

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50089098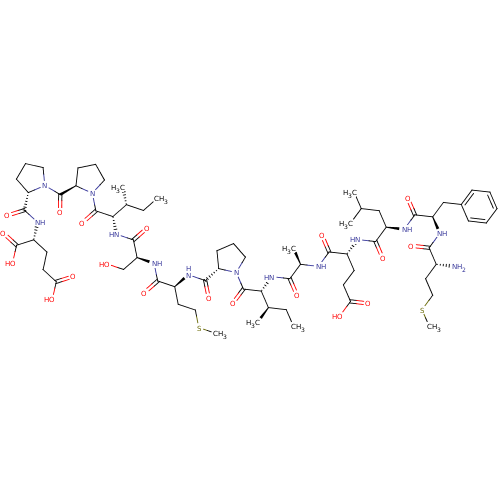 (CHEMBL266704 | Cys-Phe-Leu-Glu-Ala-Ile-Pro-Met-Asp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Oxford Centre for Molecular Sciences Curated by ChEMBL | Assay Description Competitive inhibition constant of the compound was tested against Human neutrophil elastase using the reporter substrate MeO-AAPV-pNa | Bioorg Med Chem Lett 10: 1219-21 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9VTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50089095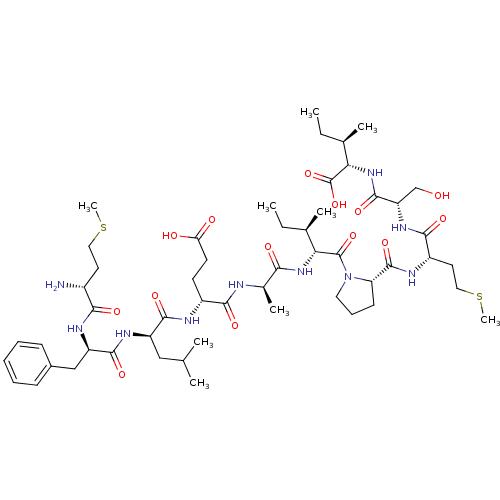 (CHEMBL216418 | Met-Phe-Leu-Glu-Ala-Ile-Pro-Met-Ser...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Oxford Centre for Molecular Sciences Curated by ChEMBL | Assay Description Competitive inhibition constant of the compound was tested against Human neutrophil elastase using the reporter substrate MeO-AAPV-pNa | Bioorg Med Chem Lett 10: 1219-21 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9VTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 2A (Sus scrofa) | BDBM50089095 (CHEMBL216418 | Met-Phe-Leu-Glu-Ala-Ile-Pro-Met-Ser...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Oxford Centre for Molecular Sciences Curated by ChEMBL | Assay Description Peptide was tested for inhibition constant for competitive inhibition using Suc-AAPA-pNa and Pancreatic elastase | Bioorg Med Chem Lett 10: 1219-21 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9VTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50089095 (CHEMBL216418 | Met-Phe-Leu-Glu-Ala-Ile-Pro-Met-Ser...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Oxford Centre for Molecular Sciences Curated by ChEMBL | Assay Description Competitive inhibition constant of the compound was tested against Chymotrypsinogen using the reporter substrate Suc-AAPA-pNa | Bioorg Med Chem Lett 10: 1219-21 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9VTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 2A (Sus scrofa) | BDBM50089098 (CHEMBL266704 | Cys-Phe-Leu-Glu-Ala-Ile-Pro-Met-Asp...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Oxford Centre for Molecular Sciences Curated by ChEMBL | Assay Description Peptide was tested for inhibition constant for competitive inhibition using Suc-AAPA-pNa and Pancreatic elastase | Bioorg Med Chem Lett 10: 1219-21 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9VTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50089094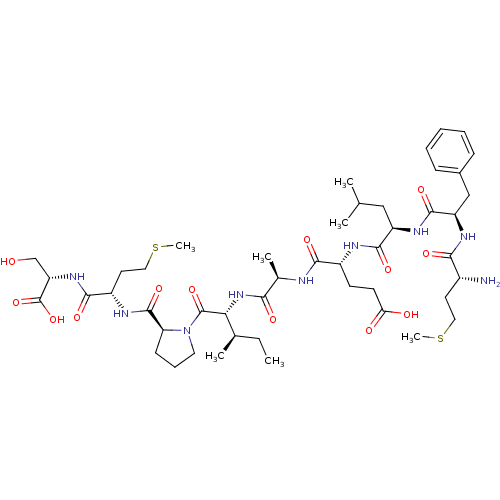 (CHEMBL414810 | Met-Phe-Leu-Glu-Ala-Ile-Pro-Met-Ser) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Oxford Centre for Molecular Sciences Curated by ChEMBL | Assay Description Competitive inhibition constant of the compound was tested against Human neutrophil elastase using the reporter substrate MeO-AAPV-pNa | Bioorg Med Chem Lett 10: 1219-21 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9VTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50089098 (CHEMBL266704 | Cys-Phe-Leu-Glu-Ala-Ile-Pro-Met-Asp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 2.