

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | US Patent | 0.0400 | -59.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222959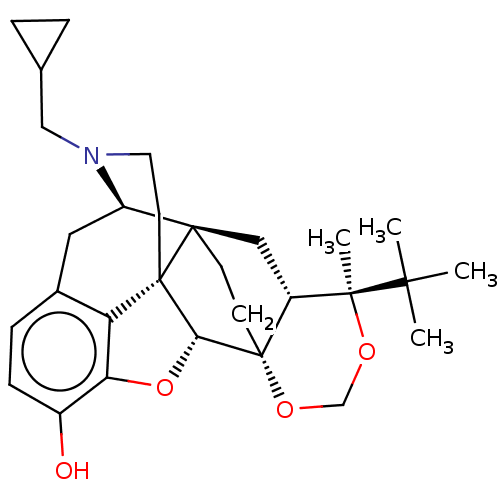 (US9315514, 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.120 | -56.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222960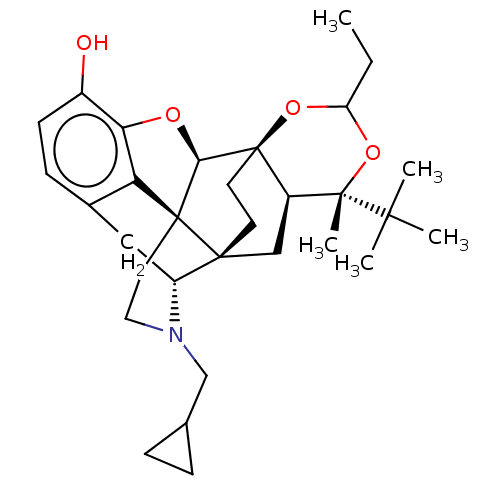 (US9315514, 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.190 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50354578 (BUPRENORPHINE | US10752592, Compound buprenorphine...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | US Patent | 0.300 | -58.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Rhodes Technologies US Patent | Assay Description Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membr... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 1 (Homo sapiens (Human)) | BDBM50063885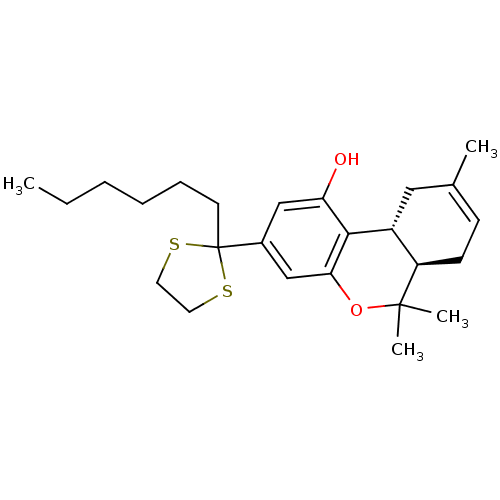 ((-)-2-(6a,7,10,10a-tetrahydro-6,6,9-trimethyl-1-hy...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation Curated by ChEMBL | Assay Description Binding affinity to CB1 receptor (unknown origin) | Bioorg Med Chem 16: 7377-87 (2008) Article DOI: 10.1016/j.bmc.2008.06.019 BindingDB Entry DOI: 10.7270/Q2TX3F6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM222960 (US9315514, 4) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.350 | -58.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Rhodes Technologies US Patent | Assay Description Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membr... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222963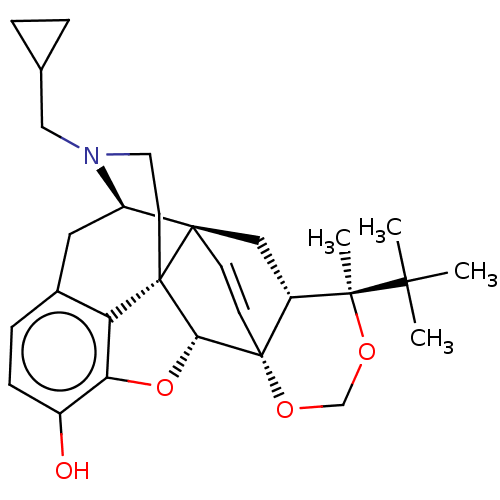 (US9315514, 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.440 | -53.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM222959 (US9315514, 3) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.450 | -57.