 Found 781 hits with Last Name = 'roche' and Initial = 'c'
Found 781 hits with Last Name = 'roche' and Initial = 'c' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50090731
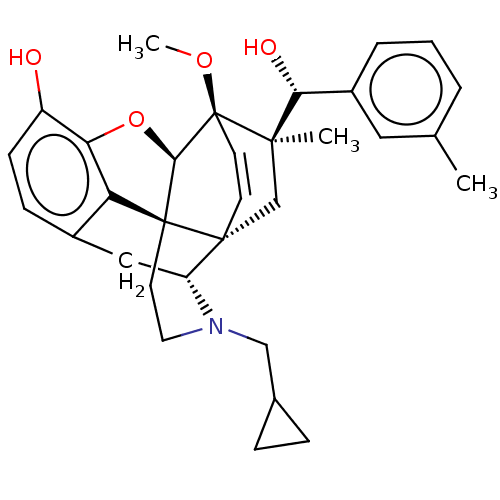
(CHEMBL3581741 | US9259422, 22, R = 3-MePh- BU10112...)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]51C[C@](C)([C@H](O)c4cccc(C)c4)[C@]2(OC)C=C1)ccc3O |r,wU:17.21,16.16,19.23,1.0,wD:30.36,21.25,7.7,c:39,TLB:21:19:16.1:34.33,THB:10:9:17:4.5.6,20:19:16.1:34.33,(-3.17,.26,;-2.2,.57,;-3.38,2.21,;-2.2,3.8,;-.81,2.99,;.55,3.8,;1.94,2.99,;1.94,1.4,;2.83,1.9,;3.33,.57,;4.33,-.58,;5.84,-.3,;7.2,-.82,;6.92,.69,;2.73,2.15,;.51,2.18,;-.81,1.4,;.55,.57,;.55,-.99,;-.81,-1.79,;-1.89,-2.38,;.53,-2.51,;1.56,-1.84,;.61,-4.05,;1.98,-4.74,;2.06,-6.28,;.77,-7.12,;-.6,-6.42,;-1.63,-7.1,;-.68,-4.89,;-2.2,-.99,;-3.6,-1.56,;-4.57,-.8,;-.88,-.72,;-.88,.28,;.55,5.4,;-.81,6.2,;-2.2,5.4,;-3.26,6.01,)| Show InChI InChI=1S/C55H75N17O13/c1-29(2)19-38(49(80)67-37(9-5-17-60-55(57)58)54(85)72-18-6-10-43(72)53(84)62-25-44(56)75)66-46(77)26-63-47(78)39(20-30-11-13-33(74)14-12-30)68-52(83)42(27-73)71-50(81)40(21-31-23-61-35-8-4-3-7-34(31)35)69-51(82)41(22-32-24-59-28-64-32)70-48(79)36-15-16-45(76)65-36/h3-4,7-8,11-14,23-24,28-29,36-43,61,73-74H,5-6,9-10,15-22,25-27H2,1-2H3,(H2,56,75)(H,59,64)(H,62,84)(H,63,78)(H,65,76)(H,66,77)(H,67,80)(H,68,83)(H,69,82)(H,70,79)(H,71,81)(H4,57,58,60)/t36?,37-,38-,39-,40-,41-,42-,43+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50090760
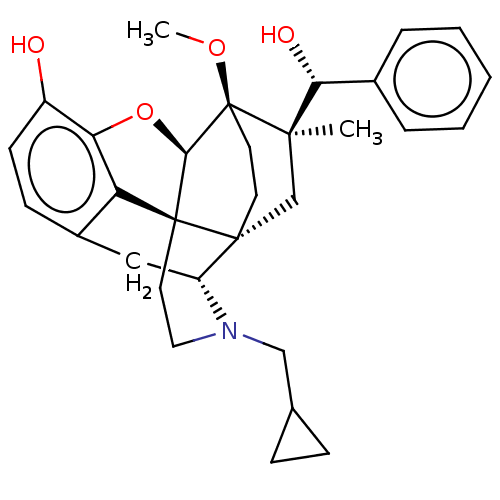
(CHEMBL3581750 | US9259422, 30, R = Ph-BU10119 | US...)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1ccccc1)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C31H37NO4/c1-28(26(34)20-6-4-3-5-7-20)18-29-12-13-31(28,35-2)27-30(29)14-15-32(17-19-8-9-19)23(29)16-21-10-11-22(33)25(36-27)24(21)30/h3-7,10-11,19,23,26-27,33-34H,8-9,12-18H2,1-2H3/t23-,26-,27-,28-,29-,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50015003

