

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cholinesterase (Homo sapiens (Human)) | BDBM50564199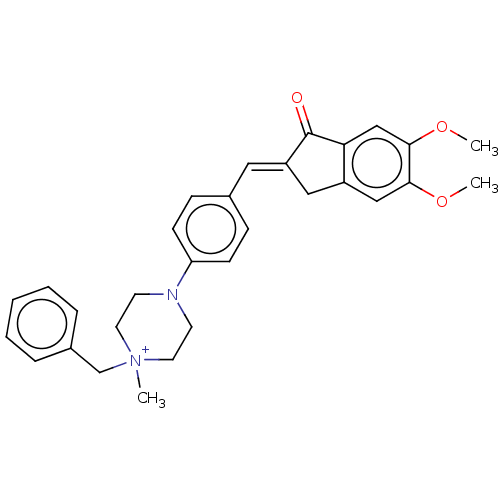 (CHEMBL4800260) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non competitive inhibition of human plasma BuChE using varying levels of butyrylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50564203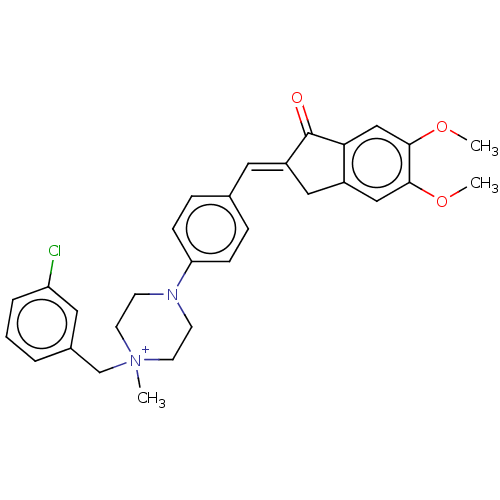 (CHEMBL4791075) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non competitive inhibition of human plasma BuChE using varying levels of butyrylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564199 (CHEMBL4800260) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Non competitive inhibition of human erythrocyte AChE using varying levels of acetylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564203 (CHEMBL4791075) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Mixed type inhibition of human erythrocyte AChE using varying levels of acetylthiocholine iodide as substrate by Lineweaver-Burk plot analysis | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde oxidase 1 (Cavia porcellus (Guinea pig)) | BDBM4078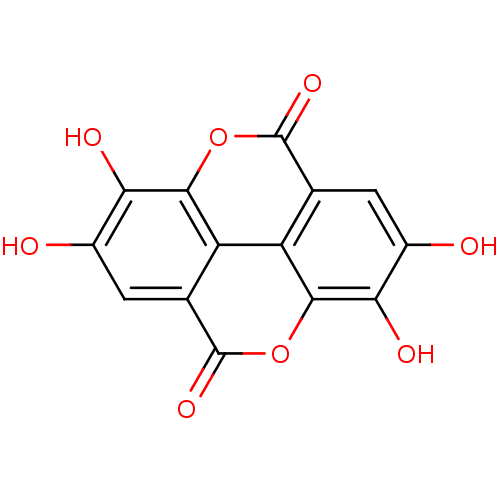 (6,7,13,14-tetrahydroxy-2,9-dioxatetracyclo[6.6.2.0...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.53E+3 | n/a | 1.45E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Kermanshah University of Medical Sciences | Assay Description Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c... | Bioorg Chem 64: 74-84 (2016) Article DOI: 10.1016/j.bioorg.2015.12.004 BindingDB Entry DOI: 10.7270/Q2MP522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde oxidase 1 (Cavia porcellus (Guinea pig)) | BDBM24778 (2-methyl-1,4-dihydronaphthalene-1,4-dione | 2-meth...