 Found 184 hits with Last Name = 'reddy' and Initial = 'pv'
Found 184 hits with Last Name = 'reddy' and Initial = 'pv' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50102258
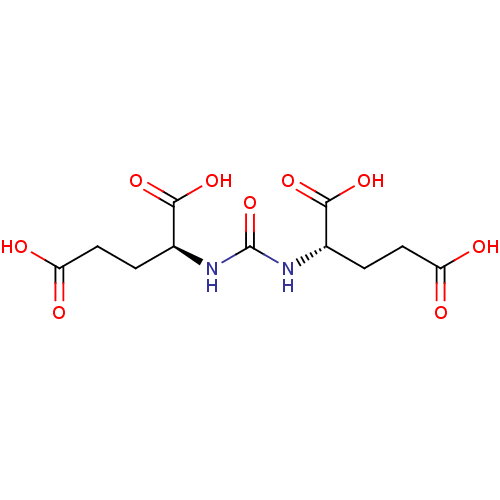
((S)-2-[3-((S)-3-Carboxy-1-carboxy-propyl)-ureido]-...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O Show InChI InChI=1S/C11H16N2O9/c14-7(15)3-1-5(9(18)19)12-11(22)13-6(10(20)21)2-4-8(16)17/h5-6H,1-4H2,(H,14,15)(H,16,17)(H,18,19)(H,20,21)(H2,12,13,22)/t5-,6-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Binding affinity PSMA (unknown origin) |
J Med Chem 58: 3094-103 (2015)
Article DOI: 10.1021/jm5018384
BindingDB Entry DOI: 10.7270/Q2MG7R6G |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363378
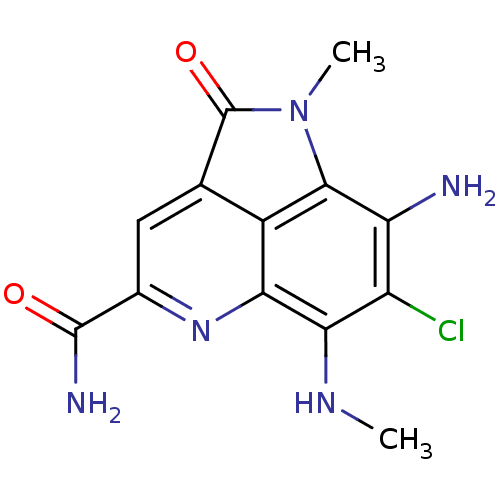
(CHEMBL1945729)Show InChI InChI=1S/C13H12ClN5O2/c1-17-10-7(14)8(15)11-6-4(13(21)19(11)2)3-5(12(16)20)18-9(6)10/h3,17H,15H2,1-2H3,(H2,16,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Glutamate carboxypeptidase 2
(Homo sapiens (Human)) | BDBM50102258

((S)-2-[3-((S)-3-Carboxy-1-carboxy-propyl)-ureido]-...)Show SMILES OC(=O)CC[C@H](NC(=O)N[C@@H](CCC(O)=O)C(O)=O)C(O)=O Show InChI InChI=1S/C11H16N2O9/c14-7(15)3-1-5(9(18)19)12-11(22)13-6(10(20)21)2-4-8(16)17/h5-6H,1-4H2,(H,14,15)(H,16,17)(H,18,19)(H,20,21)(H2,12,13,22)/t5-,6-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of PSMA (unknown origin) |
J Med Chem 58: 3094-103 (2015)
Article DOI: 10.1021/jm5018384
BindingDB Entry DOI: 10.7270/Q2MG7R6G |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363366

(CHEMBL1945406)Show InChI InChI=1S/C12H10ClN5O2/c1-18-10-5-3(12(18)20)2-4(11(16)19)17-9(5)7(14)6(13)8(10)15/h2H,14-15H2,1H3,(H2,16,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM29227