(CHEMBL3262089 | US9259422, 7a, R = Ph-BU127 | US94...)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@]([H])(C1)[C@](C)(O)c1ccccc1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C31H37NO4/c1-28(34,21-6-4-3-5-7-21)23-17-29-12-13-31(23,35-2)27-30(29)14-15-32(18-19-8-9-19)24(29)16-20-10-11-22(33)26(36-27)25(20)30/h3-7,10-11,19,23-24,27,33-34H,8-9,12-18H2,1-2H3/t23-,24-,27-,28-,29-,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0440 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50090755
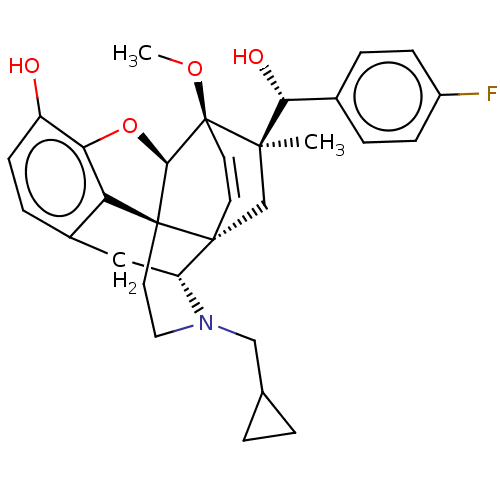
(CHEMBL3581743 | US9259422, 22, R = 4-FPh- BU10120 ...)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]51C[C@](C)([C@H](O)c4ccc(F)cc4)[C@]2(OC)C=C1)ccc3O |r,wU:17.21,16.16,19.23,1.0,wD:30.36,21.25,7.7,c:39,TLB:21:19:16.1:34.33,THB:10:9:17:4.5.6,20:19:16.1:34.33,(-3.17,.26,;-2.2,.57,;-3.38,2.21,;-2.2,3.8,;-.81,2.99,;.55,3.8,;1.94,2.99,;1.94,1.4,;2.83,1.9,;3.33,.57,;4.33,-.58,;5.84,-.3,;7.2,-.82,;6.92,.69,;2.73,2.15,;.51,2.18,;-.81,1.4,;.55,.57,;.55,-.99,;-.81,-1.79,;-1.89,-2.38,;.53,-2.51,;1.56,-1.84,;.61,-4.05,;-.68,-4.89,;-.6,-6.42,;.77,-7.12,;.84,-8.35,;2.06,-6.28,;1.98,-4.74,;-2.2,-.99,;-3.6,-1.56,;-4.57,-.8,;-.88,-.72,;-.88,.28,;.55,5.4,;-.81,6.2,;-2.2,5.4,;-3.26,6.01,)| Show InChI InChI=1S/C31H34FNO4/c1-28(26(35)19-5-8-21(32)9-6-19)17-29-11-12-31(28,36-2)27-30(29)13-14-33(16-18-3-4-18)23(29)15-20-7-10-22(34)25(37-27)24(20)30/h5-12,18,23,26-27,34-35H,3-4,13-17H2,1-2H3/t23-,26-,27-,28-,29-,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50090766
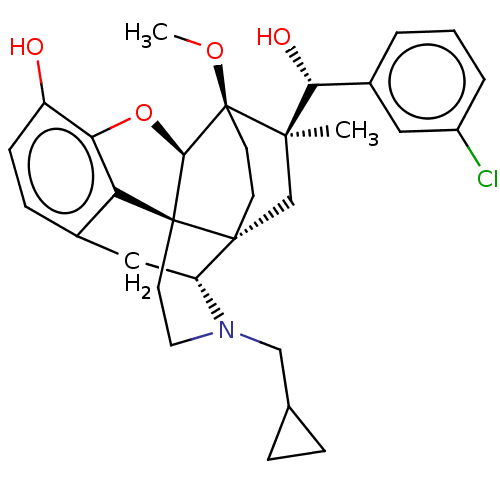
(CHEMBL3581756)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1cccc(Cl)c1)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C31H36ClNO4/c1-28(26(35)20-4-3-5-21(32)14-20)17-29-10-11-31(28,36-2)27-30(29)12-13-33(16-18-6-7-18)23(29)15-19-8-9-22(34)25(37-27)24(19)30/h3-5,8-9,14,18,23,26-27,34-35H,6-7,10-13,15-17H2,1-2H3/t23-,26-,27-,28-,29-,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50090782
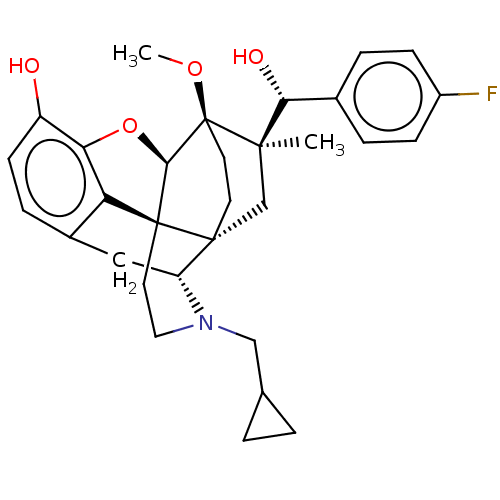
(CHEMBL3581754)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1ccc(F)cc1)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C31H36FNO4/c1-28(26(35)19-5-8-21(32)9-6-19)17-29-11-12-31(28,36-2)27-30(29)13-14-33(16-18-3-4-18)23(29)15-20-7-10-22(34)25(37-27)24(20)30/h5-10,18,23,26-27,34-35H,3-4,11-17H2,1-2H3/t23-,26-,27-,28-,29-,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50090694
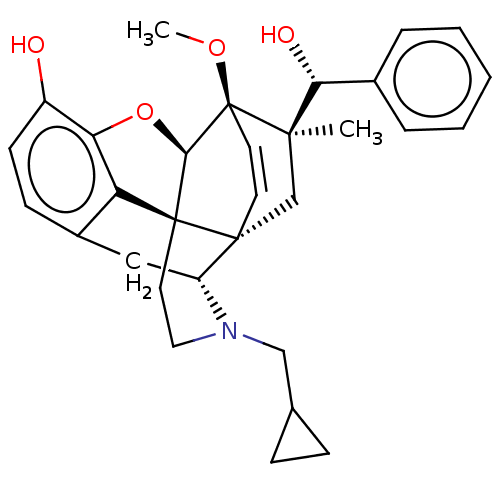
(CHEMBL3581740 | US9259422, 22, R = Ph-BU128 | US94...)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]51C[C@](C)([C@H](O)c4ccccc4)[C@]2(OC)C=C1)ccc3O |r,wU:17.21,16.16,19.23,1.0,wD:29.35,21.25,7.7,c:38,TLB:21:19:16.1:33.32,THB:10:9:17:4.5.6,20:19:16.1:33.32,(-3.17,.26,;-2.2,.57,;-3.38,2.21,;-2.2,3.8,;-.81,2.99,;.55,3.8,;1.94,2.99,;1.94,1.4,;2.83,1.9,;3.33,.57,;4.33,-.58,;5.84,-.3,;7.2,-.82,;6.92,.69,;2.73,2.15,;.51,2.18,;-.81,1.4,;.55,.57,;.55,-.99,;-.81,-1.79,;-1.89,-2.38,;.53,-2.51,;1.56,-1.84,;.61,-4.05,;-.68,-4.89,;-.6,-6.42,;.77,-7.12,;2.06,-6.28,;1.98,-4.74,;-2.2,-.99,;-3.6,-1.56,;-4.57,-.8,;-.88,-.72,;-.88,.28,;.55,5.4,;-.81,6.2,;-2.2,5.4,;-3.26,6.01,)| Show InChI InChI=1S/C19H23N5O4/c1-10(2)11-5-3-4-6-12(11)23-17-14-18(21-8-20-17)24(9-22-14)19-16(27)15(26)13(7-25)28-19/h3-6,8-10,13,15-16,19,25-27H,7H2,1-2H3,(H,20,21,23)/t13?,15?,16?,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50090694

(CHEMBL3581740 | US9259422, 22, R = Ph-BU128 | US94...)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]51C[C@](C)([C@H](O)c4ccccc4)[C@]2(OC)C=C1)ccc3O |r,wU:17.21,16.16,19.23,1.0,wD:29.35,21.25,7.7,c:38,TLB:21:19:16.1:33.32,THB:10:9:17:4.5.6,20:19:16.1:33.32,(-3.17,.26,;-2.2,.57,;-3.38,2.21,;-2.2,3.8,;-.81,2.99,;.55,3.8,;1.94,2.99,;1.94,1.4,;2.83,1.9,;3.33,.57,;4.33,-.58,;5.84,-.3,;7.2,-.82,;6.92,.69,;2.73,2.15,;.51,2.18,;-.81,1.4,;.55,.57,;.55,-.99,;-.81,-1.79,;-1.89,-2.38,;.53,-2.51,;1.56,-1.84,;.61,-4.05,;-.68,-4.89,;-.6,-6.42,;.77,-7.12,;2.06,-6.28,;1.98,-4.74,;-2.2,-.99,;-3.6,-1.56,;-4.57,-.8,;-.88,-.72,;-.88,.28,;.55,5.4,;-.81,6.2,;-2.2,5.4,;-3.26,6.01,)| Show InChI InChI=1S/C19H23N5O4/c1-10(2)11-5-3-4-6-12(11)23-17-14-18(21-8-20-17)24(9-22-14)19-16(27)15(26)13(7-25)28-19/h3-6,8-10,13,15-16,19,25-27H,7H2,1-2H3,(H,20,21,23)/t13?,15?,16?,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50354578

