

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estrogen receptor [D538G] (Homo sapiens (Human)) | BDBM50370294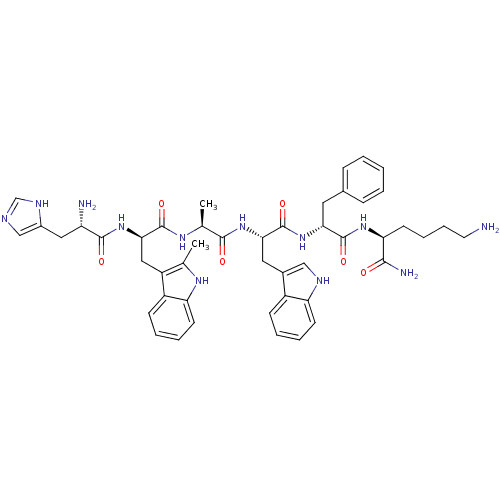 (Examorelin | HEXARELIN | US9708370, Hexareline) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 6.08 | n/a | n/a | n/a | n/a | n/a | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description GHS-R1a radioreceptor assay using LLCPK-1 membranes overexpressing GSH-R1a and radioiodinated (1-28) rat ghrelin as tracer. | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065173 ((S)-2-((S)-3-Carboxy-2-{2-[ethyl-(4-piperidin-4-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibition of radiolabeled fibrinogen binding to activated human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065188 ((S)-N-(2-Cyclohexyl-ethyl)-3-{2-[ethyl-(4-piperidi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibition of radiolabeled fibrinogen binding to activated human platelets. | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065177 ((S)-N-[(S)-1-Carboxy-2-(decahydro-naphthalen-1-yl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibition of radiolabeled fibrinogen binding to activated human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065185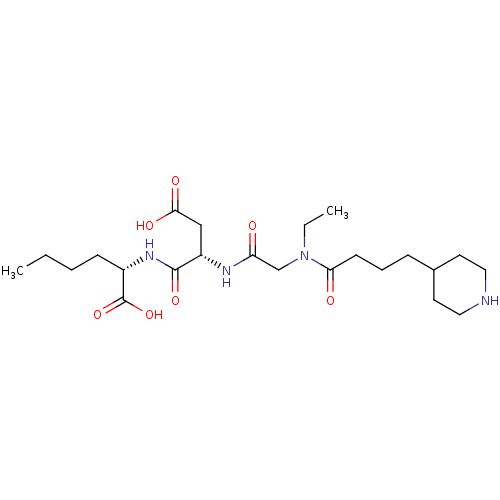 ((S)-2-((S)-3-Carboxy-2-{2-[ethyl-(4-piperidin-4-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibition of radiolabeled fibrinogen binding to activated human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065174 ((S)-N-((R)-1-Carboxy-3-cyclohexyl-propyl)-3-{2-[et...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibition of radiolabeled fibrinogen binding to activated human platelets. | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065167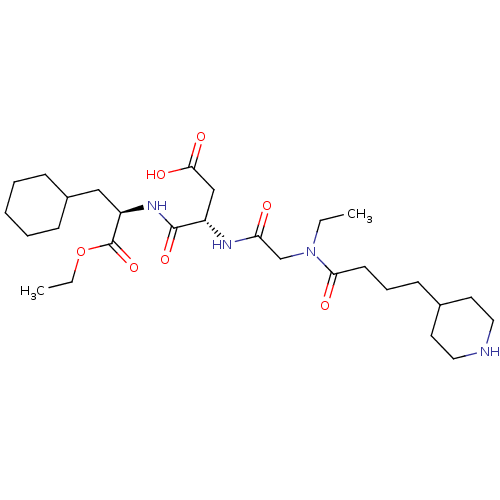 ((S)-N-((R)-2-Cyclohexyl-1-ethoxycarbonyl-ethyl)-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibition of radiolabeled fibrinogen binding to activated human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065171 ((S)-N-((S)-1-Carboxy-2-cyclohexyl-ethyl)-3-{2-[eth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibition of radiolabeled fibrinogen binding to activated human platelets. | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065177 ((S)-N-[(S)-1-Carboxy-2-(decahydro-naphthalen-1-yl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065182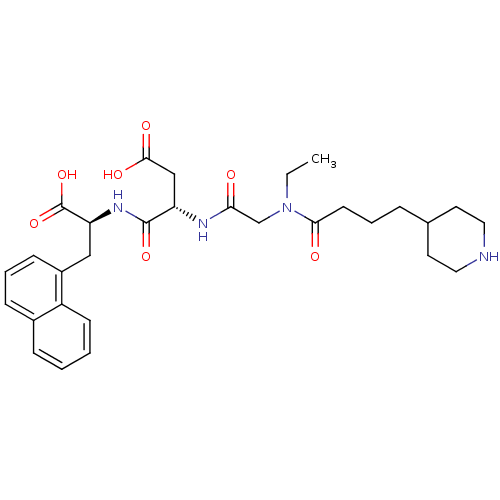 ((S)-N-((S)-1-Carboxy-2-naphthalen-1-yl-ethyl)-3-{2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibition of radiolabeled fibrinogen binding to activated human platelets. | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065172 ((S)-N-((S)-1-Carboxy-2-naphthalen-2-yl-ethyl)-3-{2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibition of radiolabeled fibrinogen binding to activated human platelets. | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065171 ((S)-N-((S)-1-Carboxy-2-cyclohexyl-ethyl)-3-{2-[eth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065173 ((S)-2-((S)-3-Carboxy-2-{2-[ethyl-(4-piperidin-4-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065166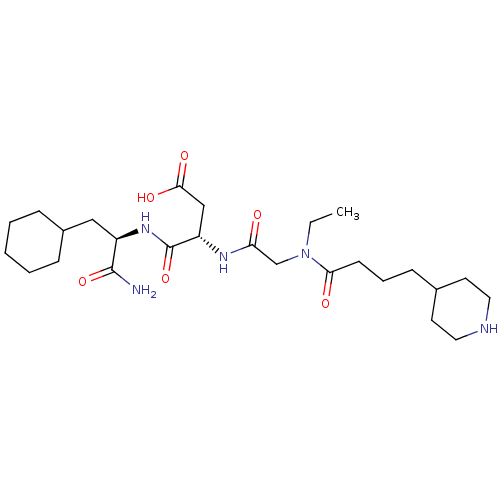 ((S)-N-((R)-1-Carbamoyl-2-cyclohexyl-ethyl)-3-{2-[e...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 28 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibition of radiolabeled fibrinogen binding to activated human platelets. | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065174 ((S)-N-((R)-1-Carboxy-3-cyclohexyl-propyl)-3-{2-[et...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065180 ((S)-N-((R)-Carboxy-cyclohexyl-methyl)-3-{2-[ethyl-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065186 ((S)-N-((R)-1-Carboxy-2-cycloheptyl-ethyl)-3-{2-[et...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065181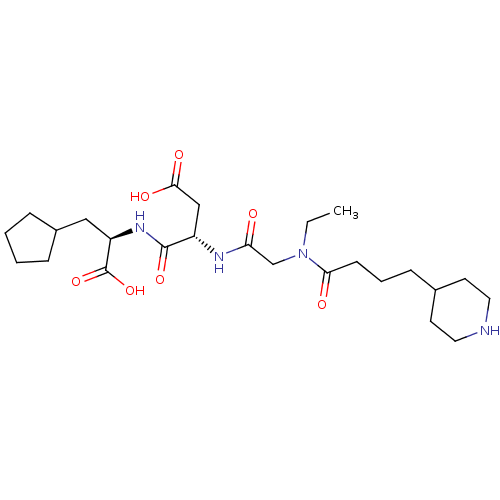 ((S)-N-((R)-1-Carboxy-2-cyclopentyl-ethyl)-3-{2-[et...