

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430967 (CHEMBL2337848) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430968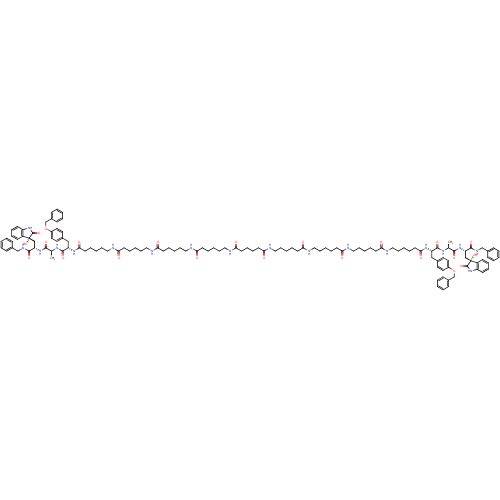 (CHEMBL2337847) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430962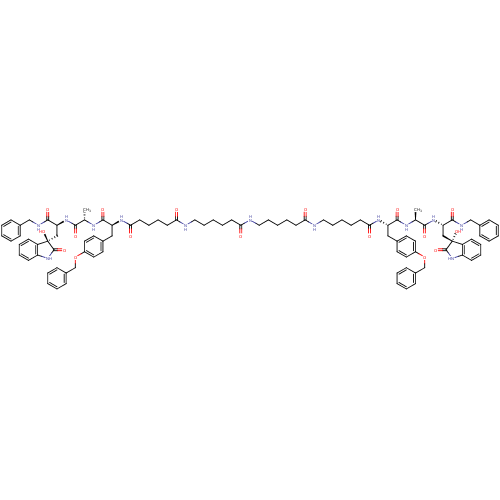 (CHEMBL2337843) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430969 (CHEMBL2337846) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430961 (CHEMBL2337844) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430970 (CHEMBL2337845) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Competitive inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as ... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485783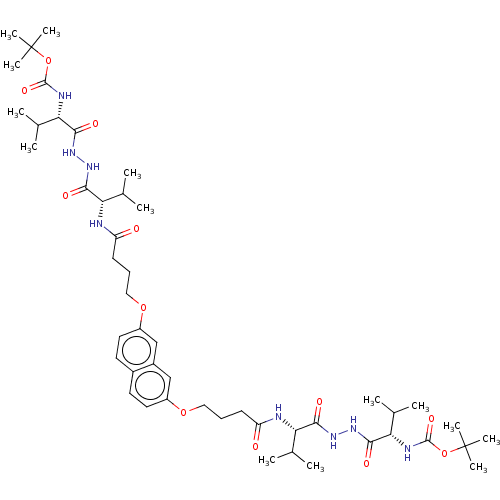 (CHEMBL2164408) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11 Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... | J Med Chem 55: 6762-75 (2012) Article DOI: 10.1021/jm300181j BindingDB Entry DOI: 10.7270/Q2125WJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430963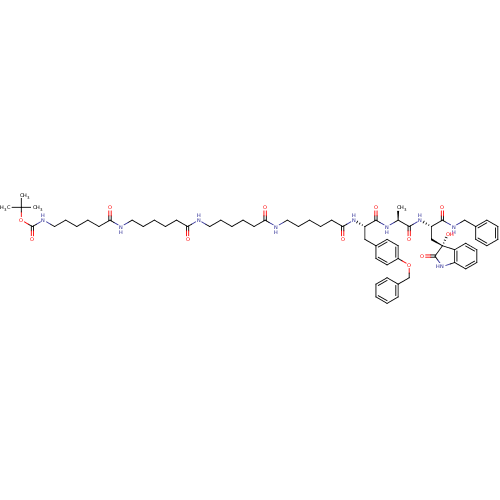 (CHEMBL2337842) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 67 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156909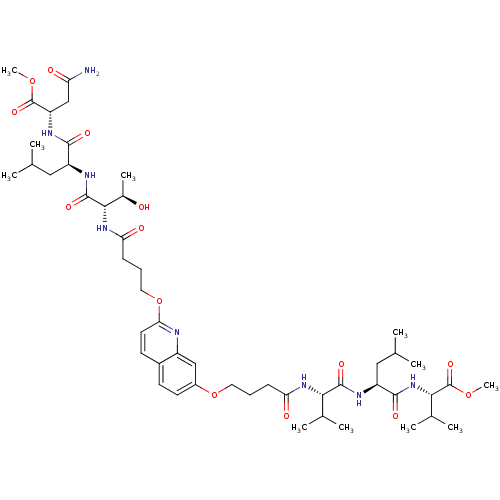 (CHEMBL376817 | methyl (2S)-3-carbamoyl-2-[(2S)-2-[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485784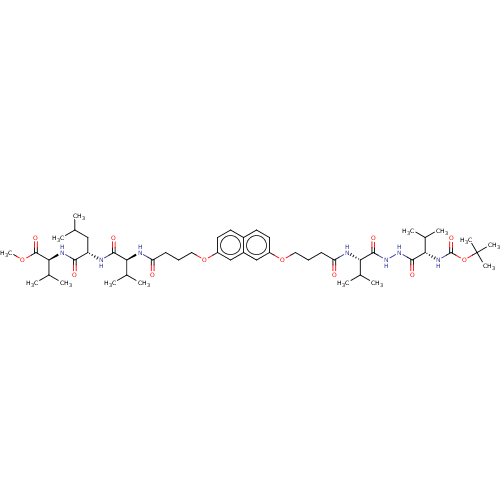 (CHEMBL2164407) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11 Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... | J Med Chem 55: 6762-75 (2012) Article DOI: 10.1021/jm300181j BindingDB Entry DOI: 10.7270/Q2125WJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50191463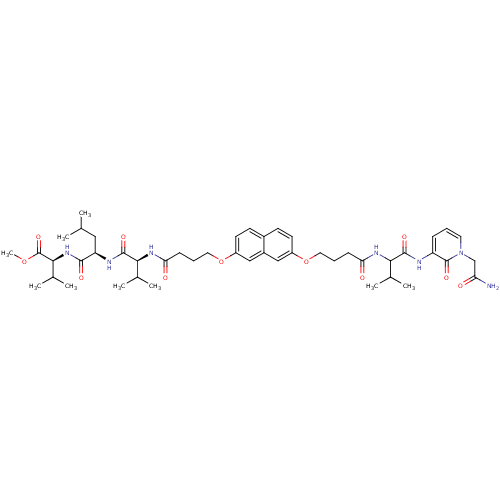 (CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease 150V mutant | J Med Chem 49: 4657-64 (2006) Article DOI: 10.1021/jm060576k BindingDB Entry DOI: 10.7270/Q2KD1XJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430964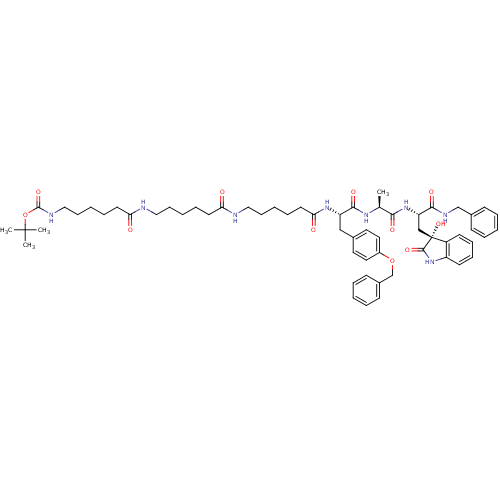 (CHEMBL2337841) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 101 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156906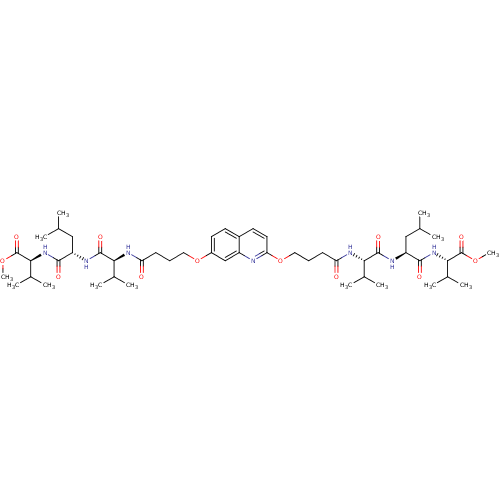 (CHEMBL375050 | methyl (2S)-2-[(2S)-2-[(2S)-2-(4-{[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485785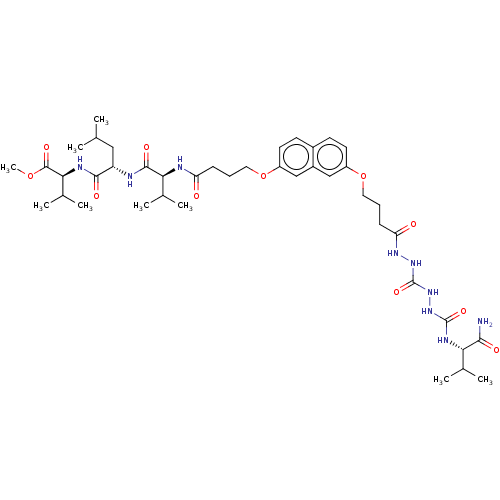 (CHEMBL2164409) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11 Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... | J Med Chem 55: 6762-75 (2012) Article DOI: 10.1021/jm300181j BindingDB Entry DOI: 10.7270/Q2125WJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430963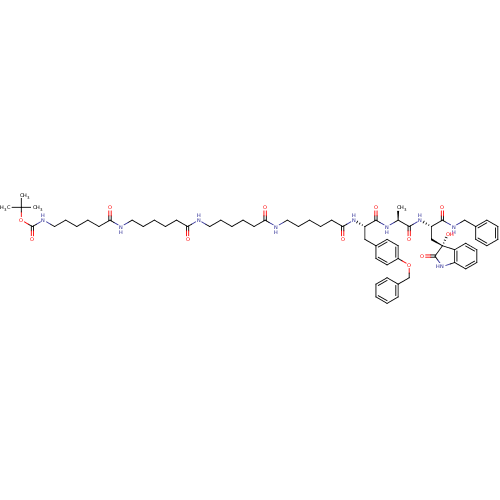 (CHEMBL2337842) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50191464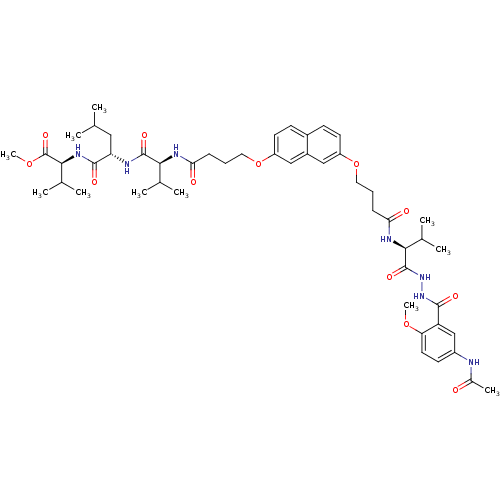 (CHEMBL212749 | methyl (2S)-2-[(2S)-2-[(2S)-2-(4-{[...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease dimerization | J Med Chem 49: 4657-64 (2006) Article DOI: 10.1021/jm060576k BindingDB Entry DOI: 10.7270/Q2KD1XJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50191463 (CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease ANAM-11 mutant | J Med Chem 49: 4657-64 (2006) Article DOI: 10.1021/jm060576k BindingDB Entry DOI: 10.7270/Q2KD1XJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156901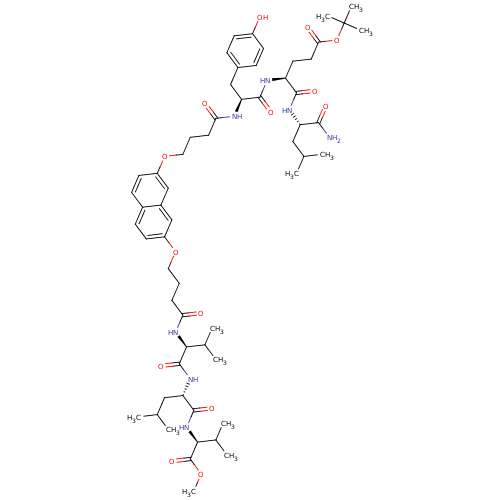 ((S)-tert-butyl 5-((S)-1-amino-4-methyl-1-oxopentan...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50191463 (CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease V82A mutant | J Med Chem 49: 4657-64 (2006) Article DOI: 10.1021/jm060576k BindingDB Entry DOI: 10.7270/Q2KD1XJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156902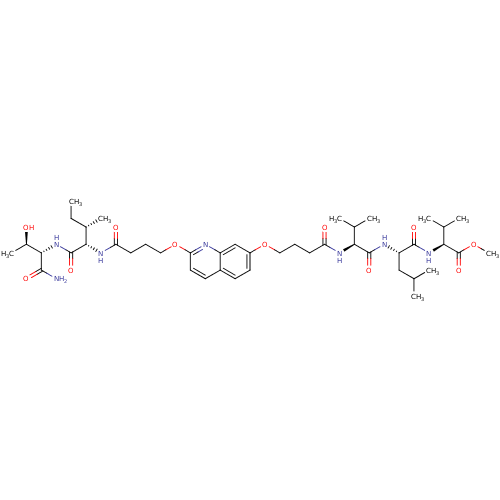 ((S)-methyl 2-((S)-2-((S)-2-(4-(2-(4-((2S,3S)-1-((2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50051763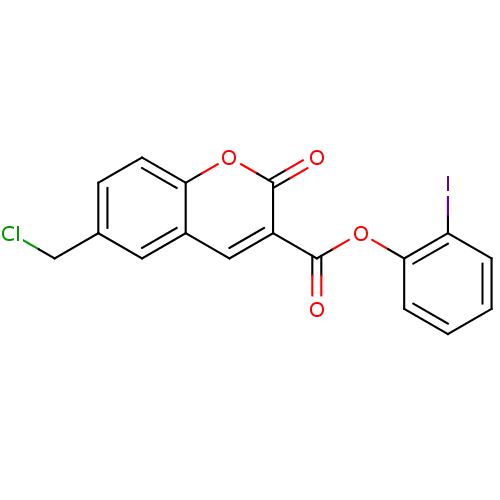 (2-iodophenyl 6-(chloromethyl)-2-oxo-2H-chromene-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Liège Curated by ChEMBL | Assay Description Compound was tested for the binding affinity against alpha-chymotrypsin | J Med Chem 39: 2579-85 (1996) Article DOI: 10.1021/jm960090b BindingDB Entry DOI: 10.7270/Q2QJ7GC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430964 (CHEMBL2337841) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 316 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM23563 (3-Carboxylate-coumarin deriv., 3 | CHEMBL13357 | p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Liège Curated by ChEMBL | Assay Description Compound was tested for the binding affinity against alpha-chymotrypsin | J Med Chem 39: 2579-85 (1996) Article DOI: 10.1021/jm960090b BindingDB Entry DOI: 