(BUPRENORPHINE | US10752592, Compound buprenorphine...)Show SMILES CO[C@@]12CC[C@@]3(C[C@@H]1[C@](C)(O)C(C)(C)C)[C@H]1Cc4ccc(O)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |r| Show InChI InChI=1S/C29H41NO4/c1-25(2,3)26(4,32)20-15-27-10-11-29(20,33-5)24-28(27)12-13-30(16-17-6-7-17)21(27)14-18-8-9-19(31)23(34-24)22(18)28/h8-9,17,20-21,24,31-32H,6-7,10-16H2,1-5H3/t20-,21-,24-,26+,27-,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0890 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50090767

(CHEMBL3581757)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1cccc(F)c1)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C31H36FNO4/c1-28(26(35)20-4-3-5-21(32)14-20)17-29-10-11-31(28,36-2)27-30(29)12-13-33(16-18-6-7-18)23(29)15-19-8-9-22(34)25(37-27)24(19)30/h3-5,8-9,14,18,23,26-27,34-35H,6-7,10-13,15-17H2,1-2H3/t23-,26-,27-,28-,29-,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0900 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50090766

(CHEMBL3581756)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1cccc(Cl)c1)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C31H36ClNO4/c1-28(26(35)20-4-3-5-21(32)14-20)17-29-10-11-31(28,36-2)27-30(29)12-13-33(16-18-6-7-18)23(29)15-19-8-9-22(34)25(37-27)24(19)30/h3-5,8-9,14,18,23,26-27,34-35H,6-7,10-13,15-17H2,1-2H3/t23-,26-,27-,28-,29-,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50090765
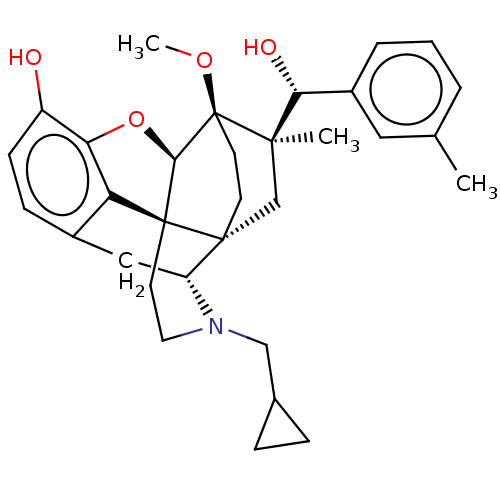
(CHEMBL3581752)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1cccc(C)c1)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C32H39NO4/c1-19-5-4-6-22(15-19)27(35)29(2)18-30-11-12-32(29,36-3)28-31(30)13-14-33(17-20-7-8-20)24(30)16-21-9-10-23(34)26(37-28)25(21)31/h4-6,9-10,15,20,24,27-28,34-35H,7-8,11-14,16-18H2,1-3H3/t24-,27-,28-,29-,30-,31+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50090760

(CHEMBL3581750 | US9259422, 30, R = Ph-BU10119 | US...)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1ccccc1)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C31H37NO4/c1-28(26(34)20-6-4-3-5-7-20)18-29-12-13-31(28,35-2)27-30(29)14-15-32(17-19-8-9-19)23(29)16-21-10-11-22(33)25(36-27)24(21)30/h3-7,10-11,19,23,26-27,33-34H,8-9,12-18H2,1-2H3/t23-,26-,27-,28-,29-,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50090782

(CHEMBL3581754)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1ccc(F)cc1)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C31H36FNO4/c1-28(26(35)19-5-8-21(32)9-6-19)17-29-11-12-31(28,36-2)27-30(29)13-14-33(16-18-3-4-18)23(29)15-20-7-10-22(34)25(37-27)24(20)30/h5-10,18,23,26-27,34-35H,3-4,11-17H2,1-2H3/t23-,26-,27-,28-,29-,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50090765

(CHEMBL3581752)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1cccc(C)c1)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C32H39NO4/c1-19-5-4-6-22(15-19)27(35)29(2)18-30-11-12-32(29,36-3)28-31(30)13-14-33(17-20-7-8-20)24(30)16-21-9-10-23(34)26(37-28)25(21)31/h4-6,9-10,15,20,24,27-28,34-35H,7-8,11-14,16-18H2,1-3H3/t24-,27-,28-,29-,30-,31+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50090768
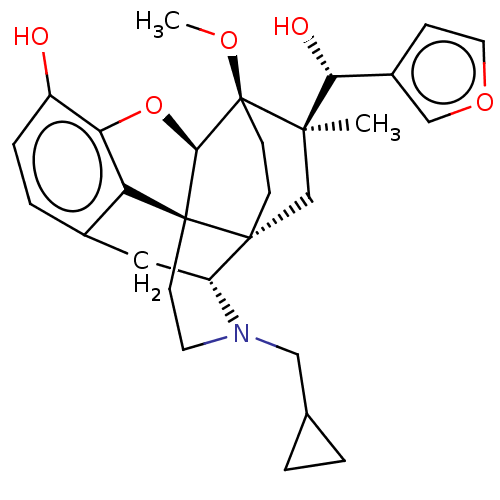
(CHEMBL3581762)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1ccoc1)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C29H35NO5/c1-26(24(32)19-7-12-34-15-19)16-27-8-9-29(26,33-2)25-28(27)10-11-30(14-17-3-4-17)21(27)13-18-5-6-20(31)23(35-25)22(18)28/h5-7,12,15,17,21,24-25,31-32H,3-4,8-11,13-14,16H2,1-2H3/t21-,24-,25-,26-,27-,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50090769
(CHEMBL3581751)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1ccccc1C)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C32H39NO4/c1-19-6-4-5-7-22(19)27(35)29(2)18-30-12-13-32(29,36-3)28-31(30)14-15-33(17-20-8-9-20)24(30)16-21-10-11-23(34)26(37-28)25(21)31/h4-7,10-11,20,24,27-28,34-35H,8-9,12-18H2,1-3H3/t24-,27-,28-,29-,30-,31+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50090767

(CHEMBL3581757)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1cccc(F)c1)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C31H36FNO4/c1-28(26(35)20-4-3-5-21(32)14-20)17-29-10-11-31(28,36-2)27-30(29)12-13-33(16-18-6-7-18)23(29)15-19-8-9-22(34)25(37-27)24(19)30/h3-5,8-9,14,18,23,26-27,34-35H,6-7,10-13,15-17H2,1-2H3/t23-,26-,27-,28-,29-,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50354578