(casimiroin analogue, 1p)Show InChI InChI=1S/C14H17NO4/c1-8-6-11(16)15(2)13-9(17-3)7-10(18-4)14(19-5)12(8)13/h6-7H,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
Aromatase inhibition was quantified by measuring the fluorescent intensity of fluorescein, the hydrolysis product of dibenzylfluorescein (DBF), by ar... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363373
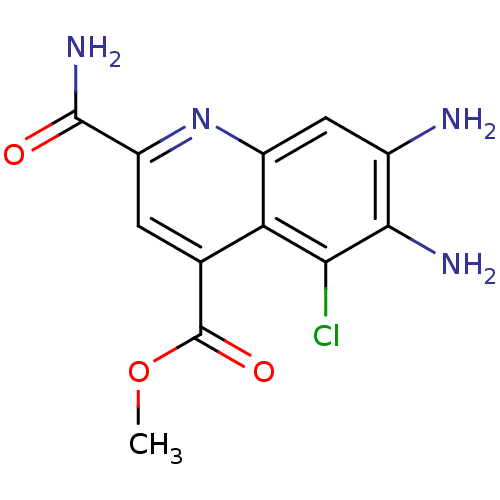
(CHEMBL1945627)Show InChI InChI=1S/C12H11ClN4O3/c1-20-12(19)4-2-7(11(16)18)17-6-3-5(14)10(15)9(13)8(4)6/h2-3H,14-15H2,1H3,(H2,16,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363372
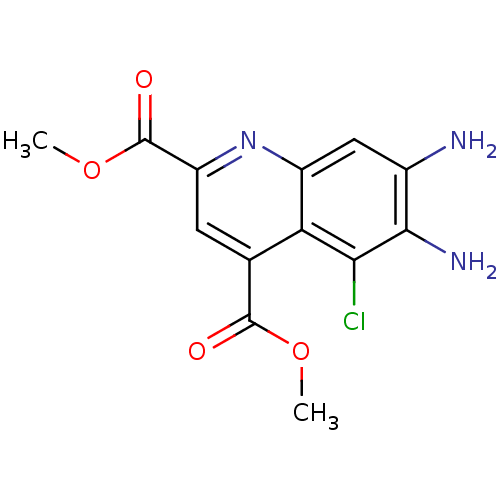
(CHEMBL1945626)Show InChI InChI=1S/C13H12ClN3O4/c1-20-12(18)5-3-8(13(19)21-2)17-7-4-6(15)11(16)10(14)9(5)7/h3-4H,15-16H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM9460

(3-(4-aminophenyl)-3-ethyl-piperidine-2,6-dione | 3...)Show InChI InChI=1S/C13H16N2O2/c1-2-13(8-7-11(16)15-12(13)17)9-3-5-10(14)6-4-9/h3-6H,2,7-8,14H2,1H3,(H,15,16,17) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
Aromatase inhibition was quantified by measuring the fluorescent intensity of fluorescein, the hydrolysis product of dibenzylfluorescein (DBF), by ar... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM29229

(casimiroin analogue, 1r)Show InChI InChI=1S/C15H19NO5/c1-8-7-9(17)16(2)11-10(8)12(18-3)14(20-5)15(21-6)13(11)19-4/h7H,1-6H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
Aromatase inhibition was quantified by measuring the fluorescent intensity of fluorescein, the hydrolysis product of dibenzylfluorescein (DBF), by ar... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM29221
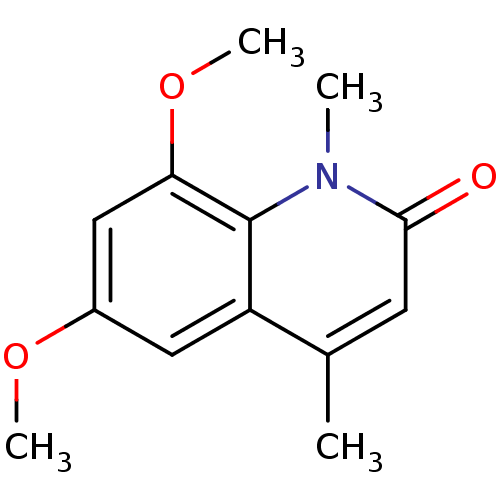
(casimiroin analogue, 1j)Show InChI InChI=1S/C13H15NO3/c1-8-5-12(15)14(2)13-10(8)6-9(16-3)7-11(13)17-4/h5-7H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
Aromatase inhibition was quantified by measuring the fluorescent intensity of fluorescein, the hydrolysis product of dibenzylfluorescein (DBF), by ar... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM29223

(casimiroin analogue, 1l)Show InChI InChI=1S/C13H15NO3/c1-8-7-11(15)14(2)13-10(17-4)6-5-9(16-3)12(8)13/h5-7H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
Aromatase inhibition was quantified by measuring the fluorescent intensity of fluorescein, the hydrolysis product of dibenzylfluorescein (DBF), by ar... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM23926

