sapiens (Human)) | BDBM50131964

(2-(4'-Chloro-biphenyl-4-ylsulfanyl)-5-(3-oxo-6,7,8...)Show SMILES OC(=O)[C@H]1[C@H](Cn2nc3CCCCCn3c2=O)CC[C@@H]1Sc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H28ClN3O3S/c27-20-10-5-17(6-11-20)18-7-12-21(13-8-18)34-22-14-9-19(24(22)25(31)32)16-30-26(33)29-15-3-1-2-4-23(29)28-30/h5-8,10-13,19,22,24H,1-4,9,14-16H2,(H,31,32)/t19-,22-,24-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-13 (MMP13) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Stromelysin-1
(Homo sapiens (Human)) | BDBM50131953

(2-(4'-Chloro-biphenyl-4-ylsulfanyl)-5-(4-oxo-4H-be...)Show SMILES OC(=O)[C@@H]1[C@@H](Cn2nnc3ccccc3c2=O)CC[C@H]1Sc1ccc(cc1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C26H22ClN3O3S/c27-19-10-5-16(6-11-19)17-7-12-20(13-8-17)34-23-14-9-18(24(23)26(32)33)15-30-25(31)21-3-1-2-4-22(21)28-29-30/h1-8,10-13,18,23-24H,9,14-15H2,(H,32,33)/t18-,23-,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-3 (MMP3) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139588

(4-(5-{[2-(3-Methoxy-phenyl)-3-oxo-2,3,4,5-tetrahyd...)Show SMILES COc1cccc(c1)N1Cc2ccccc2CC(NCc2cncn2Cc2ccc(cc2)C#N)C1=O Show InChI InChI=1S/C29H27N5O2/c1-36-27-8-4-7-25(14-27)34-19-24-6-3-2-5-23(24)13-28(29(34)35)32-17-26-16-31-20-33(26)18-22-11-9-21(15-30)10-12-22/h2-12,14,16,20,28,32H,13,17-19H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50139580
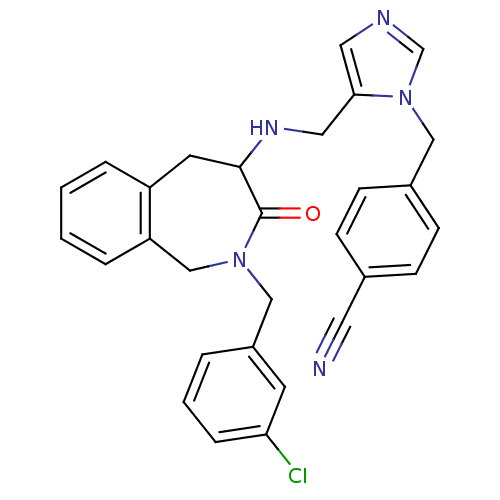
(4-(5-{[2-(3-Chloro-benzyl)-3-oxo-2,3,4,5-tetrahydr...)Show SMILES Clc1cccc(CN2Cc3ccccc3CC(NCc3cncn3Cc3ccc(cc3)C#N)C2=O)c1 Show InChI InChI=1S/C29H26ClN5O/c30-26-7-3-4-23(12-26)18-34-19-25-6-2-1-5-24(25)13-28(29(34)36)33-16-27-15-32-20-35(27)17-22-10-8-21(14-31)9-11-22/h1-12,15,20,28,33H,13,16-19H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibition of farnesyltransferase |
Bioorg Med Chem Lett 14: 767-71 (2004)
BindingDB Entry DOI: 10.7270/Q2348JSW |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM50131951

(2-(4-Oxo-4H-benzo[d][1,2,3]triazin-3-ylmethyl)-5-(...)Show SMILES OC(=O)[C@H]1[C@H](Cn2nnc3ccccc3c2=O)CC[C@@H]1Sc1ccc(cc1)-c1nccs1 Show InChI InChI=1S/C23H20N4O3S2/c28-22-17-3-1-2-4-18(17)25-26-27(22)13-15-7-10-19(20(15)23(29)30)32-16-8-5-14(6-9-16)21-24-11-12-31-21/h1-6,8-9,11-12,15,19-20H,7,10,13H2,(H,29,30)/t15-,19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >6 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut de Recherches Servier
Curated by ChEMBL
| Assay Description
Inhibitory activity against matrix metalloprotease-9 (MMP9) |
J Med Chem 46: 3840-52 (2003)
Article DOI: 10.1021/jm0307638
BindingDB Entry DOI: 10.7270/Q2CC11FF |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data