10.7270/Q2QJ7GC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50051762 (6-Chloromethyl-2-oxo-2H-chromene-3-carboxylic acid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Liège Curated by ChEMBL | Assay Description Compound was tested for the binding affinity against alpha-chymotrypsin | J Med Chem 39: 2579-85 (1996) Article DOI: 10.1021/jm960090b BindingDB Entry DOI: 10.7270/Q2QJ7GC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50191463 (CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI Curated by ChEMBL | Assay Description Inhibition of wild type HIV1 protease dimerization | J Med Chem 49: 4657-64 (2006) Article DOI: 10.1021/jm060576k BindingDB Entry DOI: 10.7270/Q2KD1XJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50135844 ((2S,8S)-2-[({[({[(1S)-1-{[(1S,2R)-2-(benzyloxy)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid Curated by ChEMBL | Assay Description Competitive inhibition against HIV-1 Protease | J Med Chem 46: 5196-207 (2003) Article DOI: 10.1021/jm030871u BindingDB Entry DOI: 10.7270/Q2C53MKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156903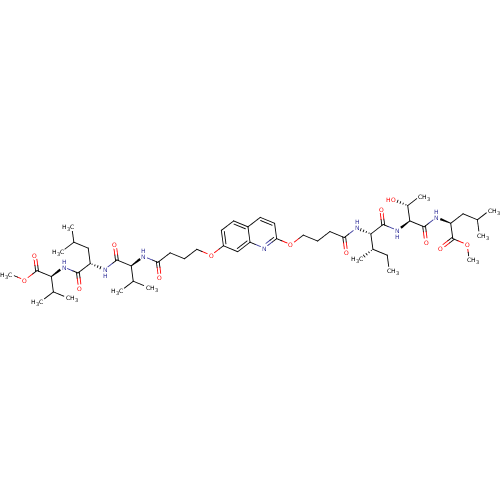 ((S)-methyl 2-((2S,3R)-3-hydroxy-2-((2S,3S)-2-(4-(7...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156910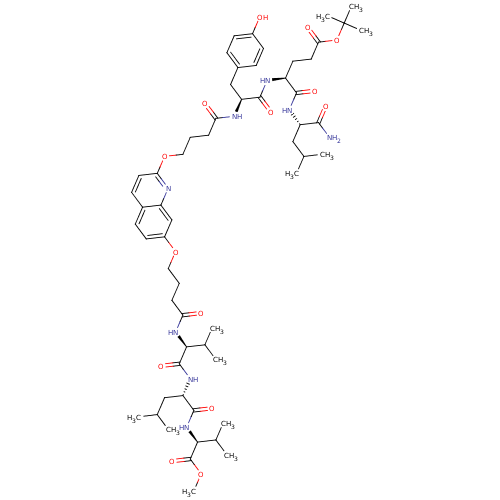 ((S)-tert-butyl 5-((S)-1-amino-4-methyl-1-oxopentan...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156908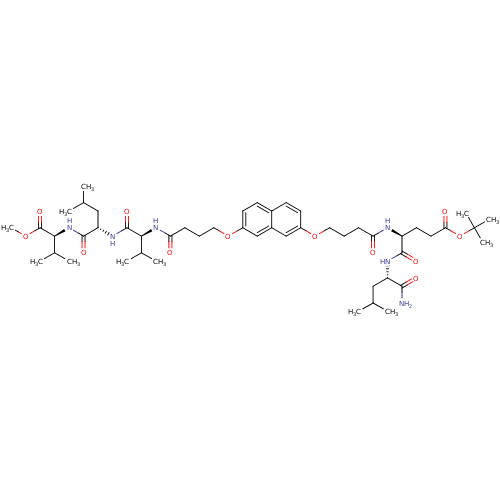 ((S)-tert-butyl 5-((S)-1-amino-4-methyl-1-oxopentan...