((E)-resveratrol | 5-[(E)-2-(4-hydroxyphenyl)etheny...)Show InChI InChI=1S/C14H12O3/c15-12-5-3-10(4-6-12)1-2-11-7-13(16)9-14(17)8-11/h1-9,15-17H/b2-1+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 960 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University
| Assay Description
The activity of QR2 under steady-state conditions was evaluated on SpectraMax Plus 384 UV/vis spectrophotometer by monitoring the increase in absorba... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosyl-DNA phosphodiesterase 1
(Homo sapiens (Human)) | BDBM50158383
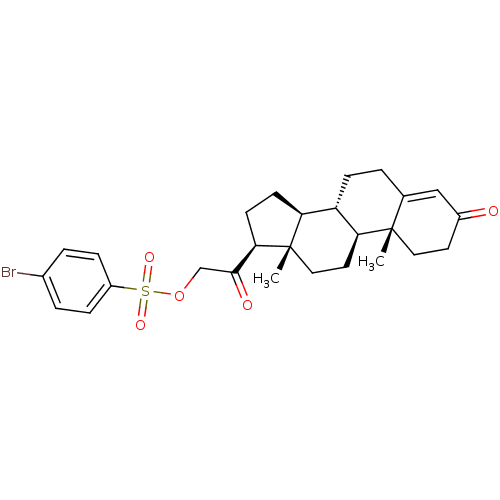
(2-((8S,9S,10R,13S,14S,17S)-10,13-dimethyl-3-oxo-2,...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)COS(=O)(=O)c1ccc(Br)cc1 |r,t:8| Show InChI InChI=1S/C27H33BrO5S/c1-26-13-11-19(29)15-17(26)3-8-21-22-9-10-24(27(22,2)14-12-23(21)26)25(30)16-33-34(31,32)20-6-4-18(28)5-7-20/h4-7,15,21-24H,3,8-14,16H2,1-2H3/t21-,22-,23-,24+,26-,27-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... |
J Med Chem 56: 182-200 (2013)
Article DOI: 10.1021/jm3014458
BindingDB Entry DOI: 10.7270/Q2MS3V3Z |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363369
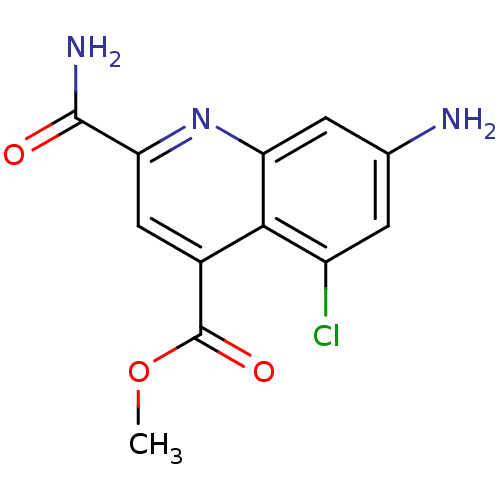
(CHEMBL1945620)Show InChI InChI=1S/C12H10ClN3O3/c1-19-12(18)6-4-9(11(15)17)16-8-3-5(14)2-7(13)10(6)8/h2-4H,14H2,1H3,(H2,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM29213

(casimiroin analogue, 1b)Show InChI InChI=1S/C12H11NO3/c1-7-5-10(14)13(2)11-8(7)3-4-9-12(11)16-6-15-9/h3-5H,6H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
Aromatase inhibition was quantified by measuring the fluorescent intensity of fluorescein, the hydrolysis product of dibenzylfluorescein (DBF), by ar... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363370
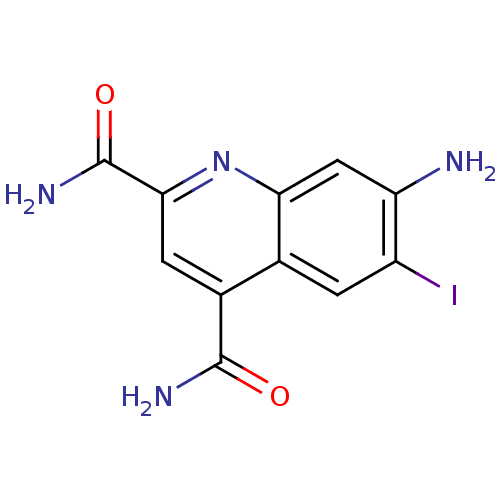
(CHEMBL1945624)Show InChI InChI=1S/C11H9IN4O2/c12-6-1-4-5(10(14)17)2-9(11(15)18)16-8(4)3-7(6)13/h1-3H,13H2,(H2,14,17)(H2,15,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363365
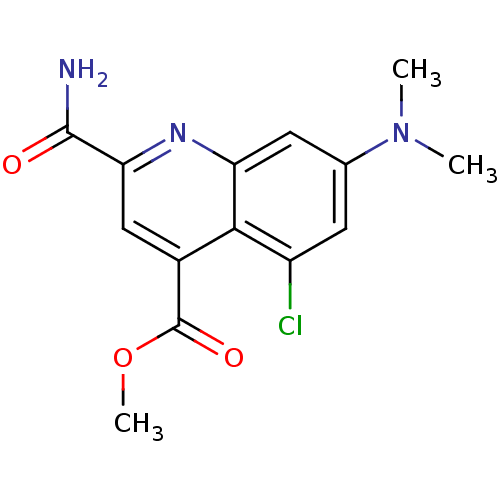
(CHEMBL1945623)Show InChI InChI=1S/C14H14ClN3O3/c1-18(2)7-4-9(15)12-8(14(20)21-3)6-11(13(16)19)17-10(12)5-7/h4-6H,1-3H3,(H2,16,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363371
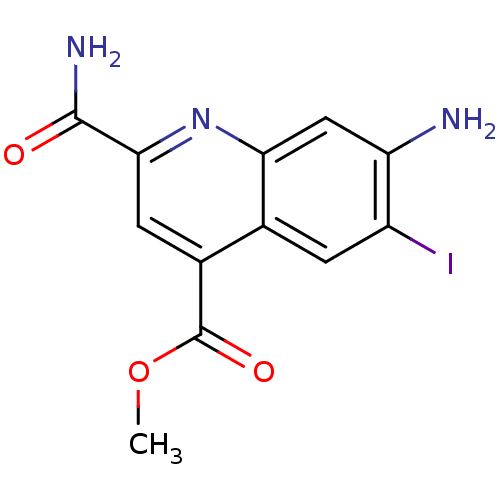
(CHEMBL1945625)Show InChI InChI=1S/C12H10IN3O3/c1-19-12(18)6-3-10(11(15)17)16-9-4-8(14)7(13)2-5(6)9/h2-4H,14H2,1H3,(H2,15,17) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM29221