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Rhodes Technologies US Patent | Assay Description Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membr... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cannabinoid receptor 2 (Homo sapiens (Human)) | BDBM50063885 ((-)-2-(6a,7,10,10a-tetrahydro-6,6,9-trimethyl-1-hy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The National Hellenic Research Foundation Curated by ChEMBL | Assay Description Binding affinity to CB2 receptor (unknown origin) | Bioorg Med Chem 16: 7377-87 (2008) Article DOI: 10.1016/j.bmc.2008.06.019 BindingDB Entry DOI: 10.7270/Q2TX3F6K | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM222961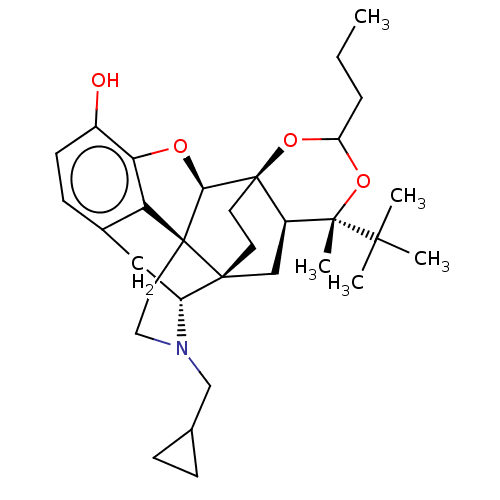 (US9315514, 5) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.540 | -57.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Rhodes Technologies US Patent | Assay Description Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membr... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222961 (US9315514, 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 0.720 | -52.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50018235 (5-Amino-2-{4-[(2,4-diamino-5-chloro-quinazolin-6-y...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase | J Med Chem 32: 1559-65 (1989) BindingDB Entry DOI: 10.7270/Q22806MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM222962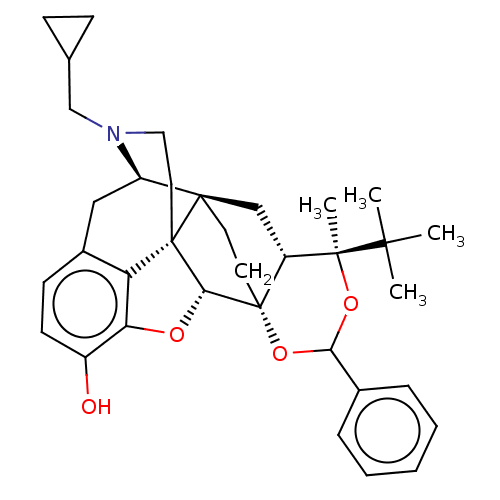 (US9315514, 6) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 2.26 | -53.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 50 |