(BUPRENORPHINE | US10752592, Compound buprenorphine...)Show SMILES CO[C@@]12CC[C@@]3(C[C@@H]1[C@](C)(O)C(C)(C)C)[C@H]1Cc4ccc(O)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |r| Show InChI InChI=1S/C29H41NO4/c1-25(2,3)26(4,32)20-15-27-10-11-29(20,33-5)24-28(27)12-13-30(16-17-6-7-17)21(27)14-18-8-9-19(31)23(34-24)22(18)28/h8-9,17,20-21,24,31-32H,6-7,10-16H2,1-5H3/t20-,21-,24-,26+,27-,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50090768

(CHEMBL3581762)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1ccoc1)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C29H35NO5/c1-26(24(32)19-7-12-34-15-19)16-27-8-9-29(26,33-2)25-28(27)10-11-30(14-17-3-4-17)21(27)13-18-5-6-20(31)23(35-25)22(18)28/h5-7,12,15,17,21,24-25,31-32H,3-4,8-11,13-14,16H2,1-2H3/t21-,24-,25-,26-,27-,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50090773
(CHEMBL3581753)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1ccc(C)cc1)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C32H39NO4/c1-19-4-8-21(9-5-19)27(35)29(2)18-30-12-13-32(29,36-3)28-31(30)14-15-33(17-20-6-7-20)24(30)16-22-10-11-23(34)26(37-28)25(22)31/h4-5,8-11,20,24,27-28,34-35H,6-7,12-18H2,1-3H3/t24-,27-,28-,29-,30-,31+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM85177
(CAS_80558-61-8 | M&B-28767 | NSC_119139)Show SMILES OC(COc1ccccc1)C=CC1CCC(=O)C1CCCCCCC(O)=O |w:11.12| Show InChI InChI=1S/C22H30O5/c23-18(16-27-19-8-4-3-5-9-19)14-12-17-13-15-21(24)20(17)10-6-1-2-7-11-22(25)26/h3-5,8-9,12,14,17-18,20,23H,1-2,6-7,10-11,13,15-16H2,(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50090755

(CHEMBL3581743 | US9259422, 22, R = 4-FPh- BU10120 ...)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]51C[C@](C)([C@H](O)c4ccc(F)cc4)[C@]2(OC)C=C1)ccc3O |r,wU:17.21,16.16,19.23,1.0,wD:30.36,21.25,7.7,c:39,TLB:21:19:16.1:34.33,THB:10:9:17:4.5.6,20:19:16.1:34.33,(-3.17,.26,;-2.2,.57,;-3.38,2.21,;-2.2,3.8,;-.81,2.99,;.55,3.8,;1.94,2.99,;1.94,1.4,;2.83,1.9,;3.33,.57,;4.33,-.58,;5.84,-.3,;7.2,-.82,;6.92,.69,;2.73,2.15,;.51,2.18,;-.81,1.4,;.55,.57,;.55,-.99,;-.81,-1.79,;-1.89,-2.38,;.53,-2.51,;1.56,-1.84,;.61,-4.05,;-.68,-4.89,;-.6,-6.42,;.77,-7.12,;.84,-8.35,;2.06,-6.28,;1.98,-4.74,;-2.2,-.99,;-3.6,-1.56,;-4.57,-.8,;-.88,-.72,;-.88,.28,;.55,5.4,;-.81,6.2,;-2.2,5.4,;-3.26,6.01,)| Show InChI InChI=1S/C31H34FNO4/c1-28(26(35)19-5-8-21(32)9-6-19)17-29-11-12-31(28,36-2)27-30(29)13-14-33(16-18-3-4-18)23(29)15-20-7-10-22(34)25(37-27)24(20)30/h5-12,18,23,26-27,34-35H,3-4,13-17H2,1-2H3/t23-,26-,27-,28-,29-,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50090773

(CHEMBL3581753)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1ccc(C)cc1)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C32H39NO4/c1-19-4-8-21(9-5-19)27(35)29(2)18-30-12-13-32(29,36-3)28-31(30)14-15-33(17-20-6-7-20)24(30)16-22-10-11-23(34)26(37-28)25(22)31/h4-5,8-11,20,24,27-28,34-35H,6-7,12-18H2,1-3H3/t24-,27-,28-,29-,30-,31+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from human kappa opioid receptor expressed in CHO cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50090731

(CHEMBL3581741 | US9259422, 22, R = 3-MePh- BU10112...)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]51C[C@](C)([C@H](O)c4cccc(C)c4)[C@]2(OC)C=C1)ccc3O |r,wU:17.21,16.16,19.23,1.0,wD:30.36,21.25,7.7,c:39,TLB:21:19:16.1:34.33,THB:10:9:17:4.5.6,20:19:16.1:34.33,(-3.17,.26,;-2.2,.57,;-3.38,2.21,;-2.2,3.8,;-.81,2.99,;.55,3.8,;1.94,2.99,;1.94,1.4,;2.83,1.9,;3.33,.57,;4.33,-.58,;5.84,-.3,;7.2,-.82,;6.92,.69,;2.73,2.15,;.51,2.18,;-.81,1.4,;.55,.57,;.55,-.99,;-.81,-1.79,;-1.89,-2.38,;.53,-2.51,;1.56,-1.84,;.61,-4.05,;1.98,-4.74,;2.06,-6.28,;.77,-7.12,;-.6,-6.42,;-1.63,-7.1,;-.68,-4.89,;-2.2,-.99,;-3.6,-1.56,;-4.57,-.8,;-.88,-.72,;-.88,.28,;.55,5.4,;-.81,6.2,;-2.2,5.4,;-3.26,6.01,)| Show InChI InChI=1S/C55H75N17O13/c1-29(2)19-38(49(80)67-37(9-5-17-60-55(57)58)54(85)72-18-6-10-43(72)53(84)62-25-44(56)75)66-46(77)26-63-47(78)39(20-30-11-13-33(74)14-12-30)68-52(83)42(27-73)71-50(81)40(21-31-23-61-35-8-4-3-7-34(31)35)69-51(82)41(22-32-24-59-28-64-32)70-48(79)36-15-16-45(76)65-36/h3-4,7-8,11-14,23-24,28-29,36-43,61,73-74H,5-6,9-10,15-22,25-27H2,1-2H3,(H2,56,75)(H,59,64)(H,62,84)(H,63,78)(H,65,76)(H,66,77)(H,67,80)(H,68,83)(H,69,82)(H,70,79)(H,71,81)(H4,57,58,60)/t36?,37-,38-,39-,40-,41-,42-,43+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50015003