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50191463 (CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease D30N mutant | J Med Chem 49: 4657-64 (2006) Article DOI: 10.1021/jm060576k BindingDB Entry DOI: 10.7270/Q2KD1XJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50191463 (CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease G48V/L90M mutant | J Med Chem 49: 4657-64 (2006) Article DOI: 10.1021/jm060576k BindingDB Entry DOI: 10.7270/Q2KD1XJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156904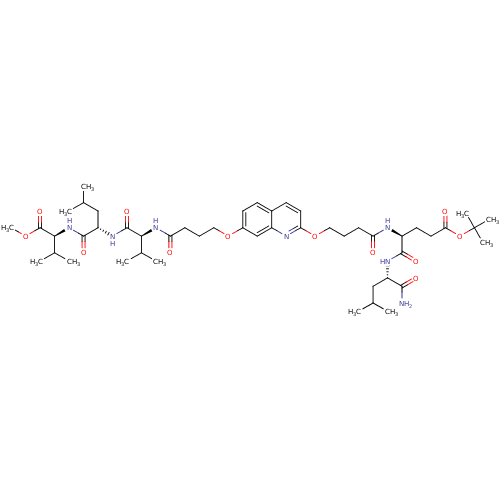 ((S)-tert-butyl 5-((S)-1-amino-4-methyl-1-oxopentan...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 530 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50074687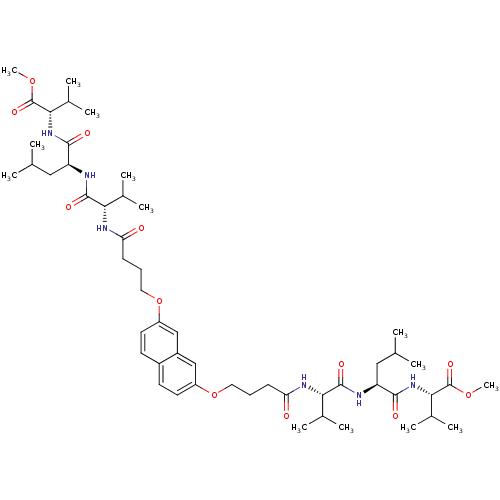 (2-[2-(2-{4-[7-(3-{1-[1-(1-Methoxycarbonyl-2-methyl...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11 Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... | J Med Chem 55: 6762-75 (2012) Article DOI: 10.1021/jm300181j BindingDB Entry DOI: 10.7270/Q2125WJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50074687 (2-[2-(2-{4-[7-(3-{1-[1-(1-Methoxycarbonyl-2-methyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 560 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485786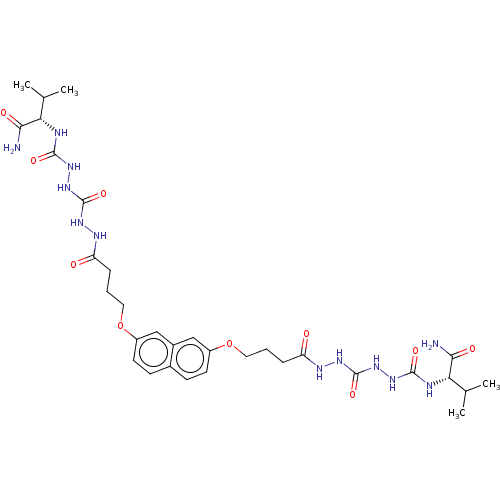 (CHEMBL2164074) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Paris-Sud 11 Curated by ChEMBL | Assay Description Inhibition of HIV1 protease dimerization expressed in Escherichia coli Rosetta(DE3) using DABCYL-gamma-abu-Ser-Gln-Asn-Tyr-Pro-Ile-Val-Gln-EDANS as s... | J Med Chem 55: 6762-75 (2012) Article