Rhodes Technologies US Patent | Assay Description Radioligand dose-displacement binding assays for μ-opioid receptors used 0.3 nM [3H]-diprenorphine (Perkin Elmer, Shelton, Conn.), with 5 mg membr... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222962 (US9315514, 6) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 4.61 | -47.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222965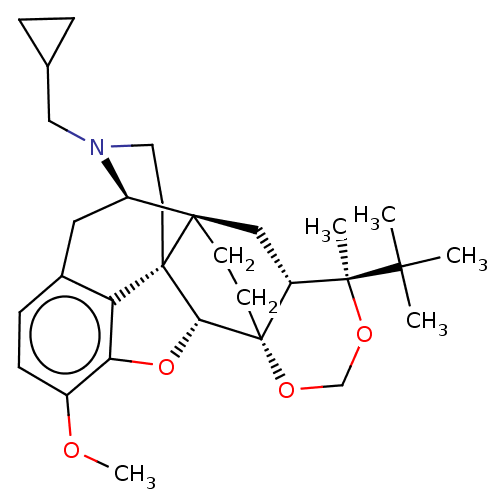 (US9315514, 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 7.22 | -46.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50002472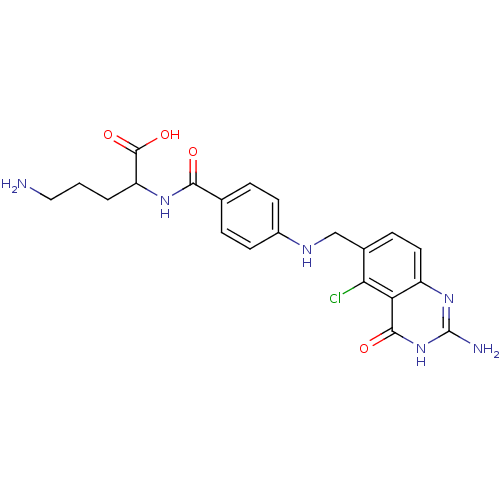 (5-Amino-2-{4-[(2-amino-5-chloro-4-oxo-3,4-dihydro-...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase | J Med Chem 32: 1559-65 (1989) BindingDB Entry DOI: 10.7270/Q22806MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50002471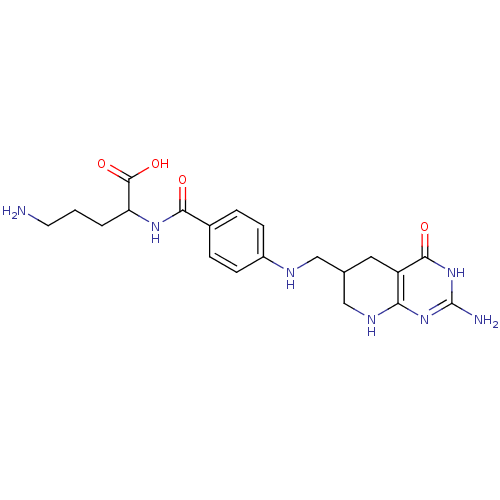 (5-Amino-2-{4-[(2-amino-4-oxo-2,3,4,4a,5,6,7,8-octa...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of mouse liver Folyl-polyglutamate synthase | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222964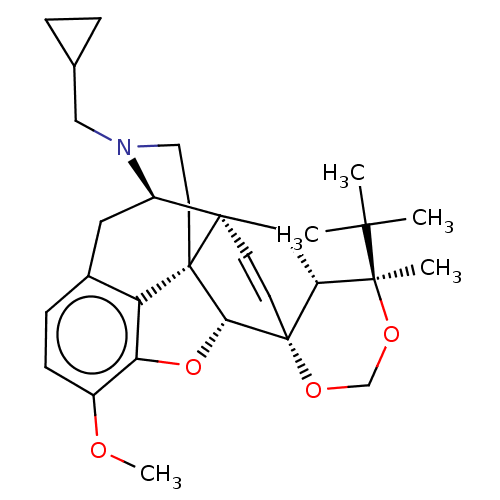 (US9315514, 12) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 30.1 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50005868 (2-{4-[(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of Glycinamide ribonucleotide formyltransferase from L1210 murine leukemia cells | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50405088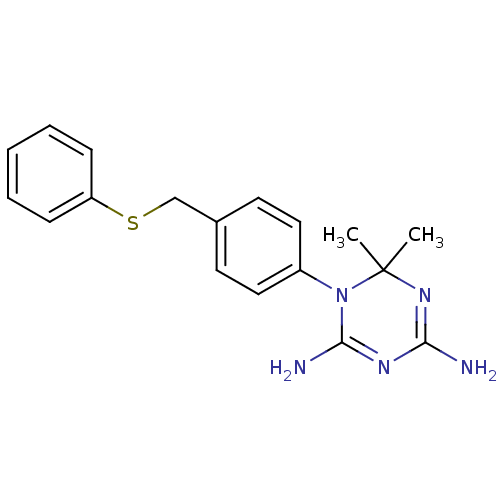 (CHEMBL31004) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) | J Med Chem 27: 144-9 (1984) BindingDB Entry DOI: 10.7270/Q28K7C96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50405074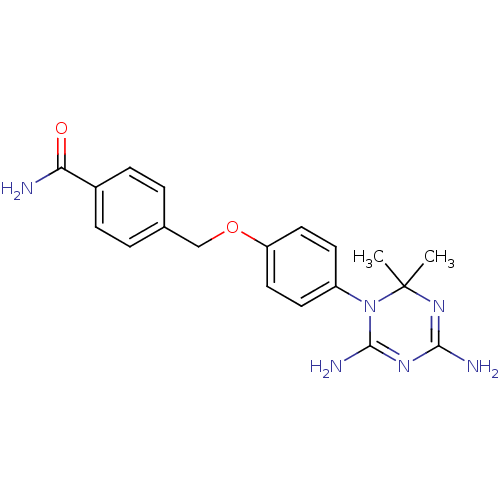 (CHEMBL34627) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 59 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) | J Med Chem 27: 144-9 (1984) BindingDB Entry DOI: 