(CHEMBL3262089 | US9259422, 7a, R = Ph-BU127 | US94...)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@]([H])(C1)[C@](C)(O)c1ccccc1)ccc3O |r,THB:10:9:17:4.5.6| Show InChI InChI=1S/C31H37NO4/c1-28(34,21-6-4-3-5-7-21)23-17-29-12-13-31(23,35-2)27-30(29)14-15-32(18-19-8-9-19)24(29)16-20-10-11-22(33)26(36-27)25(20)30/h3-7,10-11,19,23-24,27,33-34H,8-9,12-18H2,1-2H3/t23-,24-,27-,28-,29-,30+,31-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.170 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50090769

(CHEMBL3581751)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1ccccc1C)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C32H39NO4/c1-19-6-4-5-7-22(19)27(35)29(2)18-30-12-13-32(29,36-3)28-31(30)14-15-33(17-20-8-9-20)24(30)16-21-10-11-23(34)26(37-28)25(21)31/h4-7,10-11,20,24,27-28,34-35H,8-9,12-18H2,1-3H3/t24-,27-,28-,29-,30-,31+,32+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat mu opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50090760

(CHEMBL3581750 | US9259422, 30, R = Ph-BU10119 | US...)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1ccccc1)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C31H37NO4/c1-28(26(34)20-6-4-3-5-7-20)18-29-12-13-31(28,35-2)27-30(29)14-15-32(17-19-8-9-19)23(29)16-21-10-11-22(33)25(36-27)24(21)30/h3-7,10-11,19,23,26-27,33-34H,8-9,12-18H2,1-2H3/t23-,26-,27-,28-,29-,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat delta opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM85603
(CAS_5311503 | NSC_5311503 | ZK110841)Show SMILES OC(C=CC1C(O)CC(Cl)C1CC=CCCCC(O)=O)C1CCCCC1 |w:2.1,12.12| Show InChI InChI=1S/C21H33ClO4/c22-18-14-20(24)17(12-13-19(23)15-8-4-3-5-9-15)16(18)10-6-1-2-7-11-21(25)26/h1,6,12-13,15-20,23-24H,2-5,7-11,14H2,(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Prostaglandin E2 receptor EP3 subtype
(Homo sapiens (Human)) | BDBM85183
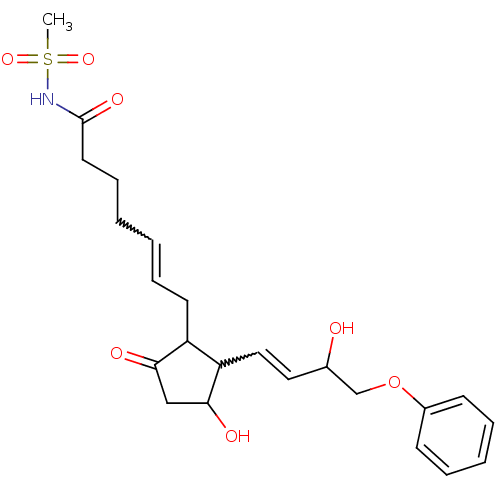
(CAS_60325-46-4 | NSC_43251 | SULPROSTONE)Show SMILES CS(=O)(=O)NC(=O)CCCC=CCC1C(C=CC(O)COc2ccccc2)C(O)CC1=O |w:10.9,15.14| Show InChI InChI=1S/C23H31NO7S/c1-32(29,30)24-23(28)12-8-3-2-7-11-19-20(22(27)15-21(19)26)14-13-17(25)16-31-18-9-5-4-6-10-18/h2,4-7,9-10,13-14,17,19-20,22,25,27H,3,8,11-12,15-16H2,1H3,(H,24,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| 0.350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50090731

(CHEMBL3581741 | US9259422, 22, R = 3-MePh- BU10112...)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]51C[C@](C)([C@H](O)c4cccc(C)c4)[C@]2(OC)C=C1)ccc3O |r,wU:17.21,16.16,19.23,1.0,wD:30.36,21.25,7.7,c:39,TLB:21:19:16.1:34.33,THB:10:9:17:4.5.6,20:19:16.1:34.33,(-3.17,.26,;-2.2,.57,;-3.38,2.21,;-2.2,3.8,;-.81,2.99,;.55,3.8,;1.94,2.99,;1.94,1.4,;2.83,1.9,;3.33,.57,;4.33,-.58,;5.84,-.3,;7.2,-.82,;6.92,.69,;2.73,2.15,;.51,2.18,;-.81,1.4,;.55,.57,;.55,-.99,;-.81,-1.79,;-1.89,-2.38,;.53,-2.51,;1.56,-1.84,;.61,-4.05,;1.98,-4.74,;2.06,-6.28,;.77,-7.12,;-.6,-6.42,;-1.63,-7.1,;-.68,-4.89,;-2.2,-.99,;-3.6,-1.56,;-4.57,-.8,;-.88,-.72,;-.88,.28,;.55,5.4,;-.81,6.2,;-2.2,5.4,;-3.26,6.01,)| Show InChI InChI=1S/C55H75N17O13/c1-29(2)19-38(49(80)67-37(9-5-17-60-55(57)58)54(85)72-18-6-10-43(72)53(84)62-25-44(56)75)66-46(77)26-63-47(78)39(20-30-11-13-33(74)14-12-30)68-52(83)42(27-73)71-50(81)40(21-31-23-61-35-8-4-3-7-34(31)35)69-51(82)41(22-32-24-59-28-64-32)70-48(79)36-15-16-45(76)65-36/h3-4,7-8,11-14,23-24,28-29,36-43,61,73-74H,5-6,9-10,15-22,25-27H2,1-2H3,(H2,56,75)(H,59,64)(H,62,84)(H,63,78)(H,65,76)(H,66,77)(H,67,80)(H,68,83)(H,69,82)(H,70,79)(H,71,81)(H4,57,58,60)/t36?,37-,38-,39-,40-,41-,42-,43+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat delta opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM85175
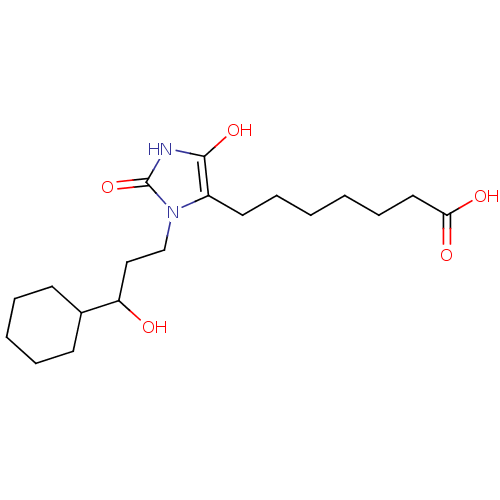
(BW245C | CAS_72814-32-5 | NSC_3080928)Show SMILES OC(CCn1c(CCCCCCC(O)=O)c(O)[nH]c1=O)C1CCCCC1 Show InChI InChI=1S/C19H32N2O5/c22-16(14-8-4-3-5-9-14)12-13-21-15(18(25)20-19(21)26)10-6-1-2-7-11-17(23)24/h14,16,22,25H,1-13H2,(H,20,26)(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50090782