(casimiroin analogue, 1j)Show InChI InChI=1S/C13H15NO3/c1-8-5-12(15)14(2)13-10(8)6-9(16-3)7-11(13)17-4/h5-7H,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University
| Assay Description
The activity of QR2 under steady-state conditions was evaluated on SpectraMax Plus 384 UV/vis spectrophotometer by monitoring the increase in absorba... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Aromatase
(Homo sapiens (Human)) | BDBM29217

(casimiroin analogue, 1f)Show InChI InChI=1S/C12H13NO2/c1-8-7-11(14)13(2)12-9(8)5-4-6-10(12)15-3/h4-7H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
Aromatase inhibition was quantified by measuring the fluorescent intensity of fluorescein, the hydrolysis product of dibenzylfluorescein (DBF), by ar... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM29225

(casimiroin analogue, 1n)Show InChI InChI=1S/C14H17NO4/c1-8-6-11(16)15(2)12-9(8)7-10(17-3)13(18-4)14(12)19-5/h6-7H,1-5H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
Aromatase inhibition was quantified by measuring the fluorescent intensity of fluorescein, the hydrolysis product of dibenzylfluorescein (DBF), by ar... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363367

(CHEMBL1945407)Show InChI InChI=1S/C13H11ClN2O4/c1-19-12(17)7-5-10(13(18)20-2)16-9-4-6(15)3-8(14)11(7)9/h3-5H,15H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM29210
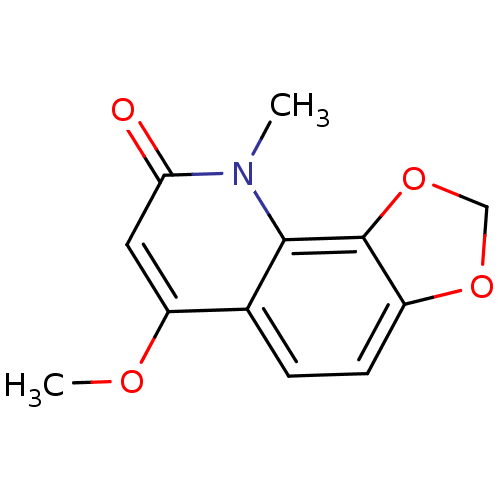
(casimiroin)Show InChI InChI=1S/C12H11NO4/c1-13-10(14)5-9(15-2)7-3-4-8-12(11(7)13)17-6-16-8/h3-5H,6H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 3.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
Aromatase inhibition was quantified by measuring the fluorescent intensity of fluorescein, the hydrolysis product of dibenzylfluorescein (DBF), by ar... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363364
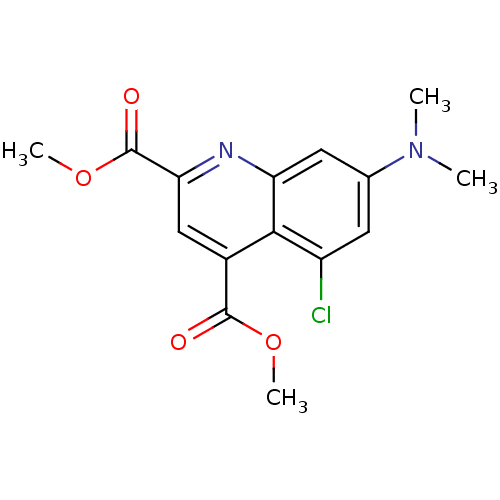
(CHEMBL1944648)Show InChI InChI=1S/C15H15ClN2O4/c1-18(2)8-5-10(16)13-9(14(19)21-3)7-12(15(20)22-4)17-11(13)6-8/h5-7H,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM29223