DOI: 10.1021/jm300181j BindingDB Entry DOI: 10.7270/Q2125WJF | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50051761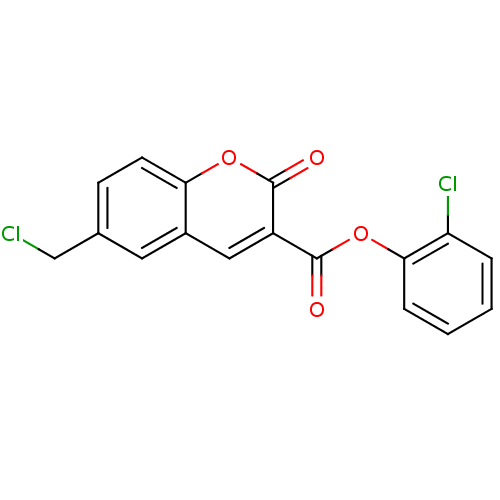 (2-chlorophenyl 6-(chloromethyl)-2-oxo-2H-chromene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Liège Curated by ChEMBL | Assay Description Compound was tested for the binding affinity against alpha-chymotrypsin | J Med Chem 39: 2579-85 (1996) Article DOI: 10.1021/jm960090b BindingDB Entry DOI: 10.7270/Q2QJ7GC1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156911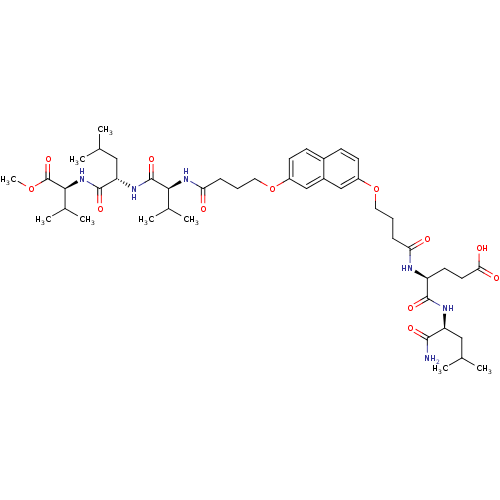 ((S)-5-((S)-1-amino-4-methyl-1-oxopentan-2-ylamino)...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156905 ((S)-methyl 2-((2S,3R)-3-hydroxy-2-(4-(7-(4-((S)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156914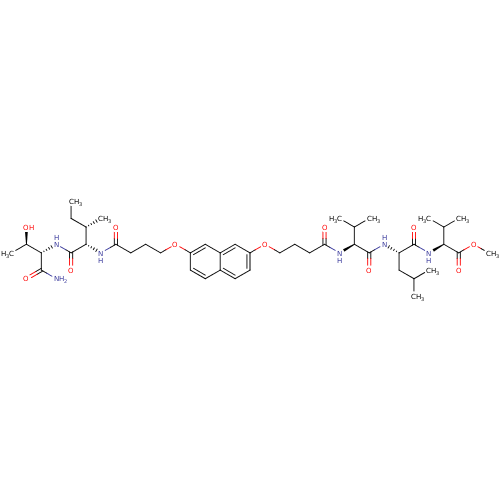 ((S)-methyl 2-((S)-2-((S)-2-(4-(7-(4-((2S,3S)-1-((2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156913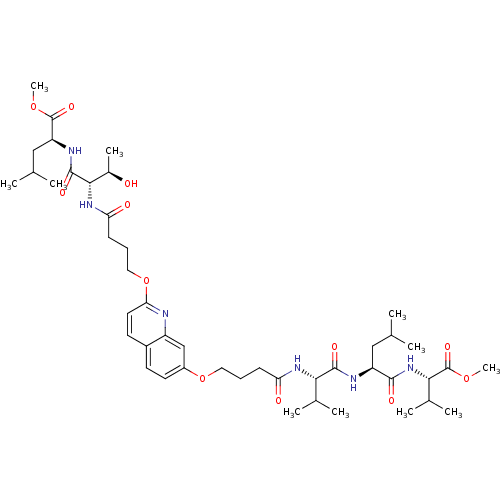 ((S)-methyl 2-((2S,3R)-3-hydroxy-2-(4-(7-(4-((S)-1-...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156912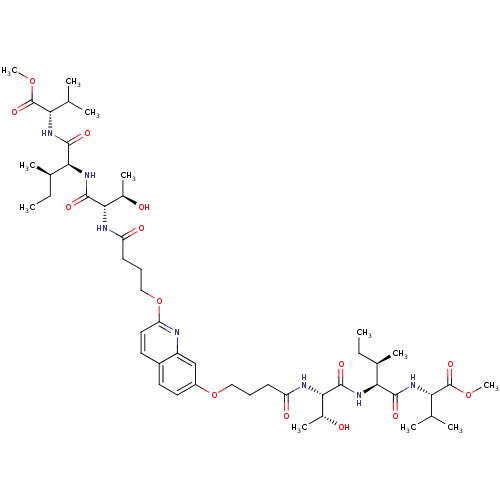 (CHEMBL376602 | methyl (2S)-2-[(2S,3R)-2-[(2S,3R)-3...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156907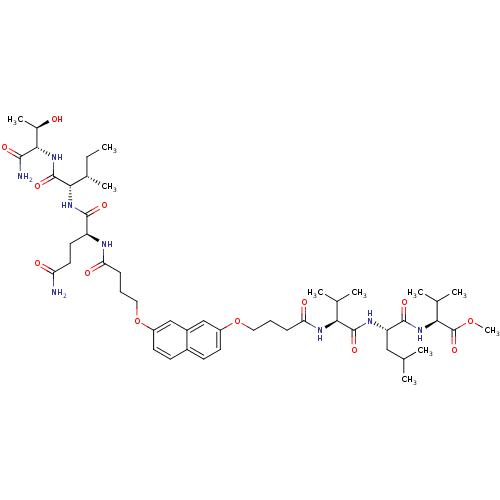 ((S)-methyl 2-((S)-2-((S)-2-(4-(7-(4-((S)-5-amino-1...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430966 (CHEMBL2337849) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.43E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50156902 ((S)-methyl 2-((S)-2-((S)-2-(4-(2-(4-((2S,3S)-1-((2...