10.7270/Q28K7C96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50405052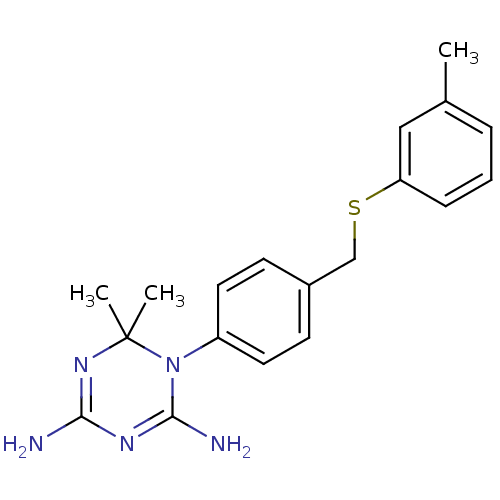 (CHEMBL283916) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) | J Med Chem 27: 144-9 (1984) BindingDB Entry DOI: 10.7270/Q28K7C96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50405070 (CHEMBL30681) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) | J Med Chem 27: 144-9 (1984) BindingDB Entry DOI: 10.7270/Q28K7C96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50024475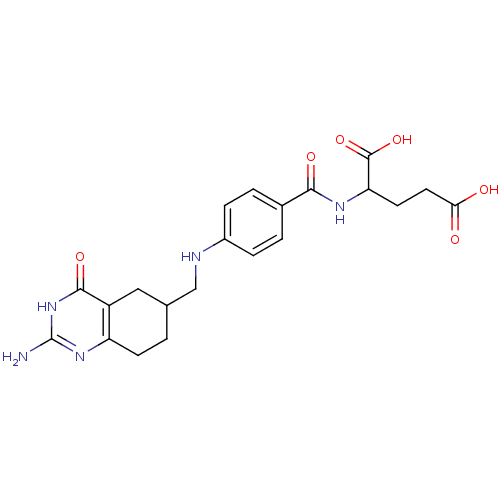 (2-{4-[(2-Amino-4-oxo-1,4,5,6,7,8-hexahydro-quinazo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 65 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of mouse GAR transformylase | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50405102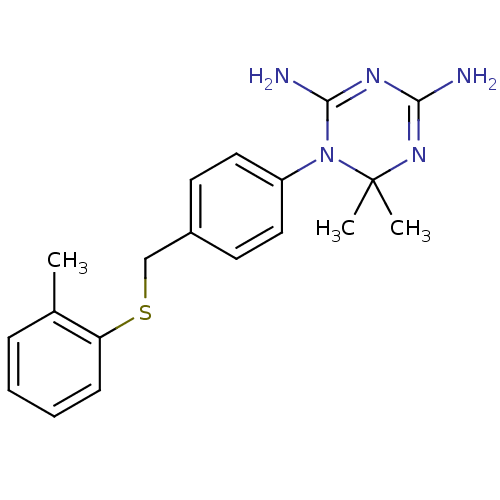 (CHEMBL30475) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 66 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) | J Med Chem 27: 144-9 (1984) BindingDB Entry DOI: 10.7270/Q28K7C96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50405072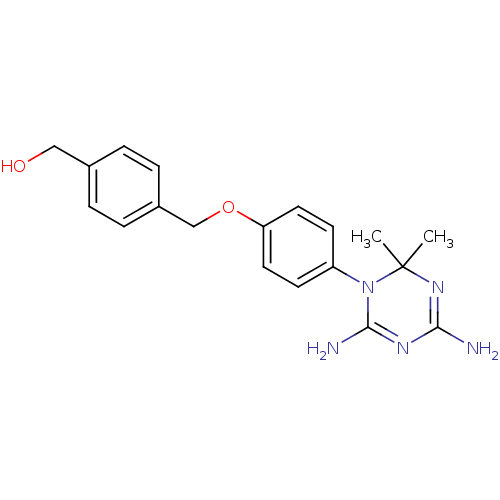 (CHEMBL33010) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) | J Med Chem 27: 144-9 (1984) BindingDB Entry DOI: 10.7270/Q28K7C96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50405055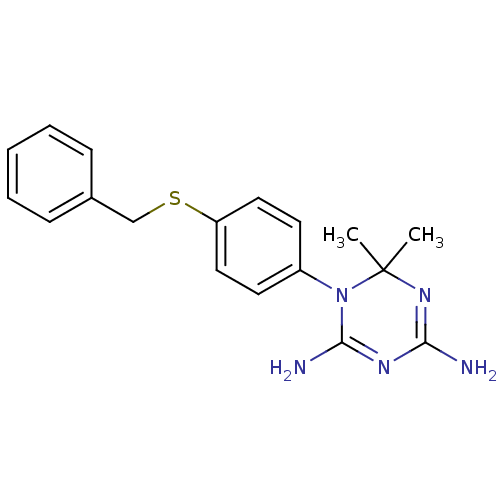 (CHEMBL283173) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 98 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) | J Med Chem 27: 144-9 (1984) BindingDB Entry DOI: 10.7270/Q28K7C96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50405049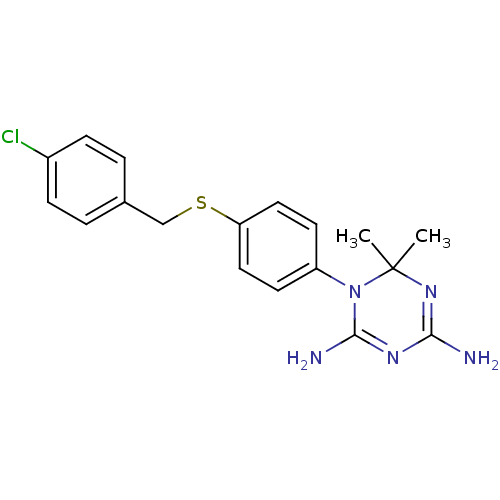 (CHEMBL285019) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 107 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) | J Med Chem 27: 144-9 (1984) BindingDB Entry DOI: 10.7270/Q28K7C96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50405043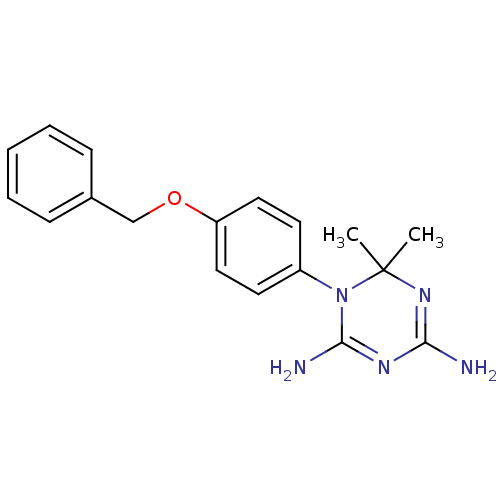 (1-(4-(Benzyloxy)phenyl)-6,6-dimethyl-1,6-dihydro-1...