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 7.23E+3 | n/a | 3.18E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Kermanshah University of Medical Sciences | Assay Description Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c... | Bioorg Chem 64: 74-84 (2016) Article DOI: 10.1016/j.bioorg.2015.12.004 BindingDB Entry DOI: 10.7270/Q2MP522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde oxidase 1 (Cavia porcellus (Guinea pig)) | BDBM175349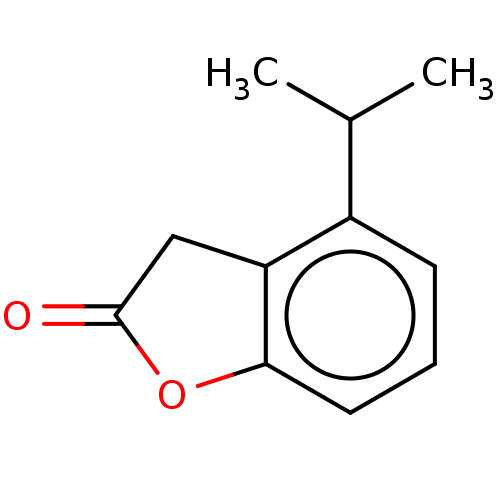 (4'-Isopropyl Benzofuranone (4'-CH(CH3)2) |...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.80E+4 | n/a | 4.94E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Kermanshah University of Medical Sciences | Assay Description Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c... | Bioorg Chem 64: 74-84 (2016) Article DOI: 10.1016/j.bioorg.2015.12.004 BindingDB Entry DOI: 10.7270/Q2MP522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde oxidase 1 (Cavia porcellus (Guinea pig)) | BDBM175350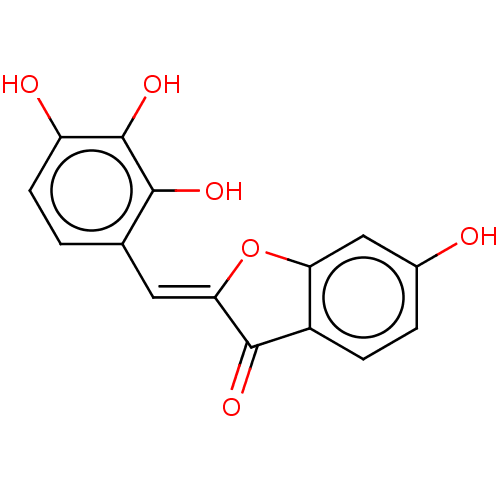 (2',3',4'-three-Hydroxyl Benzofuranone ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.21E+4 | n/a | 6.57E+4 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Kermanshah University of Medical Sciences | Assay Description Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c... | Bioorg Chem 64: 74-84 (2016) Article DOI: 10.1016/j.bioorg.2015.12.004 BindingDB Entry DOI: 10.7270/Q2MP522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde oxidase 1 (Cavia porcellus (Guinea pig)) | BDBM175351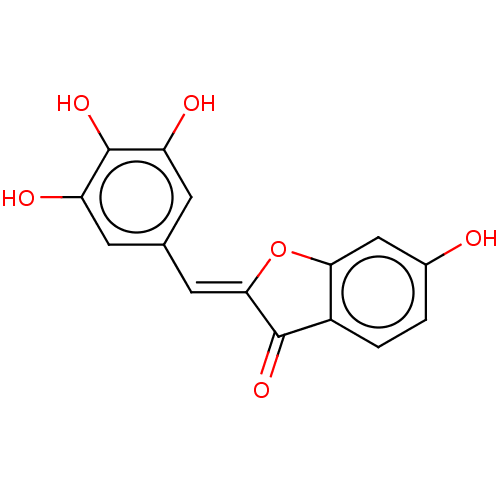 (3',4',5'-three-Hydroxyl Benzofuranone ...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Kermanshah University of Medical Sciences | Assay Description Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c... | Bioorg Chem 64: 74-84 (2016) Article DOI: 10.1016/j.bioorg.2015.12.004 BindingDB Entry DOI: 10.7270/Q2MP522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde oxidase 1 (Cavia porcellus (Guinea pig)) | BDBM175347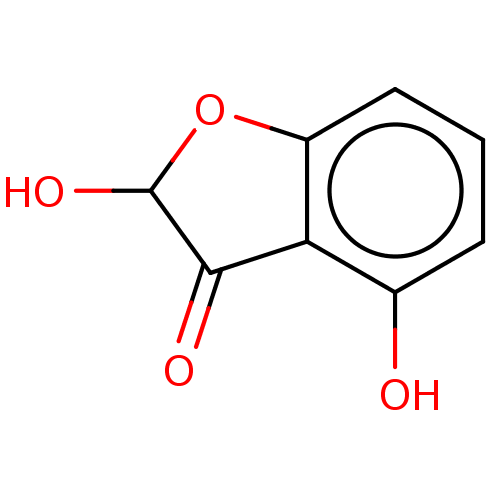 (2',4'-di-Hydroxy Benzofuranone (2',4&#...