(casimiroin analogue, 1l)Show InChI InChI=1S/C13H15NO3/c1-8-7-11(15)14(2)13-10(17-4)6-5-9(16-3)12(8)13/h5-7H,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University
| Assay Description
The activity of QR2 under steady-state conditions was evaluated on SpectraMax Plus 384 UV/vis spectrophotometer by monitoring the increase in absorba... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosyl-DNA phosphodiesterase 1
(Homo sapiens (Human)) | BDBM50425045
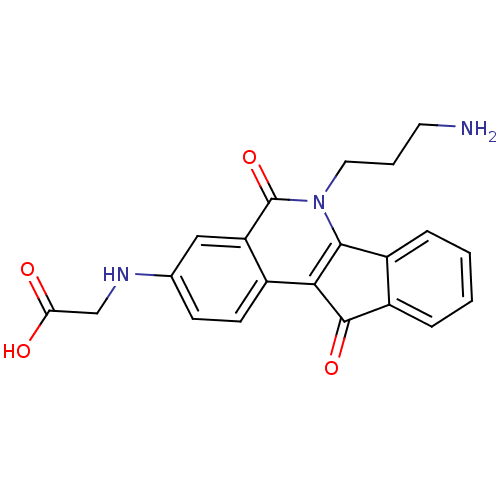
(CHEMBL2312896 | CHEMBL2322964 | US9402842, 43)Show SMILES NCCCn1c2-c3ccccc3C(=O)c2c2ccc(NCC(O)=O)cc2c1=O Show InChI InChI=1S/C21H19N3O4/c22-8-3-9-24-19-14-4-1-2-5-15(14)20(27)18(19)13-7-6-12(23-11-17(25)26)10-16(13)21(24)28/h1-2,4-7,10,23H,3,8-9,11,22H2,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... |
J Med Chem 56: 182-200 (2013)
Article DOI: 10.1021/jm3014458
BindingDB Entry DOI: 10.7270/Q2MS3V3Z |
More data for this
Ligand-Target Pair | |
Tyrosyl-DNA phosphodiesterase 1
(Homo sapiens (Human)) | BDBM50425086
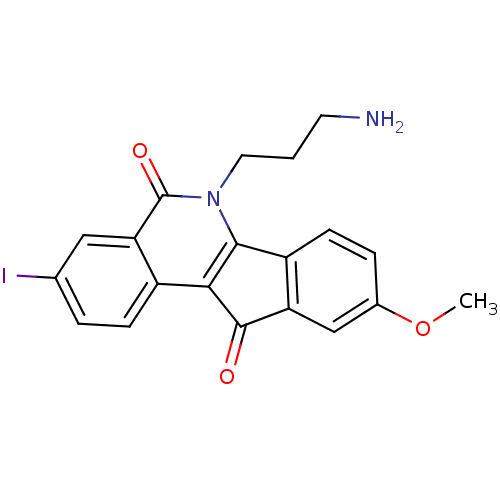
(CHEMBL2312906 | US9402842, 84)Show SMILES COc1ccc-2c(c1)C(=O)c1c-2n(CCCN)c(=O)c2cc(I)ccc12 Show InChI InChI=1S/C20H17IN2O3/c1-26-12-4-6-14-15(10-12)19(24)17-13-5-3-11(21)9-16(13)20(25)23(18(14)17)8-2-7-22/h3-6,9-10H,2,7-8,22H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... |
J Med Chem 56: 182-200 (2013)
Article DOI: 10.1021/jm3014458
BindingDB Entry DOI: 10.7270/Q2MS3V3Z |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363368
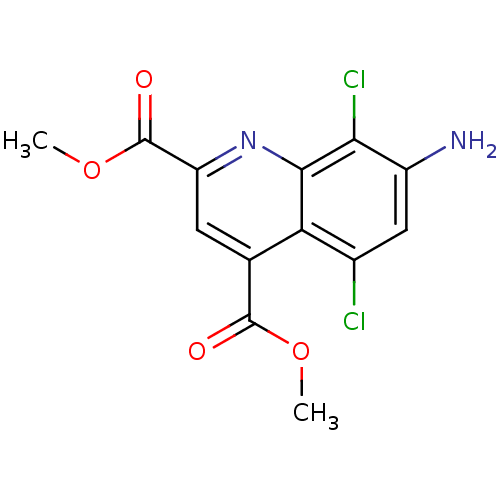
(CHEMBL1945619)Show InChI InChI=1S/C13H10Cl2N2O4/c1-20-12(18)5-3-8(13(19)21-2)17-11-9(5)6(14)4-7(16)10(11)15/h3-4H,16H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM29228
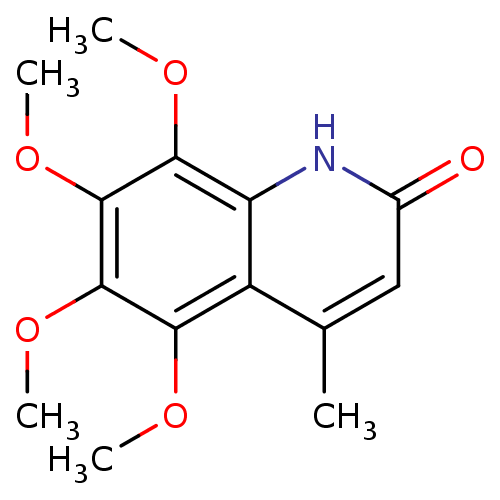
(casimiroin analogue, 1q)Show InChI InChI=1S/C14H17NO5/c1-7-6-8(16)15-10-9(7)11(17-2)13(19-4)14(20-5)12(10)18-3/h6H,1-5H3,(H,15,16) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.68E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
Aromatase inhibition was quantified by measuring the fluorescent intensity of fluorescein, the hydrolysis product of dibenzylfluorescein (DBF), by ar... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | |
Aromatase
(Homo sapiens (Human)) | BDBM29219