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065184 ((R)-2-((S)-3-Carboxy-2-{2-[ethyl-(4-piperidin-4-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065178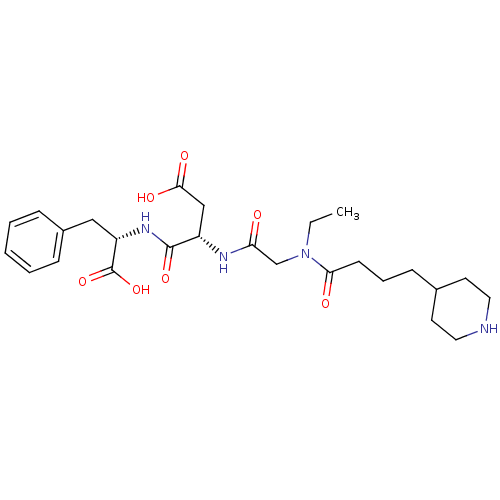 ((S)-N-((S)-1-Carboxy-2-phenyl-ethyl)-3-{2-[ethyl-(...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065167 ((S)-N-((R)-2-Cyclohexyl-1-ethoxycarbonyl-ethyl)-3-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 64 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065172 ((S)-N-((S)-1-Carboxy-2-naphthalen-2-yl-ethyl)-3-{2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065168 ((S)-2-((S)-3-Carboxy-2-{2-[ethyl-(4-piperidin-4-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibition of radiolabeled fibrinogen binding to activated human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065188 ((S)-N-(2-Cyclohexyl-ethyl)-3-{2-[ethyl-(4-piperidi...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065176 ((S)-N-((R)-1-Carboxy-2-cyclooctyl-ethyl)-3-{2-[eth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 78 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065168 ((S)-2-((S)-3-Carboxy-2-{2-[ethyl-(4-piperidin-4-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065183 ((S)-N-[(S)-1-Carboxy-2-(decahydro-naphthalen-2-yl)...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 96 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065166 ((S)-N-((R)-1-Carbamoyl-2-cyclohexyl-ethyl)-3-{2-[e...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065182 ((S)-N-((S)-1-Carboxy-2-naphthalen-1-yl-ethyl)-3-{2...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065190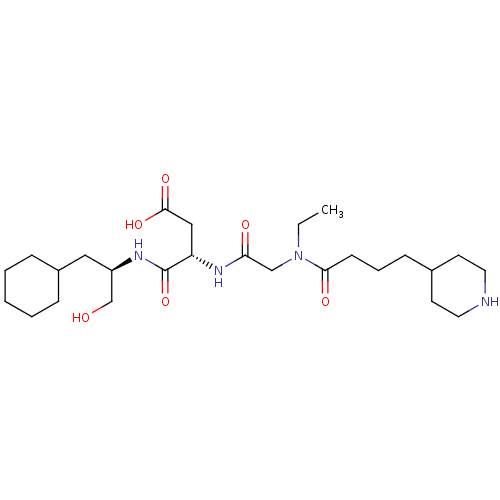 ((S)-N-((R)-2-Cyclohexyl-1-hydroxymethyl-ethyl)-3-{...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065185 ((S)-2-((S)-3-Carboxy-2-{2-[ethyl-(4-piperidin-4-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065192 ((S)-2-((S)-3-Carboxy-2-{2-[ethyl-(5-piperidin-4-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065169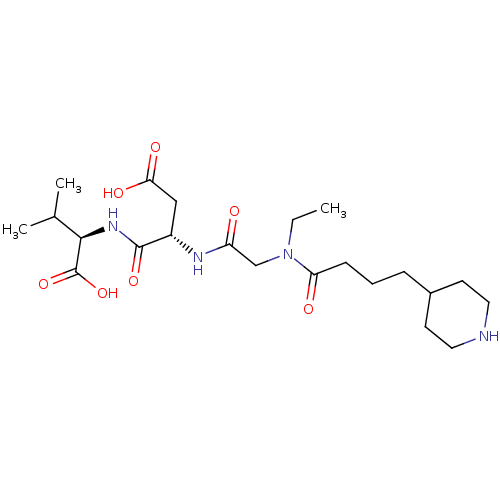 ((R)-2-((S)-3-Carboxy-2-{2-[ethyl-(4-piperidin-4-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibition of radiolabeled fibrinogen binding to activated human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065165 ((S)-2-((S)-3-Carboxy-2-{2-[ethyl-(6-guanidino-hexa...