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud Curated by ChEMBL | Assay Description Inhibition of HIV1 protease | J Med Chem 47: 6392-400 (2004) Article DOI: 10.1021/jm040833q BindingDB Entry DOI: 10.7270/Q2CV4H7H | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kallikrein-5 [D153N] (Homo sapiens (Human)) | BDBM32709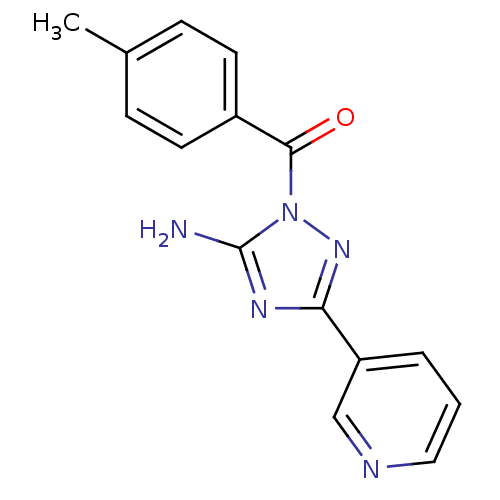 ((5-amino-3-pyridin-3-yl-1,2,4-triazol-1-yl)-(4-met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ Pierre et Marie Curie-Sorbonne Universit£s (UPMC) Curated by ChEMBL | Assay Description Inhibition of human kallikrein 5 measured after 15 mins at pH 8 by double-reciprocal plot analysis | Bioorg Med Chem Lett 23: 4547-51 (2013) Article DOI: 10.1016/j.bmcl.2013.06.039 BindingDB Entry DOI: 10.7270/Q23B61JV | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50191463 (CHEMBL378467 | methyl (2S)-2-[(2R)-2-[(2S)-2-{4-[(...) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Paris-Sud XI Curated by ChEMBL | Assay Description Inhibition of dimerization of HIV1 protease MDR-HM mutant | J Med Chem 49: 4657-64 (2006) Article DOI: 10.1021/jm060576k BindingDB Entry DOI: 10.7270/Q2KD1XJR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Saccharomyces cerevisiae) | BDBM50185622 (Apam-YET | CHEMBL210325) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
