

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 0.501 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50477152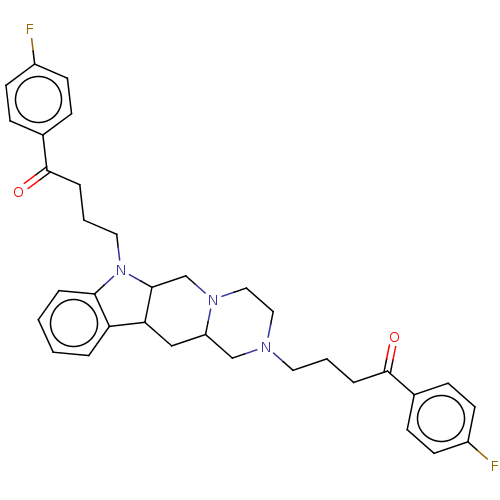 (CHEMBL393466) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.643 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]SCH-23390 from dopamine D1 receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50001885 ((risperidone)3-{2-[4-(6-Fluoro-benzo[d]isoxazol-3-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50433865 (CHEMBL2380402) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50433878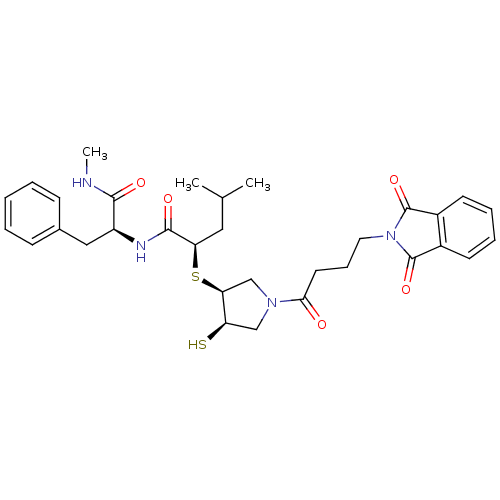 (CHEMBL2380389) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50433880 (CHEMBL2380387) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50433864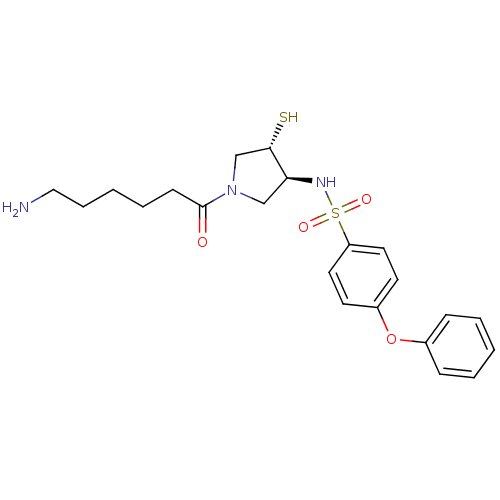 (CHEMBL2380403) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50433877 (CHEMBL2380390) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50477152 (CHEMBL393466) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50433871 (CHEMBL2380396) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50433877 (CHEMBL2380390) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-7 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50433868 (CHEMBL2380400) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50433878 (CHEMBL2380389) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-7 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50133931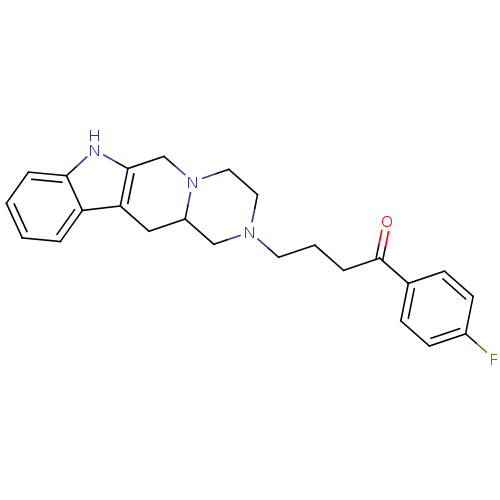 (1-(4-fluoro-phenyl)-4-(3,4,6,7,12,12a-hexahydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50433866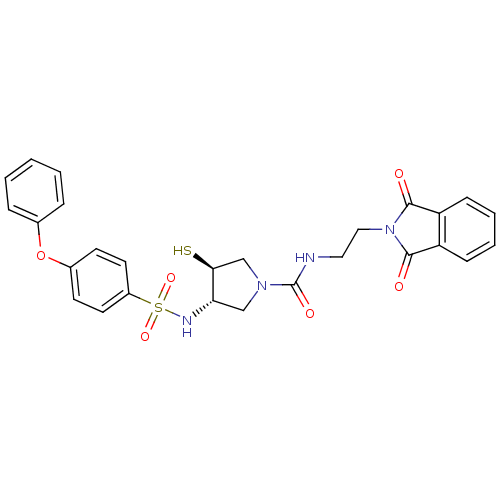 (CHEMBL2380314) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50133931 (1-(4-fluoro-phenyl)-4-(3,4,6,7,12,12a-hexahydro-1H...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 24 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50433872 (CHEMBL2380395) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50433872 (CHEMBL2380395) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 31 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50433879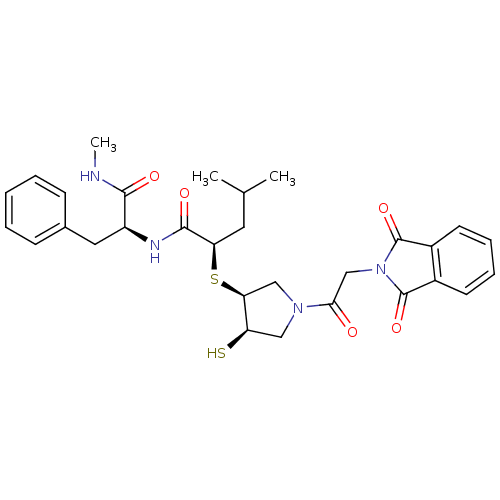 (CHEMBL2380388) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-7 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 58 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]SCH-23390 from dopamine D1 receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50433867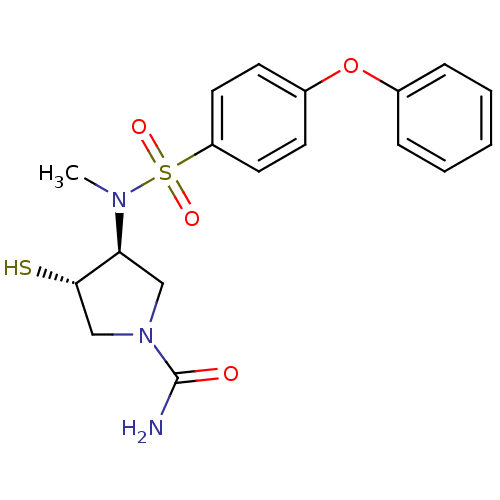 (CHEMBL2380401) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50433875 (CHEMBL2380392) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50433879 (CHEMBL2380388) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50433880 (CHEMBL2380387) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-7 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 5-hydroxytryptamine receptor 2A (Rattus norvegicus (rat)) | BDBM50477151 (CHEMBL393622) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 92 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]ketanserin from 5HT2A receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50477151 (CHEMBL393622) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 94 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM63895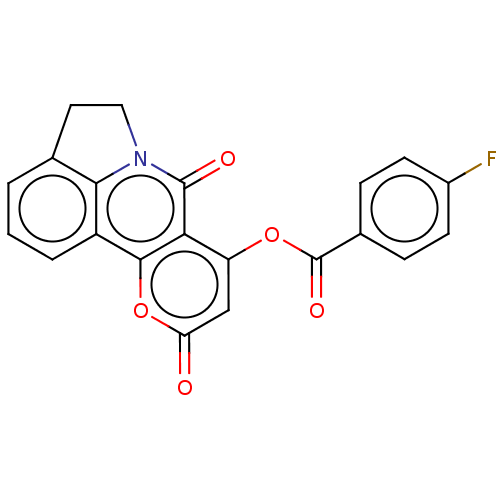 (7,10-dioxo-4,5-dihydro-7H,10H-pyrano[3,2-c]pyrrolo...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 190 | n/a | 1.41E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ecole Polytechnique Fédérale de Lausanne | Assay Description The assay principle is based on measuring the absorbance of D-dopachrome methyl ester (red), which is transformed by enzymatically active MIF to the ... | J Biol Chem 285: 26581-98 (2010) Article DOI: 10.1074/jbc.M110.113951 BindingDB Entry DOI: 10.7270/Q2XD1083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM92511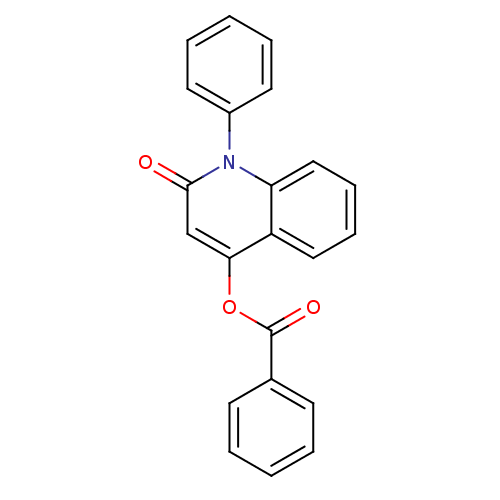 (JFD 03990, 7) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 230 | n/a | 1.55E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ecole Polytechnique Fédérale de Lausanne | Assay Description The assay principle is based on measuring the absorbance of D-dopachrome methyl ester (red), which is transformed by enzymatically active MIF to the ... | J Biol Chem 285: 26581-98 (2010) Article DOI: 10.1074/jbc.M110.113951 BindingDB Entry DOI: 