(CHEMBL3581754)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@@]51CC[C@@]2(OC)[C@](C)(C1)[C@H](O)c1ccc(F)cc1)ccc3O |r,TLB:26:23:16.1:18.19,THB:10:9:17:4.5.6,24:23:16.1:18.19| Show InChI InChI=1S/C31H36FNO4/c1-28(26(35)19-5-8-21(32)9-6-19)17-29-11-12-31(28,36-2)27-30(29)13-14-33(16-18-3-4-18)23(29)15-20-7-10-22(34)25(37-27)24(20)30/h5-10,18,23,26-27,34-35H,3-4,11-17H2,1-2H3/t23-,26-,27-,28-,29-,30+,31+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat delta opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM50085910
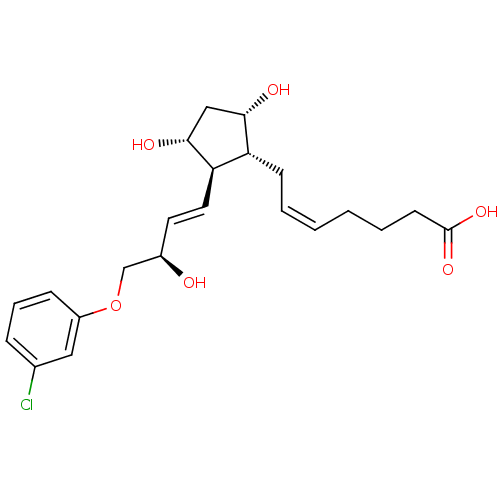
((Z)-7-{(1R,2R,3R,5S)-2-[(E)-(R)-4-(3-Chloro-phenox...)Show SMILES O[C@@H](COc1cccc(Cl)c1)\C=C\[C@H]1[C@H](O)C[C@H](O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C22H29ClO6/c23-15-6-5-7-17(12-15)29-14-16(24)10-11-19-18(20(25)13-21(19)26)8-3-1-2-4-9-22(27)28/h1,3,5-7,10-12,16,18-21,24-26H,2,4,8-9,13-14H2,(H,27,28)/b3-1-,11-10+/t16-,18-,19-,20+,21-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50090694

(CHEMBL3581740 | US9259422, 22, R = Ph-BU128 | US94...)Show SMILES [H][C@@]12Oc3c4c(C[C@@]5([H])N(CC6CC6)CC[C@@]14[C@]51C[C@](C)([C@H](O)c4ccccc4)[C@]2(OC)C=C1)ccc3O |r,wU:17.21,16.16,19.23,1.0,wD:29.35,21.25,7.7,c:38,TLB:21:19:16.1:33.32,THB:10:9:17:4.5.6,20:19:16.1:33.32,(-3.17,.26,;-2.2,.57,;-3.38,2.21,;-2.2,3.8,;-.81,2.99,;.55,3.8,;1.94,2.99,;1.94,1.4,;2.83,1.9,;3.33,.57,;4.33,-.58,;5.84,-.3,;7.2,-.82,;6.92,.69,;2.73,2.15,;.51,2.18,;-.81,1.4,;.55,.57,;.55,-.99,;-.81,-1.79,;-1.89,-2.38,;.53,-2.51,;1.56,-1.84,;.61,-4.05,;-.68,-4.89,;-.6,-6.42,;.77,-7.12,;2.06,-6.28,;1.98,-4.74,;-2.2,-.99,;-3.6,-1.56,;-4.57,-.8,;-.88,-.72,;-.88,.28,;.55,5.4,;-.81,6.2,;-2.2,5.4,;-3.26,6.01,)| Show InChI InChI=1S/C19H23N5O4/c1-10(2)11-5-3-4-6-12(11)23-17-14-18(21-8-20-17)24(9-22-14)19-16(27)15(26)13(7-25)28-19/h3-6,8-10,13,15-16,19,25-27H,7H2,1-2H3,(H,20,21,23)/t13?,15?,16?,19-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat delta opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Rattus norvegicus (rat)) | BDBM50354578

(BUPRENORPHINE | US10752592, Compound buprenorphine...)Show SMILES CO[C@@]12CC[C@@]3(C[C@@H]1[C@](C)(O)C(C)(C)C)[C@H]1Cc4ccc(O)c5O[C@@H]2[C@]3(CCN1CC1CC1)c45 |r| Show InChI InChI=1S/C29H41NO4/c1-25(2,3)26(4,32)20-15-27-10-11-29(20,33-5)24-28(27)12-13-30(16-17-6-7-17)21(27)14-18-8-9-19(31)23(34-24)22(18)28/h8-9,17,20-21,24,31-32H,6-7,10-16H2,1-5H3/t20-,21-,24-,26+,27-,28+,29+/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.480 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bath
Curated by ChEMBL
| Assay Description
Displacement of [3H]diprenorphine from rat delta opioid receptor expressed in rat C6 cell membranes incubated for 1 hr by beta counting method |
J Med Chem 58: 4242-9 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00130
BindingDB Entry DOI: 10.7270/Q2ST7RKD |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50067735
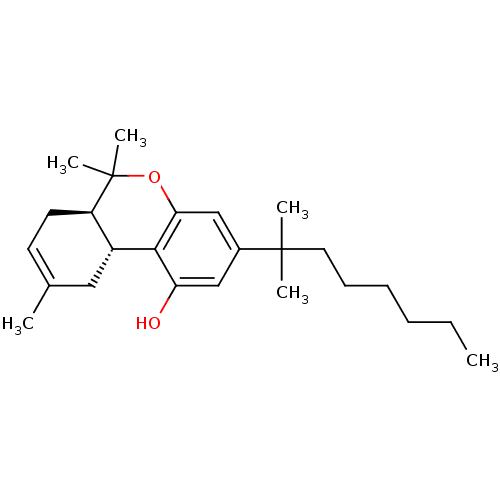
((6aR,10aR)-3-(1,1-Dimethyl-heptyl)-6,6,9-trimethyl...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:17| Show InChI InChI=1S/C25H38O2/c1-7-8-9-10-13-24(3,4)18-15-21(26)23-19-14-17(2)11-12-20(19)25(5,6)27-22(23)16-18/h11,15-16,19-20,26H,7-10,12-14H2,1-6H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.490 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Cannabinoid receptor 2 by using radioligand ([3H]-CP-55,940) assay. |
Bioorg Med Chem Lett 6: 189-194 (1996)
Article DOI: 10.1016/0960-894X(95)00573-C
BindingDB Entry DOI: 10.7270/Q2KS6RHX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50287934
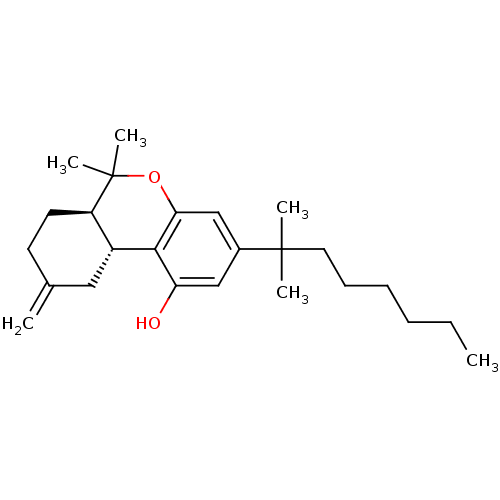
((6aR,10aR)-3-(1,1-Dimethyl-heptyl)-6,6-dimethyl-9-...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(=C)CC[C@H]3C(C)(C)Oc2c1 Show InChI InChI=1S/C25H38O2/c1-7-8-9-10-13-24(3,4)18-15-21(26)23-19-14-17(2)11-12-20(19)25(5,6)27-22(23)16-18/h15-16,19-20,26H,2,7-14H2,1,3-6H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Cannabinoid receptor 2 by using radioligand ([3H]-CP-55,940) assay. |
Bioorg Med Chem Lett 6: 189-194 (1996)
Article DOI: 10.1016/0960-894X(95)00573-C
BindingDB Entry DOI: 10.7270/Q2KS6RHX |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP4 subtype
(Homo sapiens (Human)) | BDBM35847