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor [D538G] (Homo sapiens (Human)) | BDBM50395024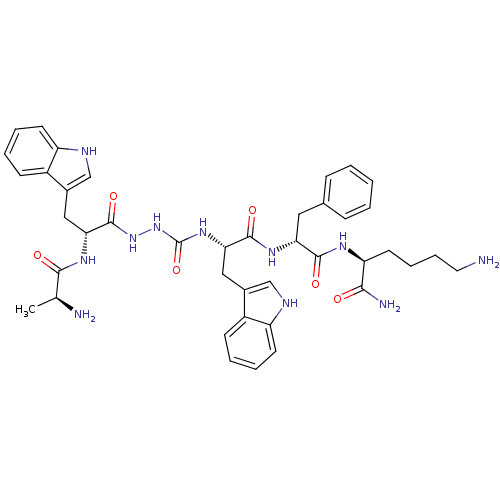 (CHEMBL2163444 | US9708370, CP-2A(v)) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 568 | n/a | n/a | n/a | n/a | n/a | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description GHS-R1a radioreceptor assay using LLCPK-1 membranes overexpressing GSH-R1a and radioiodinated (1-28) rat ghrelin as tracer. | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065187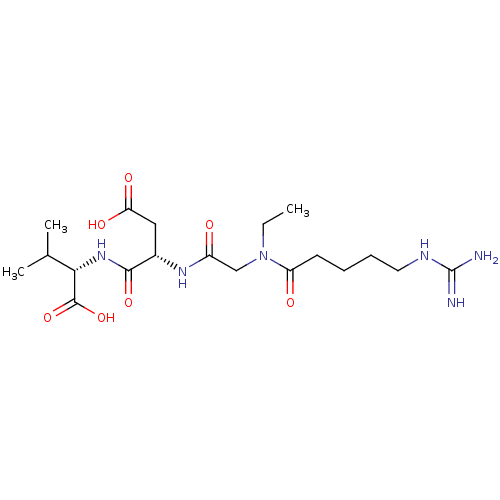 ((S)-2-((S)-3-Carboxy-2-{2-[ethyl-(5-guanidino-pent...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 630 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065169 ((R)-2-((S)-3-Carboxy-2-{2-[ethyl-(4-piperidin-4-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065189 ((S)-2-((S)-3-Carboxy-2-{2-[ethyl-(3-piperidin-4-yl...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065170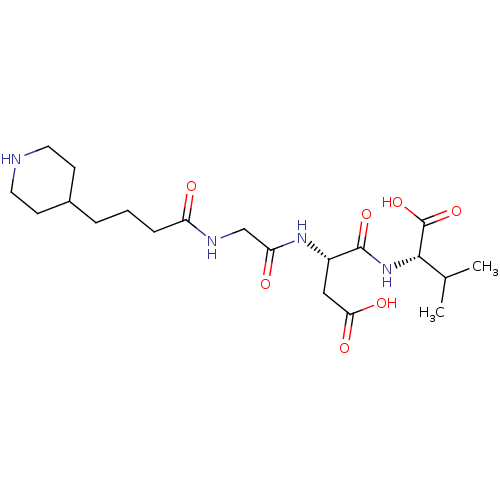 ((S)-2-{(S)-3-Carboxy-2-[2-(4-piperidin-4-yl-butyry...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395001 (CHEMBL2163478 | US9708370, DBG-253-1) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.34E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065191 ((S)-N-((R)-1-Carboxy-2-cyclohexyl-ethyl)-3-{2-[eth...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM263222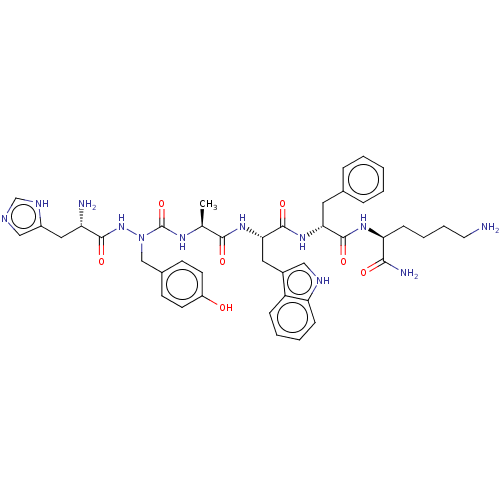 (US9708370, DBG-175p) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065179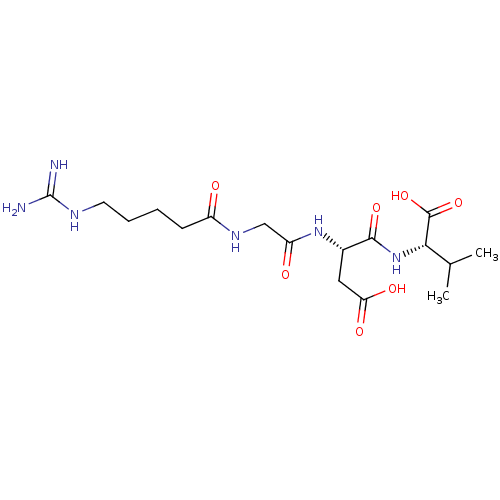 ((S)-2-{(S)-3-Carboxy-2-[2-(5-guanidino-pentanoylam...