10.7270/Q2XD1083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50433881 (CHEMBL2380406) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-7 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50433881 (CHEMBL2380406) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (RAT) | BDBM50133931 (1-(4-fluoro-phenyl)-4-(3,4,6,7,12,12a-hexahydro-1H...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 425 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]SCH-23390 from dopamine D1 receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM92518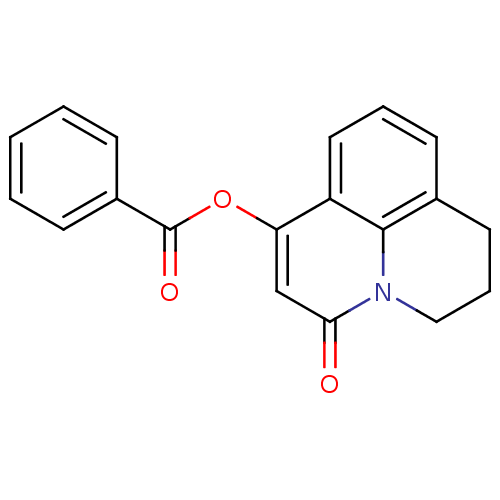 (RJC 04082, 14) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 450 | n/a | 3.02E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ecole Polytechnique Fédérale de Lausanne | Assay Description The assay principle is based on measuring the absorbance of D-dopachrome methyl ester (red), which is transformed by enzymatically active MIF to the ... | J Biol Chem 285: 26581-98 (2010) Article DOI: 10.1074/jbc.M110.113951 BindingDB Entry DOI: 10.7270/Q2XD1083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM92515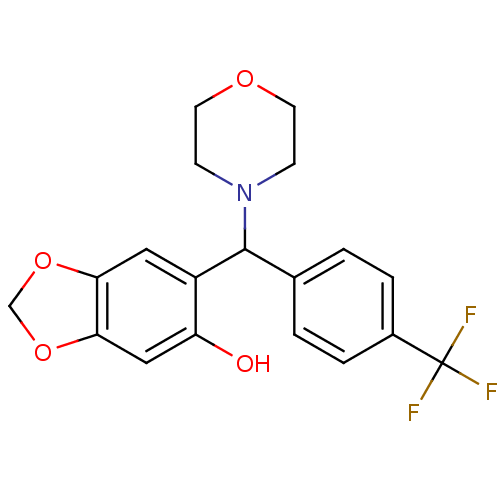 (RDR 03785, 11) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 570 | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ecole Polytechnique Fédérale de Lausanne | Assay Description The assay principle is based on measuring the absorbance of D-dopachrome methyl ester (red), which is transformed by enzymatically active MIF to the ... | J Biol Chem 285: 26581-98 (2010) Article DOI: 10.1074/jbc.M110.113951 BindingDB Entry DOI: 10.7270/Q2XD1083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM34233 (2-Phenyl-benzo[d]isoselenazol-3-one | 2-Phenyl-ben...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 570 | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ecole Polytechnique Fédérale de Lausanne | Assay Description The assay principle is based on measuring the absorbance of D-dopachrome methyl ester (red), which is transformed by enzymatically active MIF to the ... | J Biol Chem 285: 26581-98 (2010) Article DOI: 10.1074/jbc.M110.113951 BindingDB Entry DOI: 