

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50076649 (CHEMBL369848 | N-{1-methyl-3-[1-(4-methoxyphenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase(5-LO) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076666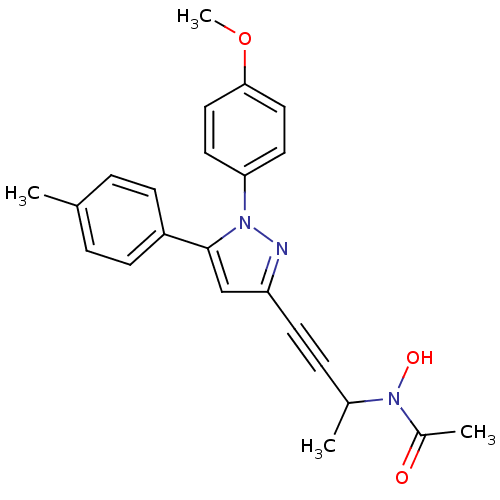 (CHEMBL175153 | N-Hydroxy-N-{3-[1-(4-methoxy-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cyclooxygenase (COX) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50086479 (4-Chloro-benzoic acid N'-p-tolyl-hydrazide | CHEMB...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human prostaglandin G/H synthase 2 in ECV-304 cells measured by the presence of PGE-2 | Bioorg Med Chem Lett 10: 601-4 (2000) BindingDB Entry DOI: 10.7270/Q2TH8KWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076661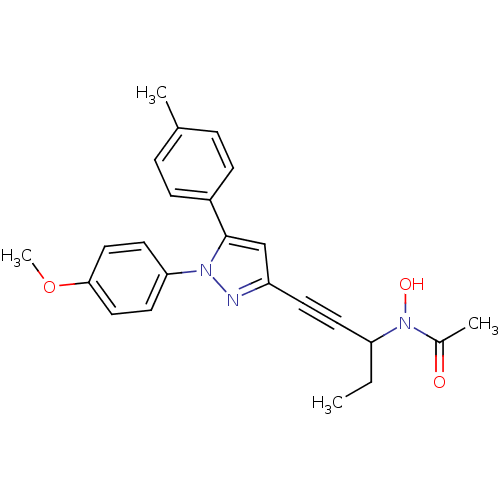 (CHEMBL177727 | N-{1-Ethyl-3-[1-(4-methoxy-phenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cyclooxygenase (COX) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| C-X-C chemokine receptor type 1 (Homo sapiens (Human)) | BDBM50150572 (1-(2-Bromo-phenyl)-3-(2,4-dihydroxy-phenyl)-urea |...) | PDB UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson and Johnson Pharmaceutical Research and Development Curated by ChEMBL | Assay Description Inhibitory concentration against interleukin-8 receptor of human neutrophils by using [125I]-IL-8 (0.125 nM) as radioligand | Bioorg Med Chem Lett 14: 4307-11 (2004) Article DOI: 10.1016/j.bmcl.2004.05.080 BindingDB Entry DOI: 10.7270/Q23F4P4M | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076637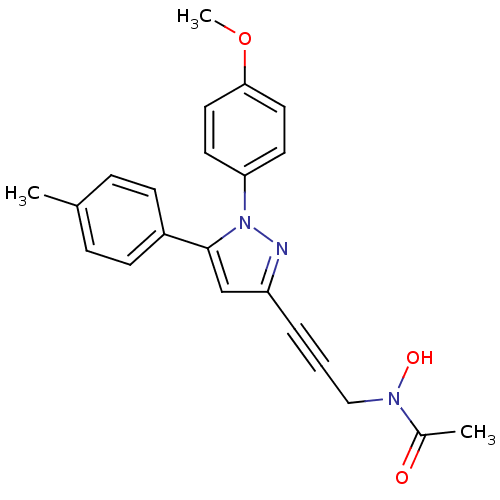 (CHEMBL369252 | N-Hydroxy-N-{3-[1-(4-methoxy-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase (5-LO) in intact rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076659 (CHEMBL435907 | N-{3-[5-(4-Chloro-phenyl)-1-(4-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cyclooxygenase (COX) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076650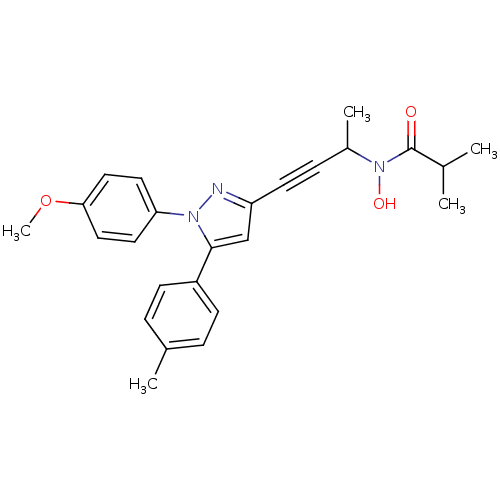 (CHEMBL368682 | N-Hydroxy-N-{3-[1-(4-methoxy-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase (5-LO) in intact rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076642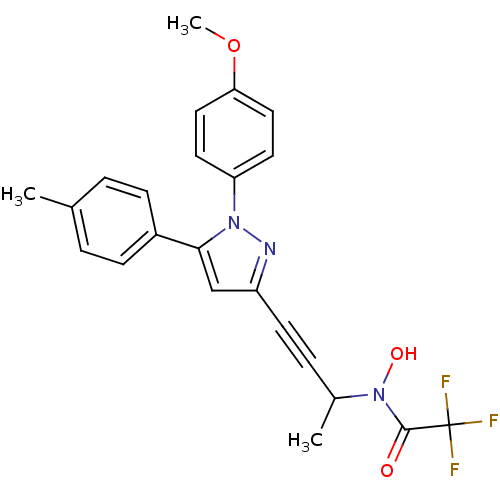 (2,2,2-Trifluoro-N-hydroxy-N-{3-[1-(4-methoxy-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cyclooxygenase (COX) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076639 (CHEMBL367010 | N-{3-[5-(4-Ethyl-phenyl)-1-(4-metho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cyclooxygenase (COX) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076640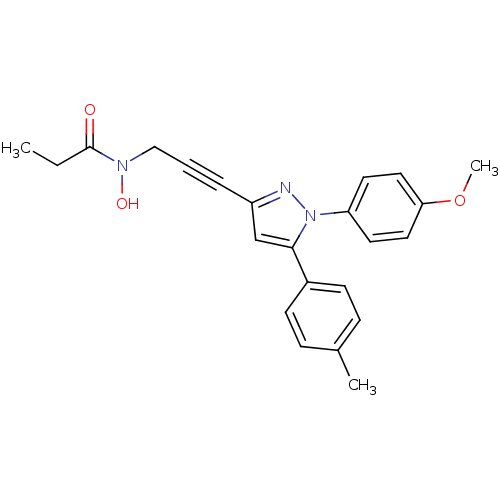 (CHEMBL173133 | N-Hydroxy-N-{3-[1-(4-methoxy-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against cyclooxygenase (COX) in intact rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50076657 (CHEMBL368546 | N-hydroxy-N-{3-[1-(4-methoxyphenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase (5-LO) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50076644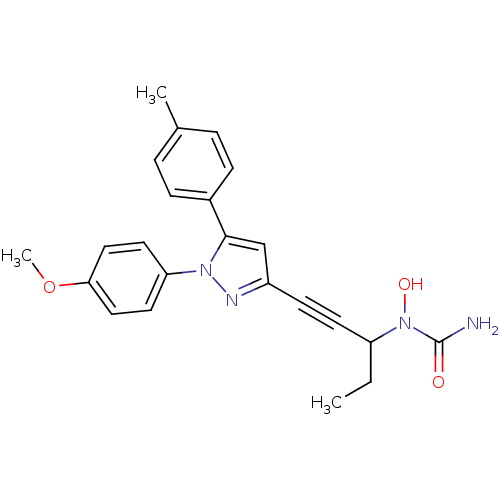 (CHEMBL175150 | N-{1-ethyl-3-[1-(4-methoxyphenyl)-5...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase(5-LO) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50076657 (CHEMBL368546 | N-hydroxy-N-{3-[1-(4-methoxyphenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase (5-LO) in intact rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50076664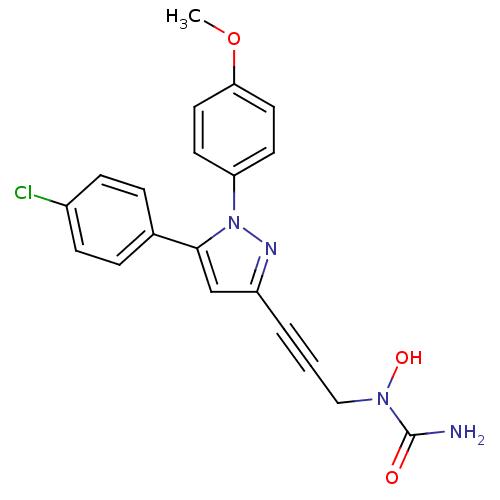 (CHEMBL368145 | N-hydroxy-N-{3-[1-(4-methoxyphenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase (5-LO) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50076666 (CHEMBL175153 | N-Hydroxy-N-{3-[1-(4-methoxy-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase(5-LO) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50076664 (CHEMBL368145 | N-hydroxy-N-{3-[1-(4-methoxyphenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase (5-LO) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076650 (CHEMBL368682 | N-Hydroxy-N-{3-[1-(4-methoxy-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase (5-LO) in intact rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50076650 (CHEMBL368682 | N-Hydroxy-N-{3-[1-(4-methoxy-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase(5-LO) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50076659 (CHEMBL435907 | N-{3-[5-(4-Chloro-phenyl)-1-(4-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase (5-LO) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076666 (CHEMBL175153 | N-Hydroxy-N-{3-[1-(4-methoxy-phenyl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against cyclooxygenase (COX) in intact rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50086492 (1,3-Diphenyl-1,4,5,6,7,8-hexahydro-cycloheptapyraz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human prostaglandin G/H synthase 1 in platelet-rich plasma measured by the presence of thromboxane A2 | Bioorg Med Chem Lett 10: 601-4 (2000) BindingDB Entry DOI: 10.7270/Q2TH8KWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50086500 (4-Chloro-benzoic acid N'-(4-methoxy-phenyl)-hydraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human prostaglandin G/H synthase 2 in ECV-304 cells measured by the presence of PGE-2 | Bioorg Med Chem Lett 10: 601-4 (2000) BindingDB Entry DOI: 10.7270/Q2TH8KWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076662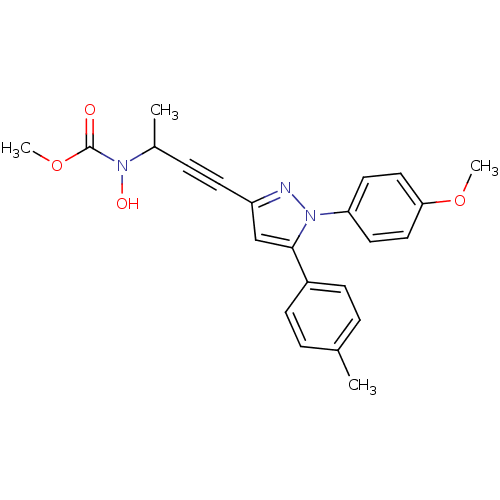 (CHEMBL174684 | methyl 1-methyl-3-[1-(4-methoxyphen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cyclooxygenase (COX) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50086498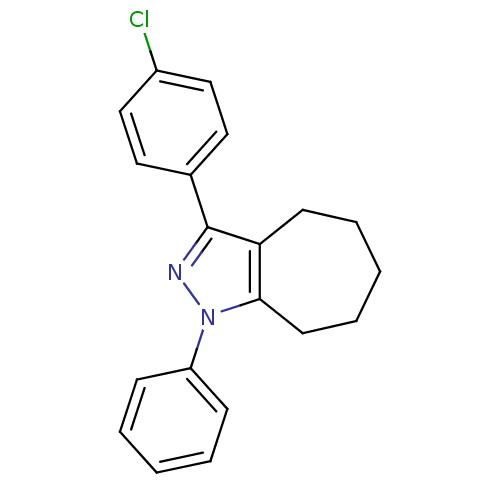 (3-(4-Chloro-phenyl)-1-phenyl-1,4,5,6,7,8-hexahydro...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human prostaglandin G/H synthase 2 in ECV-304 cells measured by the presence of PGE-2 | Bioorg Med Chem Lett 10: 601-4 (2000) BindingDB Entry DOI: 10.7270/Q2TH8KWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076659 (CHEMBL435907 | N-{3-[5-(4-Chloro-phenyl)-1-(4-meth...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against cyclooxygenase (COX) in intact rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50086492 (1,3-Diphenyl-1,4,5,6,7,8-hexahydro-cycloheptapyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human prostaglandin G/H synthase 2 in ECV-304 cells measured by the presence of PGE-2 | Bioorg Med Chem Lett 10: 601-4 (2000) BindingDB Entry DOI: 10.7270/Q2TH8KWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076641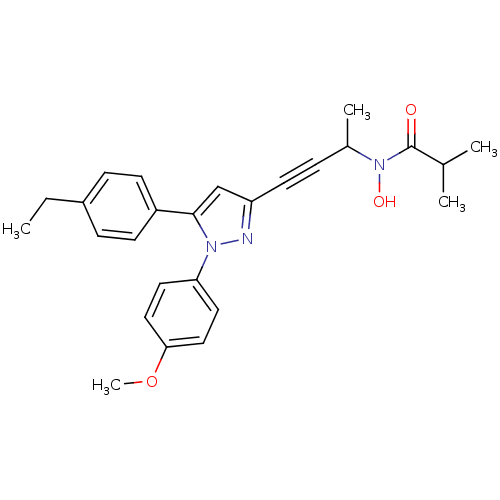 (CHEMBL176844 | N-{3-[5-(4-Ethyl-phenyl)-1-(4-metho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against cyclooxygenase (COX) in intact rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50086487 (Benzoic acid N'-(4-methoxy-phenyl)-hydrazide | CHE...