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395002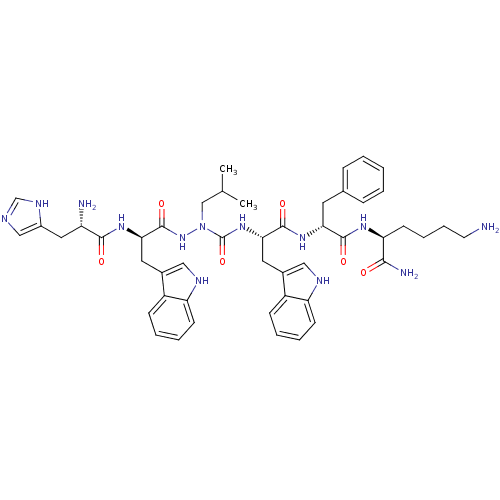 (CHEMBL2163477 | US9708370, DBG-201-A) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.89E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM263256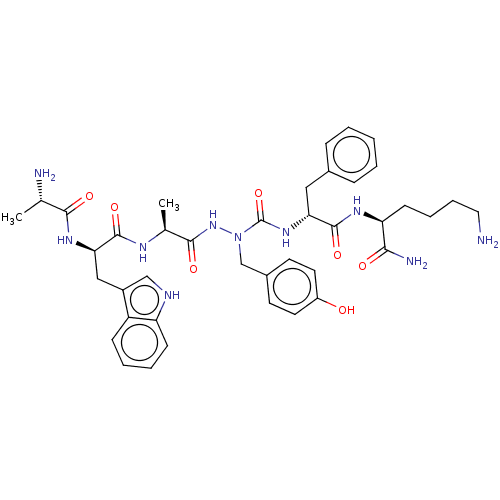 (US9708370, 67 ZS555-F40 | US9708370, ZS555-F40) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | n/a | n/a | 2.95E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50370294 (Examorelin | HEXARELIN | US9708370, Hexareline) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.97E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50370294 (Examorelin | HEXARELIN | US9708370, Hexareline) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.33E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50065175 (1-((S)-3-Carboxy-2-{2-[ethyl-(4-piperidin-4-yl-but...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Ability of the compound to inhibit the aggregation of fixed human platelets | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Integrin alpha-IIb/beta-3 (Homo sapiens (Human)) | BDBM50241180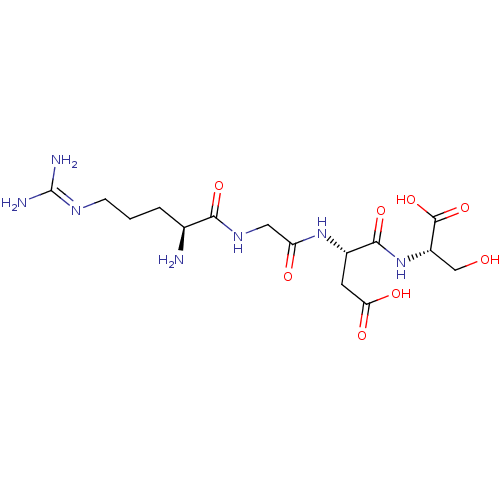 ((6S,12S,15S)-1,6-diamino-12-(carboxymethyl)-16-hyd...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer Central Research Curated by ChEMBL | Assay Description Inhibition of HUVEC adhesion to fibrinogen-coated plates | J Med Chem 41: 2492-502 (1998) Article DOI: 10.1021/jm9801096 BindingDB Entry DOI: 10.7270/Q2057F28 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Platelet glycoprotein 4 (Rattus norvegicus) | BDBM50395019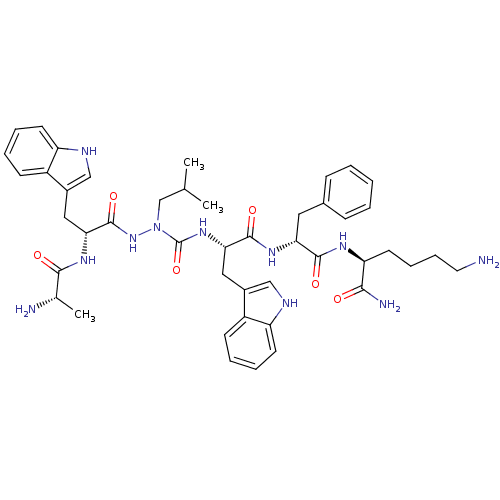 (CHEMBL2163449 | US9708370, CP-2B(v)) | PDB Reactome pathway UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.68E+3 | n/a | n/a | n/a | n/a | 7.3 | n/a |
VALORISATION-RECHERCHE, LIMITED PARTNERSHIP; VALORISATION HSJ, LIMITED PARTNERSHIP US Patent | Assay Description Competition experiments were performed by incubating 50 μg of LLC-PK1 membranes expressing human GHS-R1a with 15 fmol of 125I-Ghrelin and increa... | US Patent US9708370 (2017) BindingDB Entry DOI: 10.7270/Q2XP76ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 142 total ) | Next | Last >> |