(casimiroin analogue, 1h)Show InChI InChI=1S/C13H15NO3/c1-8-7-11(15)14(2)12-9(8)5-6-10(16-3)13(12)17-4/h5-7H,1-4H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
| Assay Description
Aromatase inhibition was quantified by measuring the fluorescent intensity of fluorescein, the hydrolysis product of dibenzylfluorescein (DBF), by ar... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM29217

(casimiroin analogue, 1f)Show InChI InChI=1S/C12H13NO2/c1-8-7-11(14)13(2)12-9(8)5-4-6-10(12)15-3/h4-7H,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University
| Assay Description
The activity of QR2 under steady-state conditions was evaluated on SpectraMax Plus 384 UV/vis spectrophotometer by monitoring the increase in absorba... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363375

(CHEMBL1945629)Show InChI InChI=1S/C12H7Cl2N3O2/c1-17-10-6(14)3-5(13)9-8(10)4(12(17)19)2-7(16-9)11(15)18/h2-3H,1H3,(H2,15,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Tyrosyl-DNA phosphodiesterase 1
(Homo sapiens (Human)) | BDBM50425087
(CHEMBL218884 | US9402842, 55)Show SMILES COc1cccc2C(=O)c3c(-c12)n(CCCN)c(=O)c1cc(ccc31)[N+]([O-])=O Show InChI InChI=1S/C20H17N3O5/c1-28-15-5-2-4-13-16(15)18-17(19(13)24)12-7-6-11(23(26)27)10-14(12)20(25)22(18)9-3-8-21/h2,4-7,10H,3,8-9,21H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... |
J Med Chem 56: 182-200 (2013)
Article DOI: 10.1021/jm3014458
BindingDB Entry DOI: 10.7270/Q2MS3V3Z |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM29226
(casimiroin analogue, 1o)Show InChI InChI=1S/C13H15NO4/c1-7-5-10(15)14-12-8(16-2)6-9(17-3)13(18-4)11(7)12/h5-6H,1-4H3,(H,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University
| Assay Description
The activity of QR2 under steady-state conditions was evaluated on SpectraMax Plus 384 UV/vis spectrophotometer by monitoring the increase in absorba... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM29213

(casimiroin analogue, 1b)Show InChI InChI=1S/C12H11NO3/c1-7-5-10(14)13(2)11-8(7)3-4-9-12(11)16-6-15-9/h3-5H,6H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.20E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University
| Assay Description
The activity of QR2 under steady-state conditions was evaluated on SpectraMax Plus 384 UV/vis spectrophotometer by monitoring the increase in absorba... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosyl-DNA phosphodiesterase 1
(Homo sapiens (Human)) | BDBM50425085
(CHEMBL2312908 | US9402842, 89)Show SMILES COc1ccc-2c(c1)C(=O)c1c-2n(CCCN)c(=O)c2cc(N)ccc12 Show InChI InChI=1S/C20H19N3O3/c1-26-12-4-6-14-15(10-12)19(24)17-13-5-3-11(22)9-16(13)20(25)23(18(14)17)8-2-7-21/h3-6,9-10H,2,7-8,21-22H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... |
J Med Chem 56: 182-200 (2013)
Article DOI: 10.1021/jm3014458
BindingDB Entry DOI: 10.7270/Q2MS3V3Z |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM29225