10.7270/Q2XD1083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM92516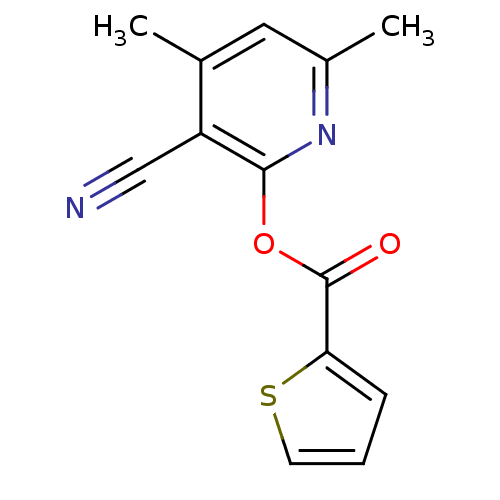 (RF 00032, 12) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 740 | n/a | 3.47E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ecole Polytechnique Fédérale de Lausanne | Assay Description The assay principle is based on measuring the absorbance of D-dopachrome methyl ester (red), which is transformed by enzymatically active MIF to the ... | J Biol Chem 285: 26581-98 (2010) Article DOI: 10.1074/jbc.M110.113951 BindingDB Entry DOI: 10.7270/Q2XD1083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM50240520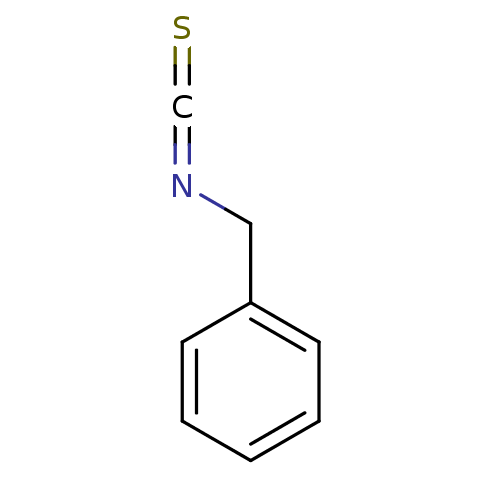 ((isothiocyanatomethyl)benzene | BITC, 17 | Benzyls...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents | Article PubMed | 790 | n/a | 6.50E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ecole Polytechnique Fédérale de Lausanne | Assay Description The assay principle is based on measuring the absorbance of D-dopachrome methyl ester (red), which is transformed by enzymatically active MIF to the ... | J Biol Chem 285: 26581-98 (2010) Article DOI: 10.1074/jbc.M110.113951 BindingDB Entry DOI: 10.7270/Q2XD1083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM92506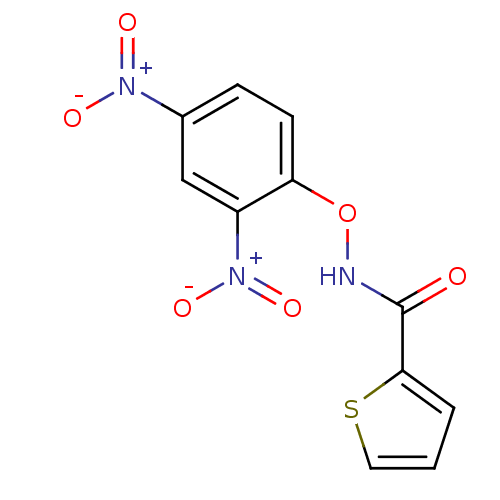 (BTB 09588, 1) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 860 | n/a | 2.45E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ecole Polytechnique Fédérale de Lausanne | Assay Description The assay principle is based on measuring the absorbance of D-dopachrome methyl ester (red), which is transformed by enzymatically active MIF to the ... | J Biol Chem 285: 26581-98 (2010) Article DOI: 10.1074/jbc.M110.113951 BindingDB Entry DOI: 10.7270/Q2XD1083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50433873 (CHEMBL2380394) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM32847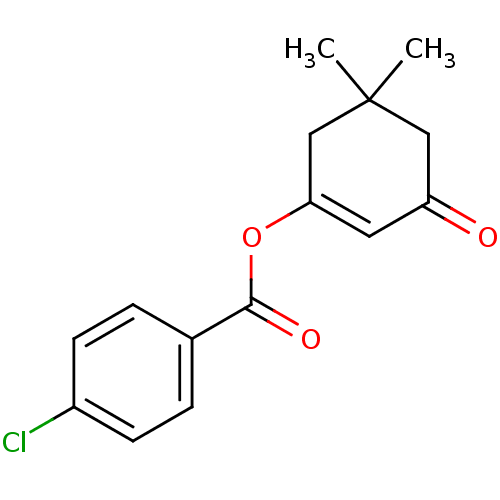 ((5,5-dimethyl-3-oxidanylidene-cyclohexen-1-yl) 4-c...