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.40E+5 | n/a | 2.01E+5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Kermanshah University of Medical Sciences | Assay Description Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c... | Bioorg Chem 64: 74-84 (2016) Article DOI: 10.1016/j.bioorg.2015.12.004 BindingDB Entry DOI: 10.7270/Q2MP522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde oxidase 1 (Cavia porcellus (Guinea pig)) | BDBM19461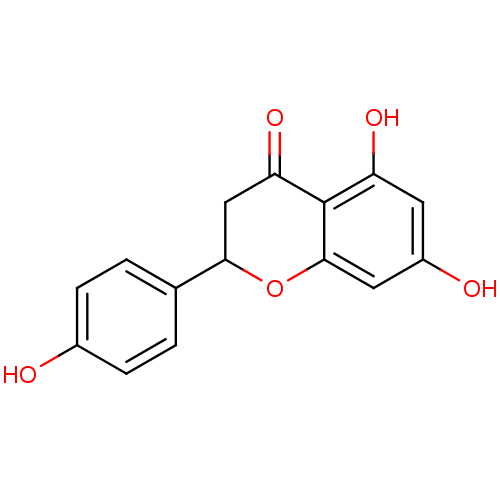 (α-CA inhibitor, 5 | 5,7-dihydroxy-2-(4-hydrox...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.48E+5 | n/a | 2.12E+5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Kermanshah University of Medical Sciences | Assay Description Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c... | Bioorg Chem 64: 74-84 (2016) Article DOI: 10.1016/j.bioorg.2015.12.004 BindingDB Entry DOI: 10.7270/Q2MP522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde oxidase 1 (Cavia porcellus (Guinea pig)) | BDBM175348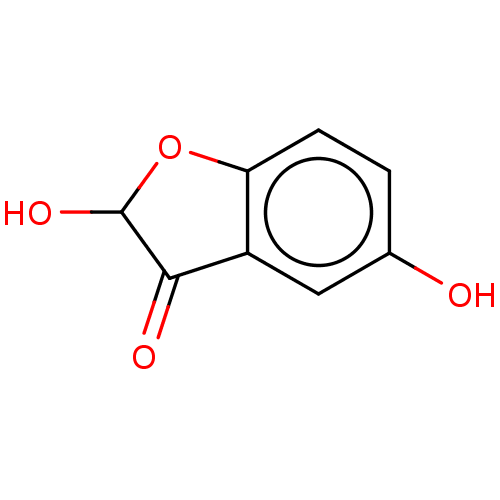 (2',5'-di-Hydroxy Benzofuranone (2',5&#...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.60E+5 | n/a | 1.15E+5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Kermanshah University of Medical Sciences | Assay Description Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c... | Bioorg Chem 64: 74-84 (2016) Article DOI: 10.1016/j.bioorg.2015.12.004 BindingDB Entry DOI: 10.7270/Q2MP522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde oxidase 1 (Cavia porcellus (Guinea pig)) | BDBM175345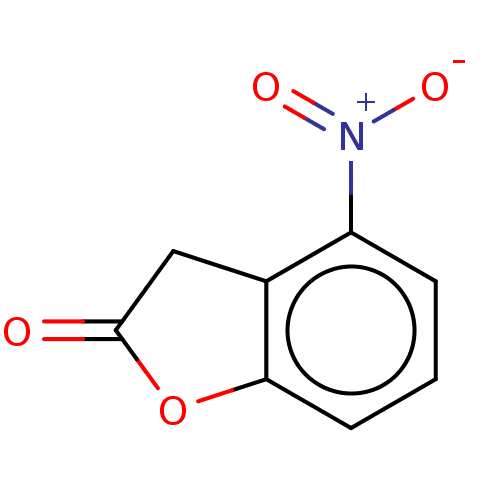 (4'-Nitro Benzofuranone (4'-NO2) | 6-Hydrox...