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Oxford Centre for Molecular Sciences Curated by ChEMBL | Assay Description Competitive inhibition constant of the compound was tested against Chymotrypsinogen using the reporter substrate Suc-AAPA-pNa | Bioorg Med Chem Lett 10: 1219-21 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9VTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50089096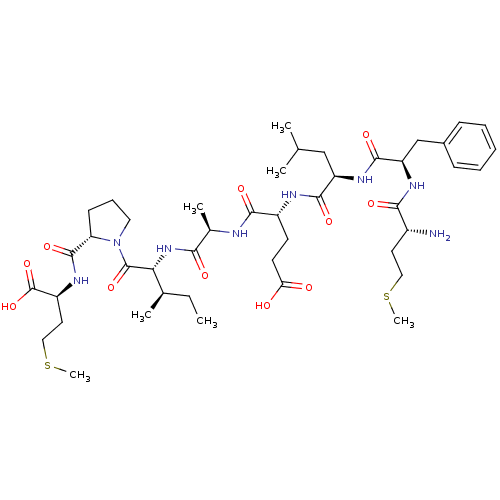 (CHEMBL278549 | Met-Phe-Leu-Glu-Ala-Ile-Pro-Met | e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.70E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Oxford Centre for Molecular Sciences Curated by ChEMBL | Assay Description Competitive inhibition constant of the compound was tested against Human neutrophil elastase using the reporter substrate MeO-AAPV-pNa | Bioorg Med Chem Lett 10: 1219-21 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9VTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine protease 1 (Homo sapiens (Human)) | BDBM50089097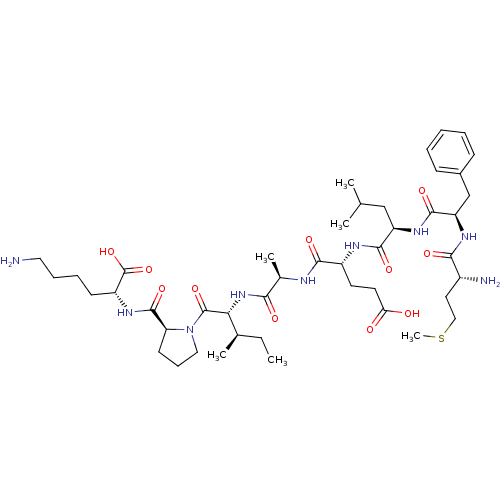 (CHEMBL21028 | Met-Phe-Leu-Glu-Ala-Ile-Pro-Lys) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.40E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Oxford Centre for Molecular Sciences Curated by ChEMBL | Assay Description Competitive inhibition constant of the compound was tested against trypsin using the reporter substrate Ac-R-pNa | Bioorg Med Chem Lett 10: 1219-21 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9VTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 2A (Sus scrofa) | BDBM50089099 ((S)-2-{(S)-2-[(S)-2-((S)-2-Amino-4-methylsulfanyl-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | PubMed | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Oxford Centre for Molecular Sciences Curated by ChEMBL | Assay Description Peptide was tested for inhibition constant for competitive inhibition using Suc-AAPA-pNa and Pancreatic elastase | Bioorg Med Chem Lett 10: 1219-21 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9VTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50089096 (CHEMBL278549 | Met-Phe-Leu-Glu-Ala-Ile-Pro-Met | e...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.30E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Oxford Centre for Molecular Sciences Curated by ChEMBL | Assay Description Competitive inhibition constant of the compound was tested against Chymotrypsinogen using the reporter substrate Suc-AAPA-pNa | Bioorg Med Chem Lett 10: 1219-21 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9VTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsinogen A (Bos taurus (bovine)) | BDBM50089094 (CHEMBL414810 | Met-Phe-Leu-Glu-Ala-Ile-Pro-Met-Ser) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Oxford Centre for Molecular Sciences Curated by ChEMBL | Assay Description Competitive inhibition constant of the compound was tested against Chymotrypsinogen using the reporter substrate Suc-AAPA-pNa | Bioorg Med Chem Lett 10: 1219-21 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9VTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like elastase family member 2A (Sus scrofa) | BDBM50089094 (CHEMBL414810 | Met-Phe-Leu-Glu-Ala-Ile-Pro-Met-Ser) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 5.80E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Oxford Centre for Molecular Sciences Curated by ChEMBL | Assay Description Peptide was tested for inhibition constant for competitive inhibition using Suc-AAPA-pNa and Pancreatic elastase | Bioorg Med Chem Lett 10: 1219-21 (2000) BindingDB Entry DOI: 10.7270/Q2FQ9VTD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||