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 117 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) | J Med Chem 27: 144-9 (1984) BindingDB Entry DOI: 10.7270/Q28K7C96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50016343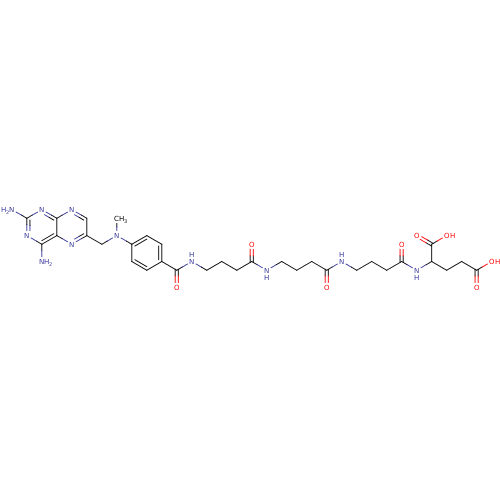 (2-{4-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50011885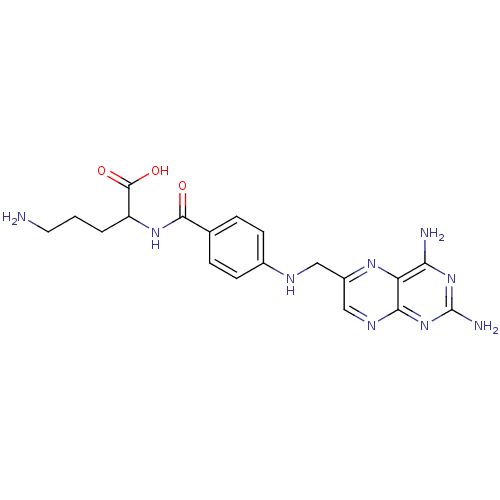 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-am...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit Folyl-polyglutamate synthase from mouse liver | J Med Chem 29: 655-60 (1986) BindingDB Entry DOI: 10.7270/Q2RR1ZTR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50016340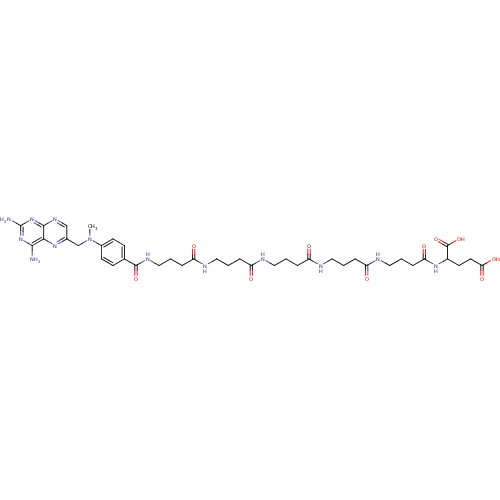 (2-[4-(4-{4-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmet...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 172 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50016339 (2-(4-{4-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 189 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Trifunctional purine biosynthetic protein adenosine-3 (Mus musculus) | BDBM50005865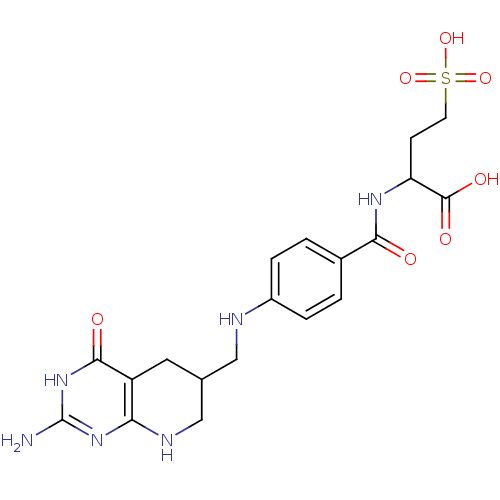 (2-{4-[(2-Amino-4-oxo-3,4,5,6,7,8-hexahydro-pyrido[...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description In vitro inhibition of Glycinamide ribonucleotide formyltransferase from L1210 murine leukemia cells | J Med Chem 35: 1578-88 (1992) BindingDB Entry DOI: 10.7270/Q2028S6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50016342 (2-[4-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 205 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50020107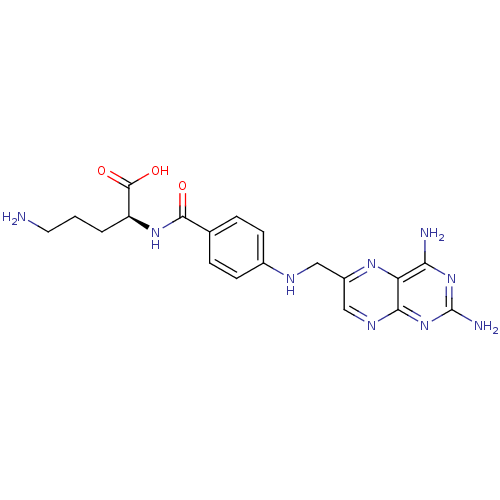 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-am...