((15S)-prostaglandin E2 | (5Z,11alpha,13E,15S)-11,1...)Show SMILES CCCCC[C@H](O)\C=C\[C@H]1[C@H](O)CC(=O)[C@@H]1C\C=C/CCCC(O)=O Show InChI InChI=1S/C20H32O5/c1-2-3-6-9-15(21)12-13-17-16(18(22)14-19(17)23)10-7-4-5-8-11-20(24)25/h4,7,12-13,15-17,19,21,23H,2-3,5-6,8-11,14H2,1H3,(H,24,25)/b7-4-,13-12+/t15-,16+,17+,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| 0.790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50067735

((6aR,10aR)-3-(1,1-Dimethyl-heptyl)-6,6,9-trimethyl...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |c:17| Show InChI InChI=1S/C25H38O2/c1-7-8-9-10-13-24(3,4)18-15-21(26)23-19-14-17(2)11-12-20(19)25(5,6)27-22(23)16-18/h11,15-16,19-20,26H,7-10,12-14H2,1-6H3/t19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 0.830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human cannabinoid receptor by using radioligand ([3H]-CP-55,940) assay. |
Bioorg Med Chem Lett 6: 189-194 (1996)
Article DOI: 10.1016/0960-894X(95)00573-C
BindingDB Entry DOI: 10.7270/Q2KS6RHX |
More data for this
Ligand-Target Pair | |
Hematopoietic prostaglandin D synthase
(Homo sapiens (Human)) | BDBM82213
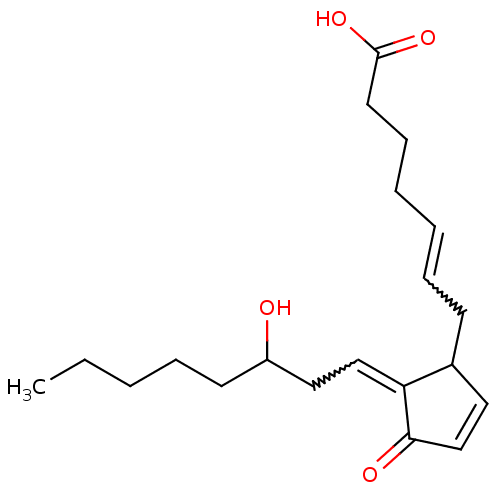
(CAS_41598-07-6 | NSC_114678 | PGD2)Show SMILES CCCCCC(O)CC=C1C(CC=CCCCC(O)=O)C=CC1=O |w:8.7,12.11,c:20| Show InChI InChI=1S/C20H30O4/c1-2-3-6-10-17(21)13-14-18-16(12-15-19(18)22)9-7-4-5-8-11-20(23)24/h4,7,12,14-17,21H,2-3,5-6,8-11,13H2,1H3,(H,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50287941
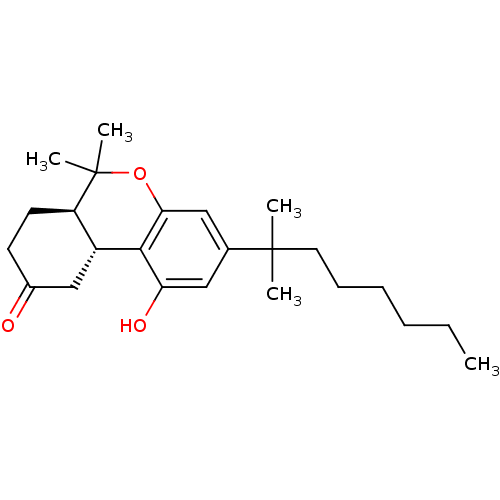
((6aR,10aR)-1-hydroxy-6,6-dimethyl-3-(2-methyloctan...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(=O)CC[C@H]3C(C)(C)Oc2c1 Show InChI InChI=1S/C24H36O3/c1-6-7-8-9-12-23(2,3)16-13-20(26)22-18-15-17(25)10-11-19(18)24(4,5)27-21(22)14-16/h13-14,18-19,26H,6-12,15H2,1-5H3/t18-,19-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Cannabinoid receptor 2 by using radioligand ([3H]-CP-55,940) assay. |
Bioorg Med Chem Lett 6: 189-194 (1996)
Article DOI: 10.1016/0960-894X(95)00573-C
BindingDB Entry DOI: 10.7270/Q2KS6RHX |
More data for this
Ligand-Target Pair | |
Prostaglandin E2 receptor EP1 subtype
(Homo sapiens (Human)) | BDBM85603

(CAS_5311503 | NSC_5311503 | ZK110841)Show SMILES OC(C=CC1C(O)CC(Cl)C1CC=CCCCC(O)=O)C1CCCCC1 |w:2.1,12.12| Show InChI InChI=1S/C21H33ClO4/c22-18-14-20(24)17(12-13-19(23)15-8-4-3-5-9-15)16(18)10-6-1-2-7-11-21(25)26/h1,6,12-13,15-20,23-24H,2-5,7-11,14H2,(H,25,26) | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50287934