CNRS-Université Paris VI Curated by ChEMBL | Assay Description Inhibition of chymotrypsin like activity of yeast 20S proteasome | Bioorg Med Chem Lett 16: 3277-81 (2006) Article DOI: 10.1016/j.bmcl.2006.03.033 BindingDB Entry DOI: 10.7270/Q280527Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Proteasome subunit beta type-5 (Homo sapiens (Human)) | BDBM50430966 (CHEMBL2337849) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.03E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University Paris 6 Curated by ChEMBL | Assay Description Mixed type inhibition of chymotrypsin-like activity of human constitutive 20s proteasome beta-5 subunit using Suc-LLVY-AMC as substrate assessed as i... | J Med Chem 56: 3367-78 (2013) Article DOI: 10.1021/jm4002007 BindingDB Entry DOI: 10.7270/Q2F19135 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50135845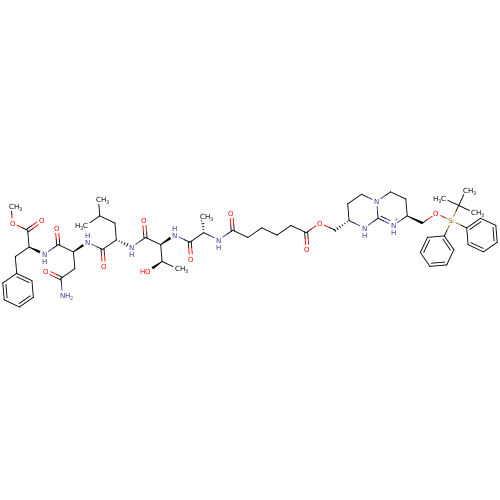 (Bicyclic Guanidinium Subunit | CHEMBL266754) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid Curated by ChEMBL | Assay Description Competitive inhibition against HIV-1 Protease | J Med Chem 46: 5196-207 (2003) Article DOI: 10.1021/jm030871u BindingDB Entry DOI: 10.7270/Q2C53MKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [489-587] (Human immunodeficiency virus type 1) | BDBM50135842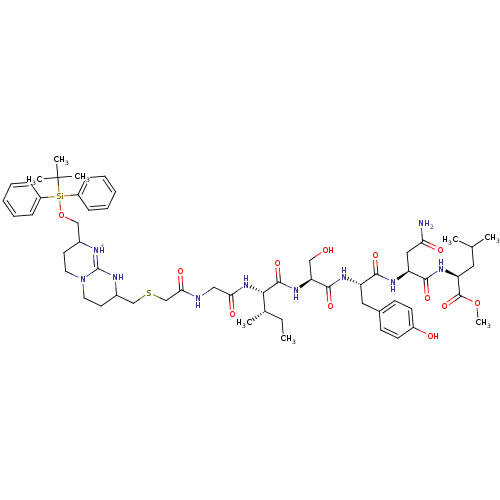 (Bicyclic Guanidinium Subunit | CHEMBL386994) | PDB MMDB UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universidad Aut£noma de Madrid Curated by ChEMBL | Assay Description Competitive inhibition against HIV-1 Protease | J Med Chem 46: 5196-207 (2003) Article DOI: 10.1021/jm030871u BindingDB Entry DOI: 10.7270/Q2C53MKZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 400 total ) | Next | Last >> |