(casimiroin analogue, 1n)Show InChI InChI=1S/C14H17NO4/c1-8-6-11(16)15(2)12-9(8)7-10(17-3)13(18-4)14(12)19-5/h6-7H,1-5H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 7.00E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University
| Assay Description
The activity of QR2 under steady-state conditions was evaluated on SpectraMax Plus 384 UV/vis spectrophotometer by monitoring the increase in absorba... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363376
(CHEMBL1945630)Show SMILES CN1C(=O)c2cc(nc3c(Cl)cc(Cl)c1c23)C(=O)NCc1ccccc1 Show InChI InChI=1S/C19H13Cl2N3O2/c1-24-17-13(21)8-12(20)16-15(17)11(19(24)26)7-14(23-16)18(25)22-9-10-5-3-2-4-6-10/h2-8H,9H2,1H3,(H,22,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM29220
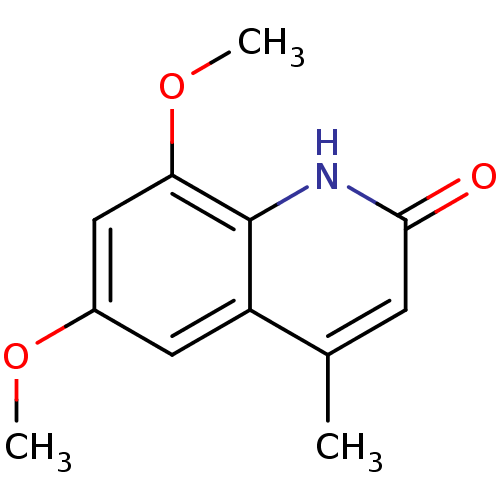
(casimiroin analogue, 1i)Show InChI InChI=1S/C12H13NO3/c1-7-4-11(14)13-12-9(7)5-8(15-2)6-10(12)16-3/h4-6H,1-3H3,(H,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University
| Assay Description
The activity of QR2 under steady-state conditions was evaluated on SpectraMax Plus 384 UV/vis spectrophotometer by monitoring the increase in absorba... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM50363363
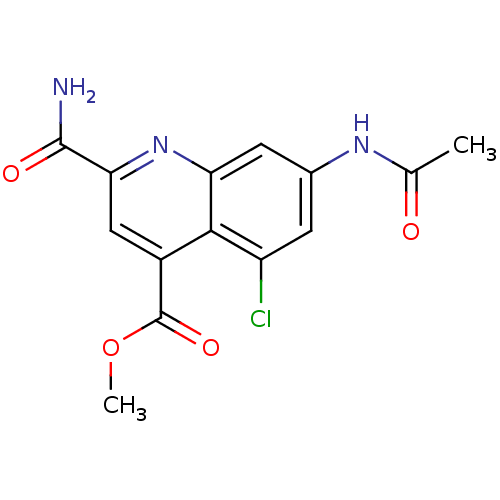
(CHEMBL1945622)Show InChI InChI=1S/C14H12ClN3O4/c1-6(19)17-7-3-9(15)12-8(14(21)22-2)5-11(13(16)20)18-10(12)4-7/h3-5H,1-2H3,(H2,16,20)(H,17,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of human quinone reductase 2 using NMeH as substrate by MTT assay |
J Med Chem 55: 367-77 (2012)
Article DOI: 10.1021/jm201251c
BindingDB Entry DOI: 10.7270/Q228083N |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM29219

(casimiroin analogue, 1h)Show InChI InChI=1S/C13H15NO3/c1-8-7-11(15)14(2)12-9(8)5-6-10(16-3)13(12)17-4/h5-7H,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University
| Assay Description
The activity of QR2 under steady-state conditions was evaluated on SpectraMax Plus 384 UV/vis spectrophotometer by monitoring the increase in absorba... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM29224

(casimiroin analogue, 1m)Show InChI InChI=1S/C13H15NO4/c1-7-5-10(15)14-11-8(7)6-9(16-2)12(17-3)13(11)18-4/h5-6H,1-4H3,(H,14,15) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University
| Assay Description
The activity of QR2 under steady-state conditions was evaluated on SpectraMax Plus 384 UV/vis spectrophotometer by monitoring the increase in absorba... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM29222

(casimiroin analogue, 1k)Show InChI InChI=1S/C12H13NO3/c1-7-6-10(14)13-12-9(16-3)5-4-8(15-2)11(7)12/h4-6H,1-3H3,(H,13,14) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University
| Assay Description
The activity of QR2 under steady-state conditions was evaluated on SpectraMax Plus 384 UV/vis spectrophotometer by monitoring the increase in absorba... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM29212

(casimiroin analogue, 1a)Show InChI InChI=1S/C11H9NO3/c1-6-4-9(13)12-10-7(6)2-3-8-11(10)15-5-14-8/h2-4H,5H2,1H3,(H,12,13) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University
| Assay Description
The activity of QR2 under steady-state conditions was evaluated on SpectraMax Plus 384 UV/vis spectrophotometer by monitoring the increase in absorba... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM29227