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.30E+3 | n/a | 3.31E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ecole Polytechnique Fédérale de Lausanne | Assay Description The assay principle is based on measuring the absorbance of D-dopachrome methyl ester (red), which is transformed by enzymatically active MIF to the ... | J Biol Chem 285: 26581-98 (2010) Article DOI: 10.1074/jbc.M110.113951 BindingDB Entry DOI: 10.7270/Q2XD1083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM92510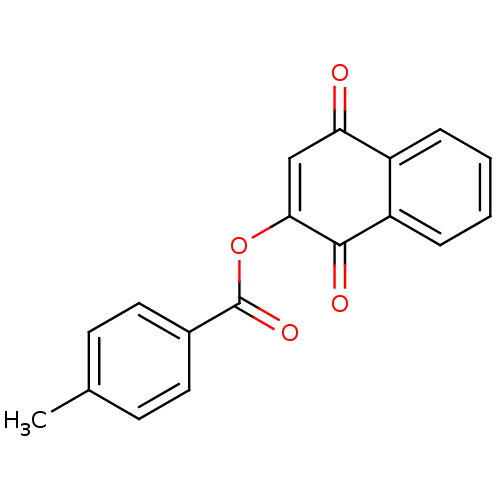 (JFD 03186, 6) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.58E+3 | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ecole Polytechnique Fédérale de Lausanne | Assay Description The assay principle is based on measuring the absorbance of D-dopachrome methyl ester (red), which is transformed by enzymatically active MIF to the ... | J Biol Chem 285: 26581-98 (2010) Article DOI: 10.1074/jbc.M110.113951 BindingDB Entry DOI: 10.7270/Q2XD1083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM92513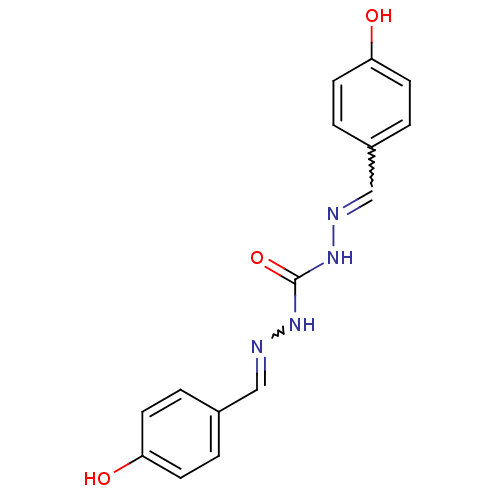 (ML 00144, 9) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | Article PubMed | 1.81E+3 | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ecole Polytechnique Fédérale de Lausanne | Assay Description The assay principle is based on measuring the absorbance of D-dopachrome methyl ester (red), which is transformed by enzymatically active MIF to the ... | J Biol Chem 285: 26581-98 (2010) Article DOI: 10.1074/jbc.M110.113951 BindingDB Entry DOI: 10.7270/Q2XD1083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM92508 (DP 00477, 3) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | 1.84E+3 | n/a | 3.31E+4 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ecole Polytechnique Fédérale de Lausanne | Assay Description The assay principle is based on measuring the absorbance of D-dopachrome methyl ester (red), which is transformed by enzymatically active MIF to the ... | J Biol Chem 285: 26581-98 (2010) Article DOI: 10.1074/jbc.M110.113951 BindingDB Entry DOI: 10.7270/Q2XD1083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(2) dopamine receptor (Rattus norvegicus (rat)) | BDBM50477152 (CHEMBL393466) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Central Drug Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]spiperone from