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory constant against purified Folyl-polyglutamate synthase obtained from human | J Med Chem 31: 1338-44 (1988) BindingDB Entry DOI: 10.7270/Q22J6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Mus musculus) | BDBM50020107 (5-Amino-2-{4-[(2,4-diamino-pteridin-6-ylmethyl)-am...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Harvard Medical School Curated by ChEMBL | Assay Description Inhibitory constant against purified Folyl-polyglutamate synthase obtained from mouse | J Med Chem 31: 1338-44 (1988) BindingDB Entry DOI: 10.7270/Q22J6CFD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50405073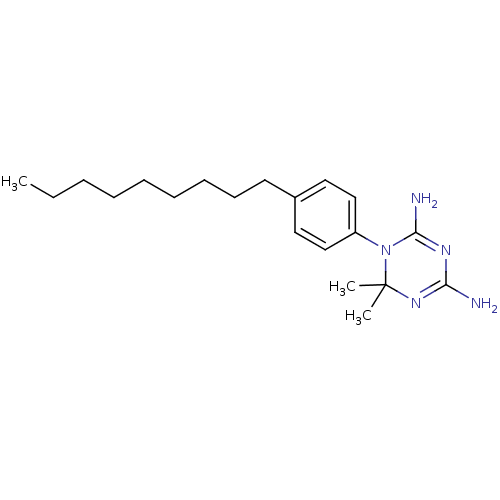 (CHEMBL33014) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 302 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) | J Med Chem 27: 144-9 (1984) BindingDB Entry DOI: 10.7270/Q28K7C96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidylate synthase (Lactobacillus casei) | BDBM50016341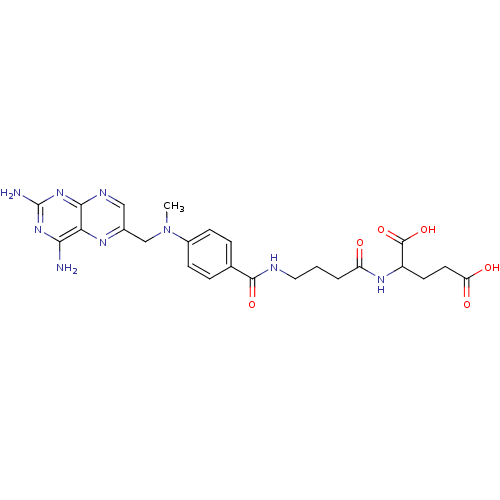 (2-(4-{4-[(2,4-Diamino-pteridin-6-ylmethyl)-methyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 336 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for its ability to inhibit thymidylate synthetase isolated from MTX-resistant Lactobacillus casei | J Med Chem 29: 1872-6 (1986) BindingDB Entry DOI: 10.7270/Q2JH3K4Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50405054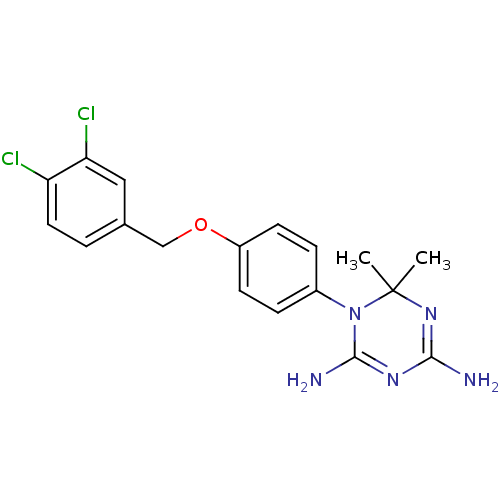 (CHEMBL7035) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 347 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) | J Med Chem 27: 144-9 (1984) BindingDB Entry DOI: 10.7270/Q28K7C96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50018236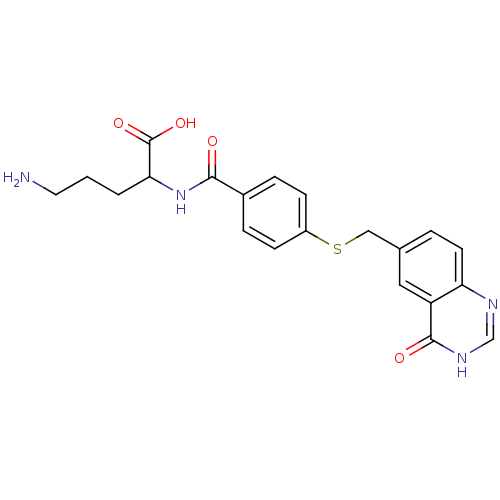 (5-Amino-2-[4-(4-oxo-3,4-dihydro-quinazolin-6-ylmet...