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human prostaglandin G/H synthase 2 in ECV-304 cells measured by the presence of PGE-2 | Bioorg Med Chem Lett 10: 601-4 (2000) BindingDB Entry DOI: 10.7270/Q2TH8KWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076642 (2,2,2-Trifluoro-N-hydroxy-N-{3-[1-(4-methoxy-pheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against cyclooxygenase (COX) in intact rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076661 (CHEMBL177727 | N-{1-Ethyl-3-[1-(4-methoxy-phenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against cyclooxygenase (COX) in intact rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50076653 (CHEMBL366595 | N-{1-isopropyl-3-[1-(4-methoxypheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase(5-LO) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50076653 (CHEMBL366595 | N-{1-isopropyl-3-[1-(4-methoxypheny...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 240 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase(5-LO) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50076662 (CHEMBL174684 | methyl 1-methyl-3-[1-(4-methoxyphen...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase(5-LO) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076651 (CHEMBL174976 | methyl-3-[1-(4-methoxyphenyl)-5-(4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase (5-LO) in intact rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076658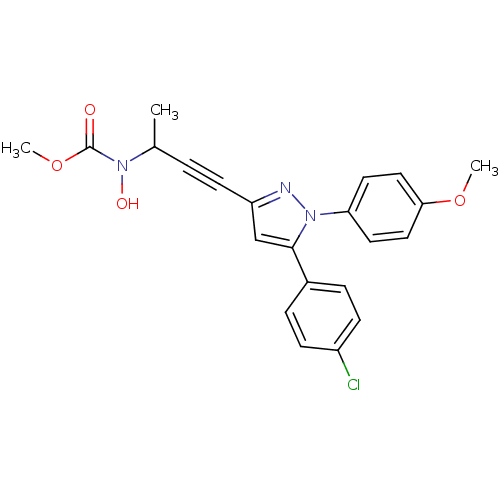 (CHEMBL174501 | methyl 3-[5-(4-chlorophenyl)-1-(4-m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against cyclooxygenase (COX) in intact rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076657 (CHEMBL368546 | N-hydroxy-N-{3-[1-(4-methoxyphenyl)...