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.60E+5 | n/a | 1.71E+5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Kermanshah University of Medical Sciences | Assay Description Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c... | Bioorg Chem 64: 74-84 (2016) Article DOI: 10.1016/j.bioorg.2015.12.004 BindingDB Entry DOI: 10.7270/Q2MP522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde oxidase 1 (Cavia porcellus (Guinea pig)) | BDBM175344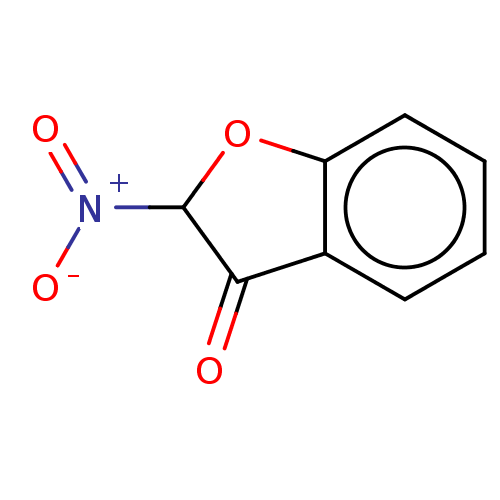 (2'-Nitro Benzofuranone (2'-NO2) | 6-Hydrox...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.53E+5 | n/a | 1.93E+5 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Kermanshah University of Medical Sciences | Assay Description Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c... | Bioorg Chem 64: 74-84 (2016) Article DOI: 10.1016/j.bioorg.2015.12.004 BindingDB Entry DOI: 10.7270/Q2MP522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Aldehyde oxidase 1 (Cavia porcellus (Guinea pig)) | BDBM175346 (2'-Hydroxy Benzofuranone (2'-OH) | 6-Hydro...) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 4.42E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.0 | n/a |
Kermanshah University of Medical Sciences | Assay Description Guinea pig AO activity was assayed spectrophotometrically using phenanthridine as a substrate at 322 nm. All spectrophotometric determinations were c... | Bioorg Chem 64: 74-84 (2016) Article DOI: 10.1016/j.bioorg.2015.12.004 BindingDB Entry DOI: 10.7270/Q2MP522Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564213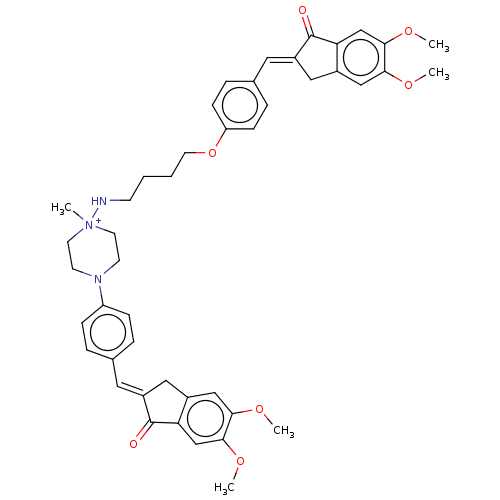 (CHEMBL4780352) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564203 (CHEMBL4791075) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564198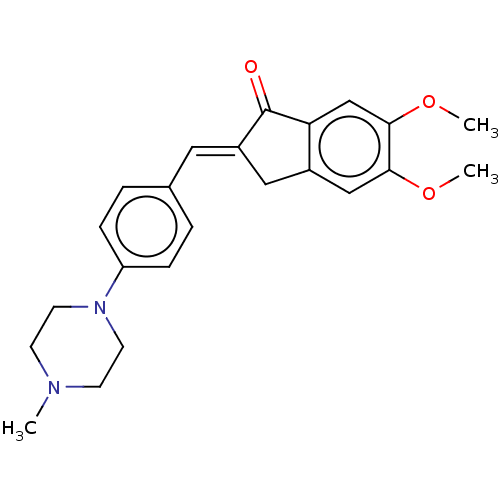 (CHEMBL4786658) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564199 (CHEMBL4800260) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564205 (CHEMBL4784191) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50564203 (CHEMBL4791075) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human plasma BuChE using butyrylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564210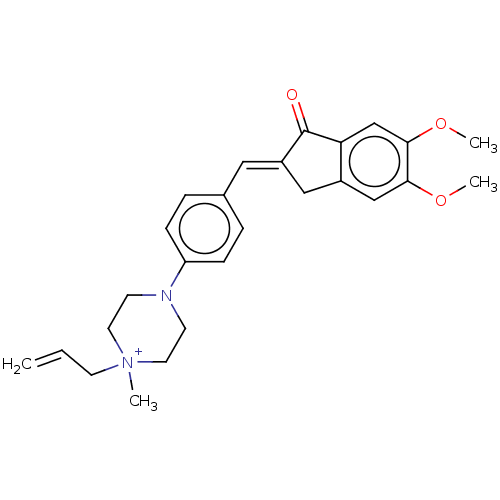 (CHEMBL4798336) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50564199 (CHEMBL4800260) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human plasma BuChE using butyrylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564204 (CHEMBL4787855) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564208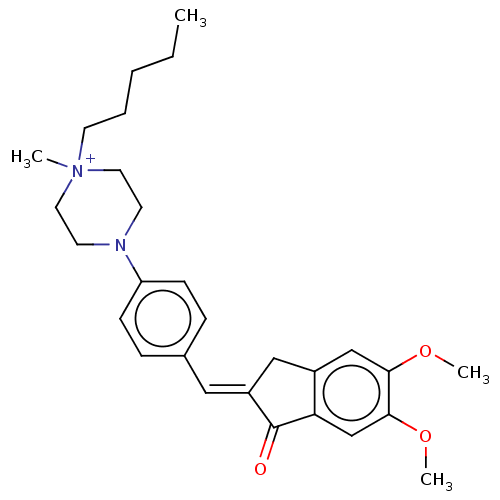 (CHEMBL4797677) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564207 (CHEMBL4776999) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564202 (CHEMBL4794208) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50564201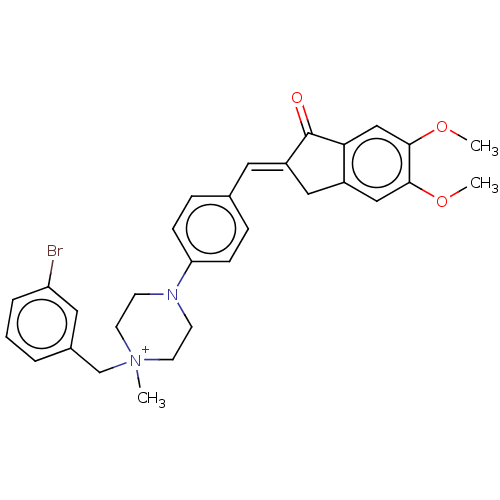 (CHEMBL4778690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human plasma BuChE using butyrylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50564213 (CHEMBL4780352) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human plasma BuChE using butyrylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564211 (CHEMBL4790642) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50564202 (CHEMBL4794208) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human plasma BuChE using butyrylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564201 (CHEMBL4778690) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.99E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564206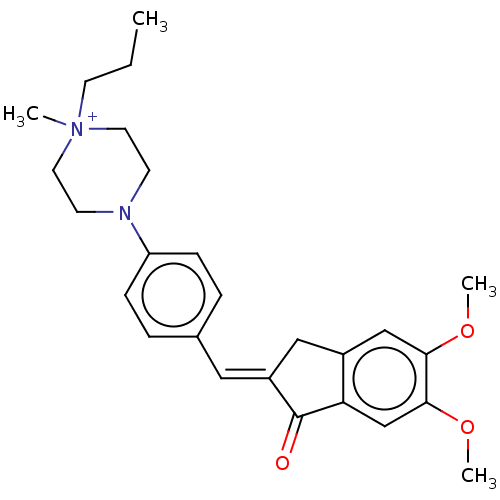 (CHEMBL4794690) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50564204 (CHEMBL4787855) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human plasma BuChE using butyrylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564200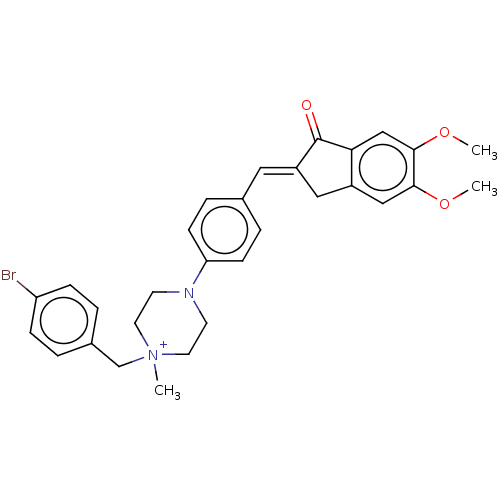 (CHEMBL4780628) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50564205 (CHEMBL4784191) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human plasma BuChE using butyrylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50564208 (CHEMBL4797677) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human plasma BuChE using butyrylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50564209 (CHEMBL4794354) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human plasma BuChE using butyrylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564209 (CHEMBL4794354) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50564200 (CHEMBL4780628) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human plasma BuChE using butyrylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50564211 (CHEMBL4790642) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human plasma BuChE using butyrylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human plasma BuChE using butyrylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50564212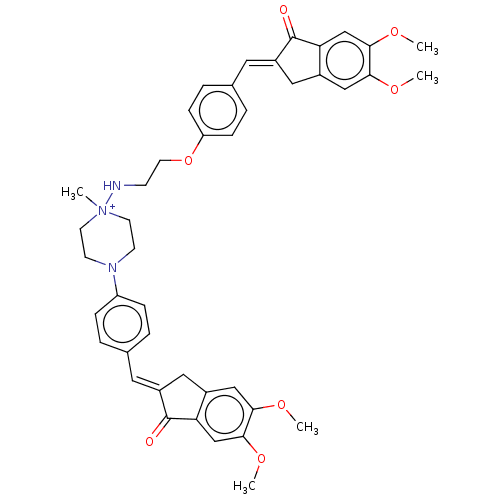 (CHEMBL4794250) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human plasma BuChE using butyrylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50564206 (CHEMBL4794690) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human plasma BuChE using butyrylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50564207 (CHEMBL4776999) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human plasma BuChE using butyrylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50564212 (CHEMBL4794250) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human erythrocyte AChE using acetylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM50564210 (CHEMBL4798336) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human plasma BuChE using butyrylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human plasma BuChE using butyrylthiocholine as substrate measured after 5 mins by Ellman's method | Citation and Details Article DOI: 10.1016/j.ejmech.2020.112140 BindingDB Entry DOI: 10.7270/Q2RV0SGV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||