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase | J Med Chem 32: 1559-65 (1989) BindingDB Entry DOI: 10.7270/Q22806MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50405046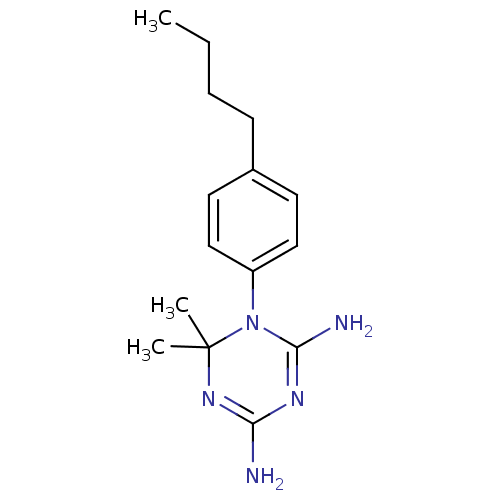 (CHEMBL6962) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 537 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) | J Med Chem 27: 144-9 (1984) BindingDB Entry DOI: 10.7270/Q28K7C96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM18792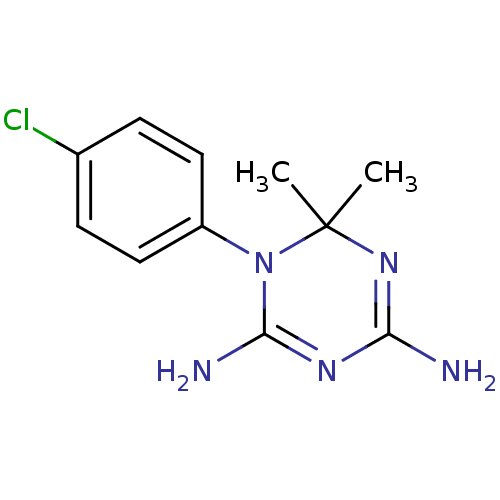 (1-(4-chlorophenyl)-6,6-dimethyl-1,6-dihydro-1,3,5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 631 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) | J Med Chem 27: 144-9 (1984) BindingDB Entry DOI: 10.7270/Q28K7C96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50090050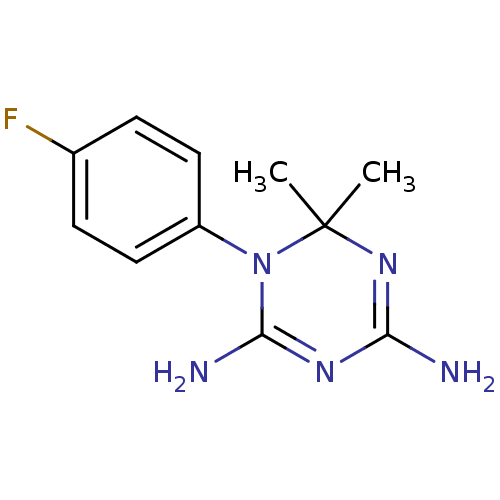 (1-(4-Fluoro-phenyl)-6,6-dimethyl-1,6-dihydro-[1,3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 708 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) | J Med Chem 27: 144-9 (1984) BindingDB Entry DOI: 10.7270/Q28K7C96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM222966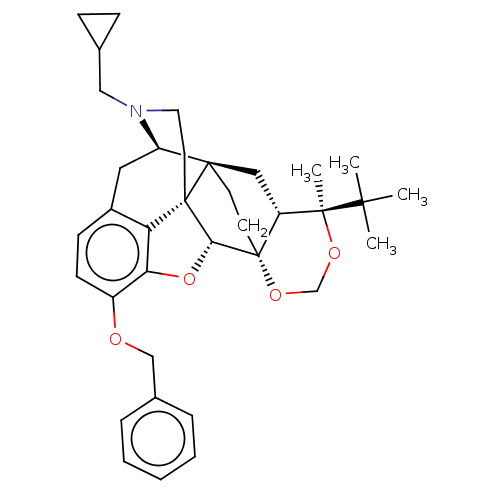 (US9315514, 7) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 757 | -34.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Rhodes Technologies US Patent | Assay Description Radioligand dose displacement assays used 0.4 nM [3H]-U69,593 (GE Healthcare, Piscataway, N.J.; 40 Ci/mmole) with 15 μg membrane protein (recombin... | US Patent US9315514 (2016) BindingDB Entry DOI: 10.7270/Q2KW5DWC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50405051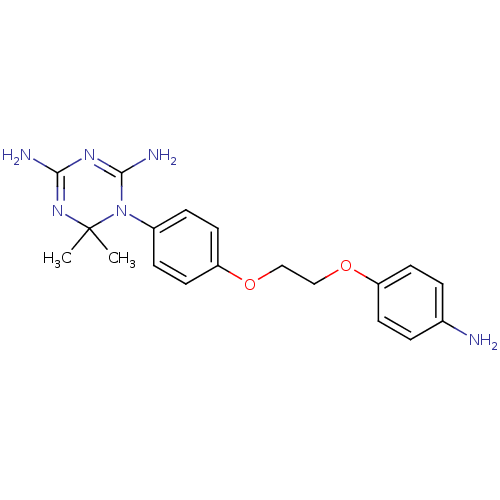 (CHEMBL33389) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) | J Med Chem 27: 144-9 (1984) BindingDB Entry DOI: 10.7270/Q28K7C96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50090051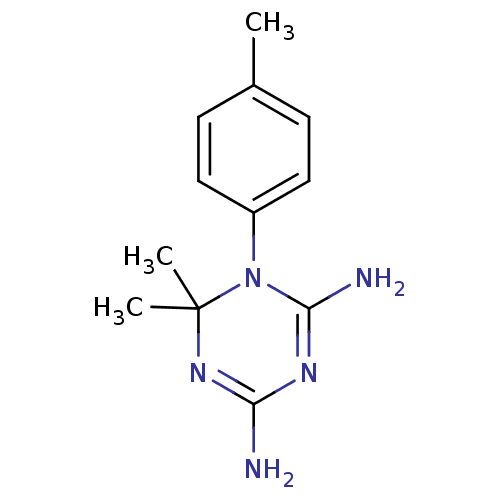 (6,6-Dimethyl-1-p-tolyl-1,6-dihydro-[1,3,5]triazine...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.07E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) | J Med Chem 27: 144-9 (1984) BindingDB Entry DOI: 10.7270/Q28K7C96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Folylpolyglutamate synthase, mitochondrial (Homo sapiens (Human)) | BDBM50018232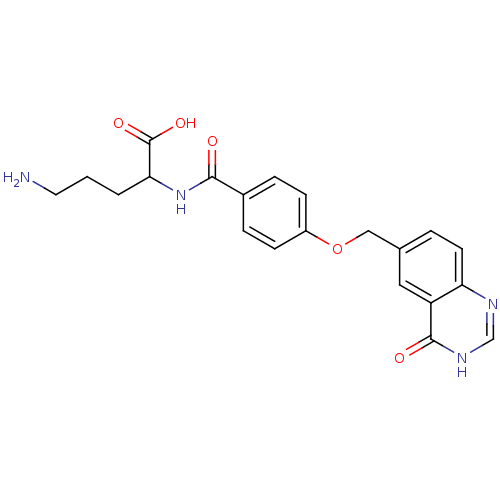 (5-Amino-2-[4-(4-oxo-3,4-dihydro-quinazolin-6-ylmet...) | Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Medical University of South Carolina Curated by ChEMBL | Assay Description The apparent Km of compound as a substrate for partially purified mouse liver Folyl-polyglutamate synthase | J Med Chem 32: 1559-65 (1989) BindingDB Entry DOI: 10.7270/Q22806MJ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50405100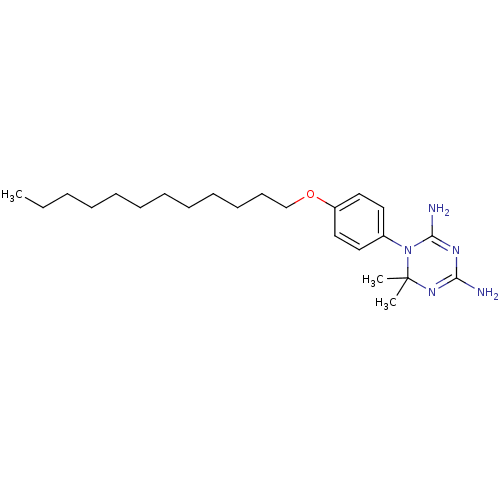 (CHEMBL32304) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) | J Med Chem 27: 144-9 (1984) BindingDB Entry DOI: 10.7270/Q28K7C96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Dihydrofolate reductase (Homo sapiens (Human)) | BDBM50090067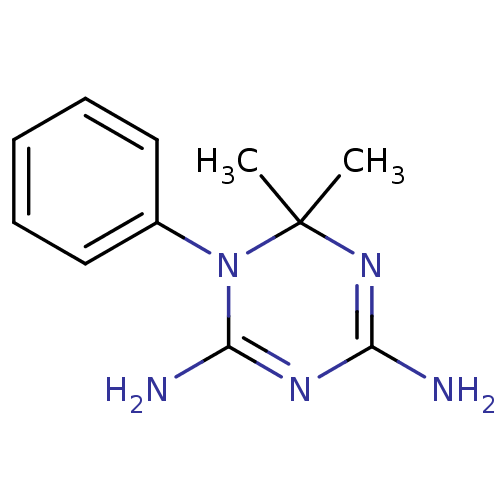 (6,6-Dimethyl-1-phenyl-1,6-dihydro-[1,3,5]triazine-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | 1.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibitory activity against human lymphoblastoid cell (WIL2) dihydrofolate reductase (DHFR) | J Med Chem 27: 144-9 (1984) BindingDB Entry DOI: 10.7270/Q28K7C96 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 339 total ) | Next | Last >> |