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cyclooxygenase (COX) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50076666 (CHEMBL175153 | N-Hydroxy-N-{3-[1-(4-methoxy-phenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cyclooxygenase using sheep seminal vesicle (SSV) enzyme (COX-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50086481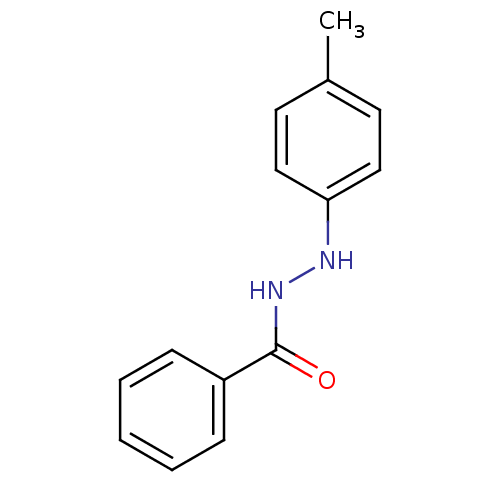 (Benzoic acid N'-p-tolyl-hydrazide | CHEMBL157950) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human prostaglandin G/H synthase 2 in ECV-304 cells measured by the presence of PGE-2 | Bioorg Med Chem Lett 10: 601-4 (2000) BindingDB Entry DOI: 10.7270/Q2TH8KWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50086476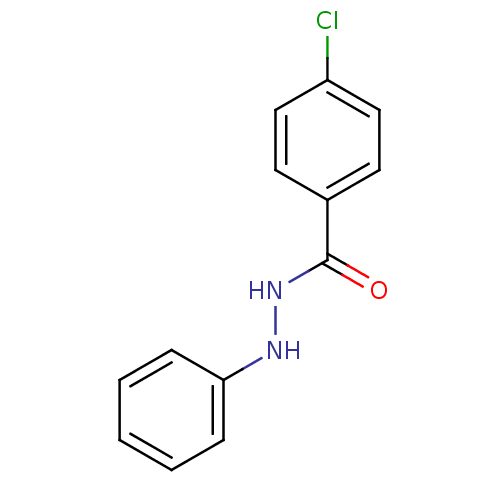 (4-Chloro-benzoic acid N'-phenyl-hydrazide | CHEMBL...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 330 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human prostaglandin G/H synthase 2 in ECV-304 cells measured by the presence of PGE-2 | Bioorg Med Chem Lett 10: 601-4 (2000) BindingDB Entry DOI: 10.7270/Q2TH8KWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1/2 (RAT) | BDBM50076639 (CHEMBL367010 | N-{3-[5-(4-Ethyl-phenyl)-1-(4-metho...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against cyclooxygenase (COX) in intact rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50086488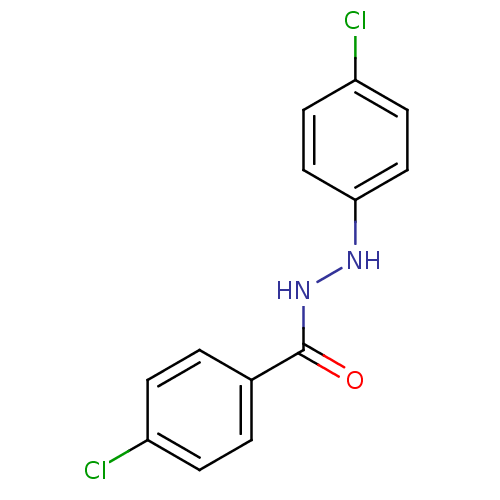 (4-Chloro-benzoic acid N'-(4-chloro-phenyl)-hydrazi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human prostaglandin G/H synthase 2 in ECV-304 cells measured by the presence of PGE-2 | Bioorg Med Chem Lett 10: 601-4 (2000) BindingDB Entry DOI: 10.7270/Q2TH8KWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50076656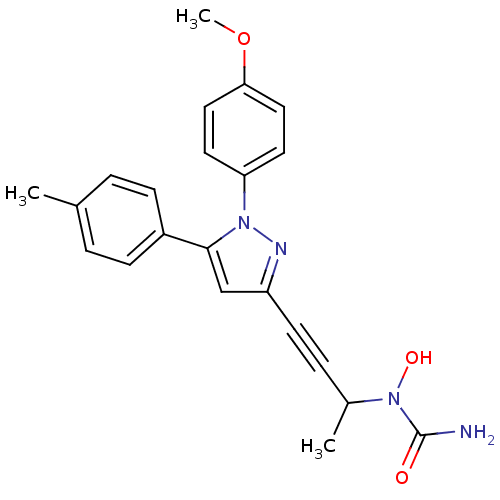 (CHEMBL173939 | N-{1-methyl-3-[1-(4-methoxyphenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase(5-LO) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Rattus norvegicus) | BDBM50076656 (CHEMBL173939 | N-{1-methyl-3-[1-(4-methoxyphenyl)-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 370 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against 5-lipoxygenase(5-LO) using broken rat barophilic leukemia cells (RBL-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50076650 (CHEMBL368682 | N-Hydroxy-N-{3-[1-(4-methoxy-phenyl...