dopamine D2 receptor in rat brain | Bioorg Med Chem 15: 7361-7 (2007) Article DOI: 10.1016/j.bmc.2007.07.018 BindingDB Entry DOI: 10.7270/Q251420V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM92507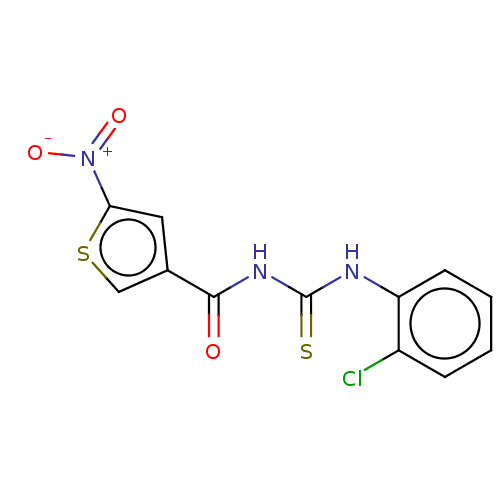 (DFP 00129, 2) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.47E+3 | n/a | 7.08E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ecole Polytechnique Fédérale de Lausanne | Assay Description The assay principle is based on measuring the absorbance of D-dopachrome methyl ester (red), which is transformed by enzymatically active MIF to the ... | J Biol Chem 285: 26581-98 (2010) Article DOI: 10.1074/jbc.M110.113951 BindingDB Entry DOI: 10.7270/Q2XD1083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Macrophage migration inhibitory factor (Homo sapiens (Human)) | BDBM92509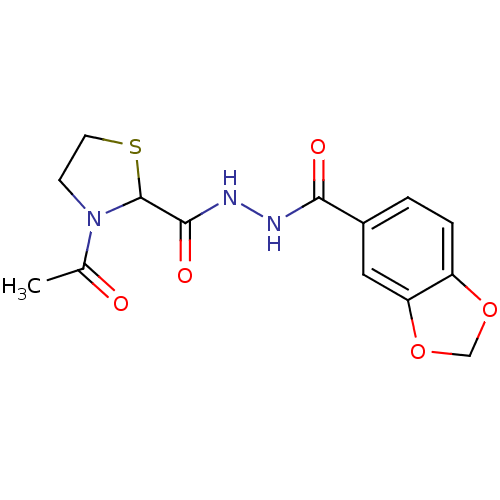 (HTS 11308, 4) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.85E+3 | n/a | 4.57E+3 | n/a | n/a | n/a | n/a | 6.5 | n/a |
Ecole Polytechnique Fédérale de Lausanne | Assay Description The assay principle is based on measuring the absorbance of D-dopachrome methyl ester (red), which is transformed by enzymatically active MIF to the ... | J Biol Chem 285: 26581-98 (2010) Article DOI: 10.1074/jbc.M110.113951 BindingDB Entry DOI: 10.7270/Q2XD1083 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50433865 (CHEMBL2380402) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-7 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-14 (Homo sapiens (Human)) | BDBM50433874 (CHEMBL2380393) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-14 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrilysin (Homo sapiens (Human)) | BDBM50433864 (CHEMBL2380403) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Florida State University Curated by ChEMBL | Assay Description Inhibition of MMP-7 (unknown origin) using Mca-PLGLDpa-AR-NH2 as substrate preincubated for 30 mins by fluorescence assay | J Med Chem 56: 4357-73 (2013) Article DOI: 10.1021/jm400529f BindingDB Entry DOI: 10.7270/Q21Z45TX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 520 total ) | Next | Last >> |