((6aR,10aR)-3-(1,1-Dimethyl-heptyl)-6,6-dimethyl-9-...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(=C)CC[C@H]3C(C)(C)Oc2c1 Show InChI InChI=1S/C25H38O2/c1-7-8-9-10-13-24(3,4)18-15-21(26)23-19-14-17(2)11-12-20(19)25(5,6)27-22(23)16-18/h15-16,19-20,26H,2,7-14H2,1,3-6H3/t19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human cannabinoid receptor by using radioligand ([3H]-CP-55,940) assay. |
Bioorg Med Chem Lett 6: 189-194 (1996)
Article DOI: 10.1016/0960-894X(95)00573-C
BindingDB Entry DOI: 10.7270/Q2KS6RHX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50287935
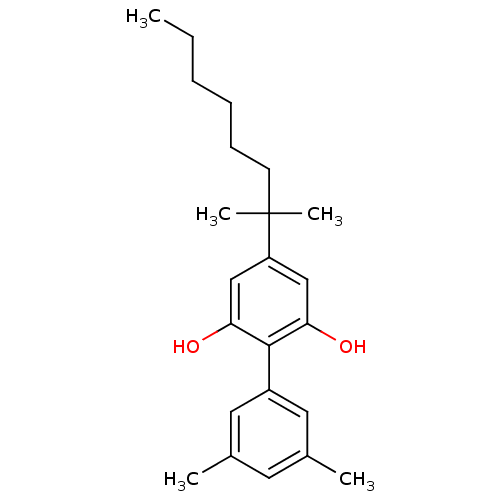
(4-(1,1-Dimethyl-heptyl)-3',5'-dimethyl-biphenyl-2,...)Show SMILES CCCCCCC(C)(C)c1cc(O)c(c(O)c1)-c1cc(C)cc(C)c1 Show InChI InChI=1S/C23H32O2/c1-6-7-8-9-10-23(4,5)19-14-20(24)22(21(25)15-19)18-12-16(2)11-17(3)13-18/h11-15,24-25H,6-10H2,1-5H3 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Cannabinoid receptor 2 by using radioligand ([3H]-CP-55,940) assay. |
Bioorg Med Chem Lett 6: 189-194 (1996)
Article DOI: 10.1016/0960-894X(95)00573-C
BindingDB Entry DOI: 10.7270/Q2KS6RHX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50287930
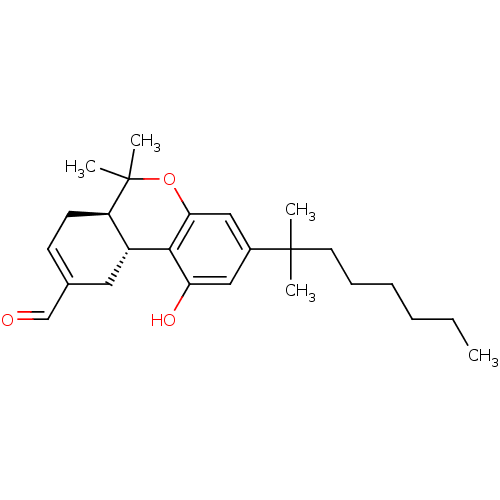
((6aR,10aR)-3-(1,1-Dimethyl-heptyl)-1-hydroxy-6,6-d...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(C=O)=CC[C@H]3C(C)(C)Oc2c1 |c:18| Show InChI InChI=1S/C25H36O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h10,14-16,19-20,27H,6-9,11-13H2,1-5H3/t19-,20-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human cannabinoid receptor by using radioligand ([3H]-CP-55,940) assay. |
Bioorg Med Chem Lett 6: 189-194 (1996)
Article DOI: 10.1016/0960-894X(95)00573-C
BindingDB Entry DOI: 10.7270/Q2KS6RHX |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50287941

((6aR,10aR)-1-hydroxy-6,6-dimethyl-3-(2-methyloctan...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(=O)CC[C@H]3C(C)(C)Oc2c1 Show InChI InChI=1S/C24H36O3/c1-6-7-8-9-12-23(2,3)16-13-20(26)22-18-15-17(25)10-11-19(18)24(4,5)27-21(22)14-16/h13-14,18-19,26H,6-12,15H2,1-5H3/t18-,19-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
PC cid
PC sid
UniChem
Patents
Similars
| DrugBank
Article
| 2.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human cannabinoid receptor by using radioligand ([3H]-CP-55,940) assay. |
Bioorg Med Chem Lett 6: 189-194 (1996)
Article DOI: 10.1016/0960-894X(95)00573-C
BindingDB Entry DOI: 10.7270/Q2KS6RHX |
More data for this
Ligand-Target Pair | |
Prostaglandin F2-alpha receptor
(Homo sapiens (Human)) | BDBM85173
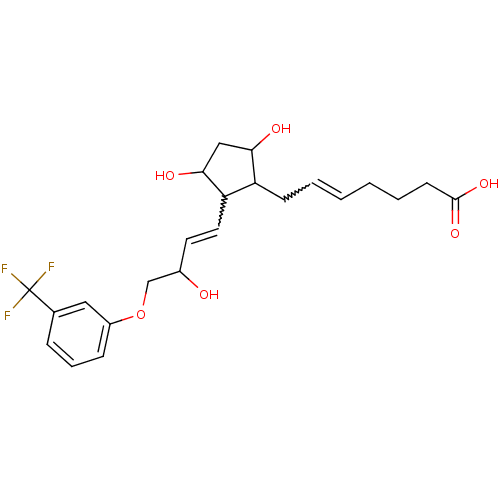
(CAS_40666-16-8 | FLUPROSTENOL | NSC_5311100)Show SMILES OC(COc1cccc(c1)C(F)(F)F)C=CC1C(O)CC(O)C1CC=CCCCC(O)=O |w:15.16,24.25| Show InChI InChI=1S/C23H29F3O6/c24-23(25,26)15-6-5-7-17(12-15)32-14-16(27)10-11-19-18(20(28)13-21(19)29)8-3-1-2-4-9-22(30)31/h1,3,5-7,10-12,16,18-21,27-29H,2,4,8-9,13-14H2,(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by PDSP Ki Database
| |
Biochim Biophys Acta 1483: 285-93 (2000)
Article DOI: 10.1016/s1388-1981(99)00164-x
BindingDB Entry DOI: 10.7270/Q2J964XQ |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50287930

((6aR,10aR)-3-(1,1-Dimethyl-heptyl)-1-hydroxy-6,6-d...)Show SMILES CCCCCCC(C)(C)c1cc(O)c2[C@@H]3CC(C=O)=CC[C@H]3C(C)(C)Oc2c1 |c:18| Show InChI InChI=1S/C25H36O3/c1-6-7-8-9-12-24(2,3)18-14-21(27)23-19-13-17(16-26)10-11-20(19)25(4,5)28-22(23)15-18/h10,14-16,19-20,27H,6-9,11-13H2,1-5H3/t19-,20-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 2.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Binding affinity against human Cannabinoid receptor 2 by using radioligand ([3H]-CP-55,940) assay. |
Bioorg Med Chem Lett 6: 189-194 (1996)
Article DOI: 10.1016/0960-894X(95)00573-C
BindingDB Entry DOI: 10.7270/Q2KS6RHX |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data