(casimiroin analogue, 1p)Show InChI InChI=1S/C14H17NO4/c1-8-6-11(16)15(2)13-9(17-3)7-10(18-4)14(19-5)12(8)13/h6-7H,1-5H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University
| Assay Description
The activity of QR2 under steady-state conditions was evaluated on SpectraMax Plus 384 UV/vis spectrophotometer by monitoring the increase in absorba... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosyl-DNA phosphodiesterase 1
(Homo sapiens (Human)) | BDBM50425088
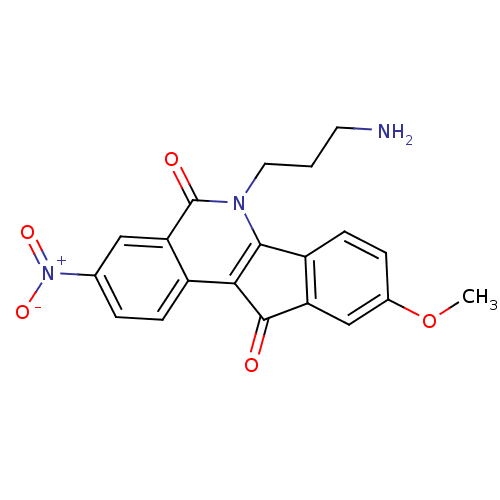
(CHEMBL375623 | US9402842, 54)Show SMILES COc1ccc-2c(c1)C(=O)c1c-2n(CCCN)c(=O)c2cc(ccc12)[N+]([O-])=O Show InChI InChI=1S/C20H17N3O5/c1-28-12-4-6-14-15(10-12)19(24)17-13-5-3-11(23(26)27)9-16(13)20(25)22(18(14)17)8-2-7-21/h3-6,9-10H,2,7-8,21H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... |
J Med Chem 56: 182-200 (2013)
Article DOI: 10.1021/jm3014458
BindingDB Entry DOI: 10.7270/Q2MS3V3Z |
More data for this
Ligand-Target Pair | |
Tyrosyl-DNA phosphodiesterase 1
(Homo sapiens (Human)) | BDBM50425084
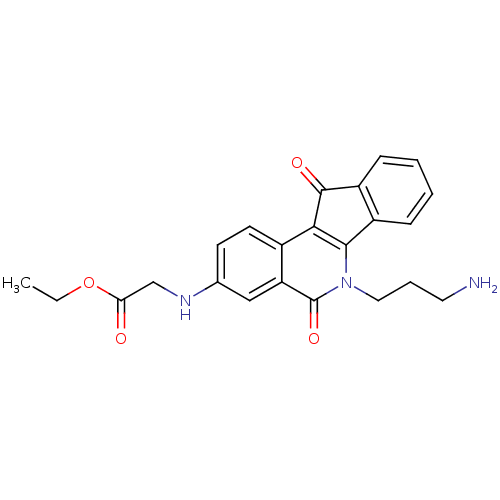
(CHEMBL2312893 | US9402842, 40)Show SMILES CCOC(=O)CNc1ccc2c3C(=O)c4ccccc4-c3n(CCCN)c(=O)c2c1 Show InChI InChI=1S/C23H23N3O4/c1-2-30-19(27)13-25-14-8-9-15-18(12-14)23(29)26(11-5-10-24)21-16-6-3-4-7-17(16)22(28)20(15)21/h3-4,6-9,12,25H,2,5,10-11,13,24H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... |
J Med Chem 56: 182-200 (2013)
Article DOI: 10.1021/jm3014458
BindingDB Entry DOI: 10.7270/Q2MS3V3Z |
More data for this
Ligand-Target Pair | |
Ribosyldihydronicotinamide dehydrogenase [quinone]
(Homo sapiens (Human)) | BDBM9019

(CHEMBL45 | Melatonin | N-[2-(5-methoxy-1H-indol-3-...)Show InChI InChI=1S/C13H16N2O2/c1-9(16)14-6-5-10-8-15-13-4-3-11(17-2)7-12(10)13/h3-4,7-8,15H,5-6H2,1-2H3,(H,14,16) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | 7.5 | 23 |
Purdue University
| Assay Description
The activity of QR2 under steady-state conditions was evaluated on SpectraMax Plus 384 UV/vis spectrophotometer by monitoring the increase in absorba... |
J Med Chem 52: 1873-84 (2009)
Article DOI: 10.1021/jm801335z
BindingDB Entry DOI: 10.7270/Q2NP22RV |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Tyrosyl-DNA phosphodiesterase 1
(Homo sapiens (Human)) | BDBM50396238

(CHEMBL2172212 | US9402842, 62)Show InChI InChI=1S/C19H17N3O2/c20-8-3-9-22-17-13-4-1-2-5-14(13)18(23)16(17)12-7-6-11(21)10-15(12)19(22)24/h1-2,4-7,10H,3,8-9,20-21H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... |
J Med Chem 56: 182-200 (2013)
Article DOI: 10.1021/jm3014458
BindingDB Entry DOI: 10.7270/Q2MS3V3Z |
More data for this
Ligand-Target Pair | |
Tyrosyl-DNA phosphodiesterase 1
(Homo sapiens (Human)) | BDBM50388546
(CHEMBL213072 | CHEMBL333363)Show InChI InChI=1S/C19H16N2O2/c20-10-5-11-21-17-13-7-2-3-8-14(13)18(22)16(17)12-6-1-4-9-15(12)19(21)23/h1-4,6-9H,5,10-11,20H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Purdue University
Curated by ChEMBL
| Assay Description
Inhibition of recombinant Tdp1 (unknown origin) using 5'-[32P]-labeled single-stranded DNA oligonucleotide containing 3'-phosphotyrosine as substrate... |
J Med Chem 56: 182-200 (2013)
Article DOI: 10.1021/jm3014458
BindingDB Entry DOI: 10.7270/Q2MS3V3Z |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data