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cyclooxygenase using sheep seminal vesicle (SSV) enzyme (COX-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50076642 (2,2,2-Trifluoro-N-hydroxy-N-{3-[1-(4-methoxy-pheny...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cyclooxygenase using sheep seminal vesicle (SSV) enzyme (COX-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Ovis aries (Sheep)) | BDBM50076661 (CHEMBL177727 | N-{1-Ethyl-3-[1-(4-methoxy-phenyl)-...) | PDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against Cyclooxygenase using sheep seminal vesicle (SSV) enzyme (COX-1) | Bioorg Med Chem Lett 9: 979-84 (1999) BindingDB Entry DOI: 10.7270/Q2Z31XV3 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50086478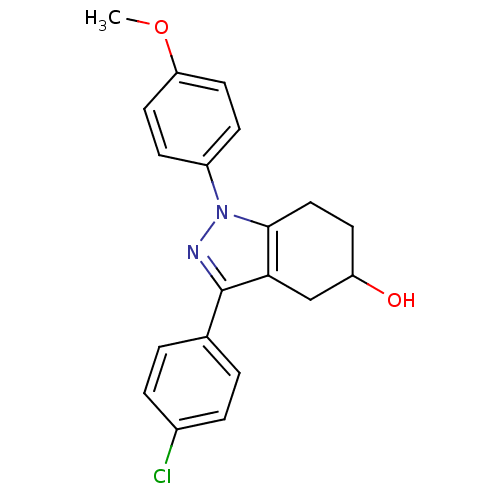 (3-(4-Chloro-phenyl)-1-(4-methoxy-phenyl)-4,5,6,7-t...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human prostaglandin G/H synthase 2 in ECV-304 cells measured by the presence of PGE-2 | Bioorg Med Chem Lett 10: 601-4 (2000) BindingDB Entry DOI: 10.7270/Q2TH8KWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50086496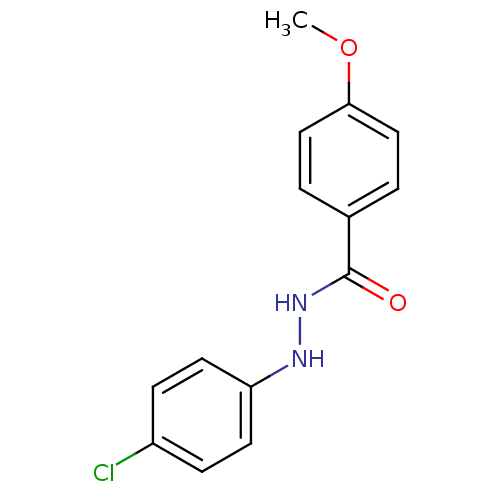 (4-Methoxy-benzoic acid N'-(4-chloro-phenyl)-hydraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human prostaglandin G/H synthase 2 in ECV-304 cells measured by the presence of PGE-2 | Bioorg Med Chem Lett 10: 601-4 (2000) BindingDB Entry DOI: 10.7270/Q2TH8KWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50086484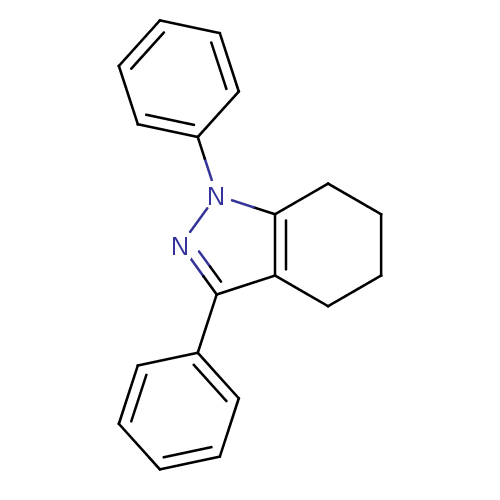 (1,3-Diphenyl-4,5,6,7-tetrahydro-1H-indazole | CHEM...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 490 | n/a | n/a | n/a | n/a | n/a | n/a |
The R.W. Johnson Pharmaceutical Research Institute Curated by ChEMBL | Assay Description Inhibitory activity against human prostaglandin G/H synthase 1 in platelet-rich plasma measured by the presence of thromboxane A2 | Bioorg Med Chem Lett 10: 601-4 (2000) BindingDB Entry DOI: 10.7270/Q2TH8KWZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 198 total ) | Next | Last >> |