 Found 55 hits with Last Name = 'sakkiah' and Initial = 's'
Found 55 hits with Last Name = 'sakkiah' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Renin
(Homo sapiens (Human)) | BDBM50330355
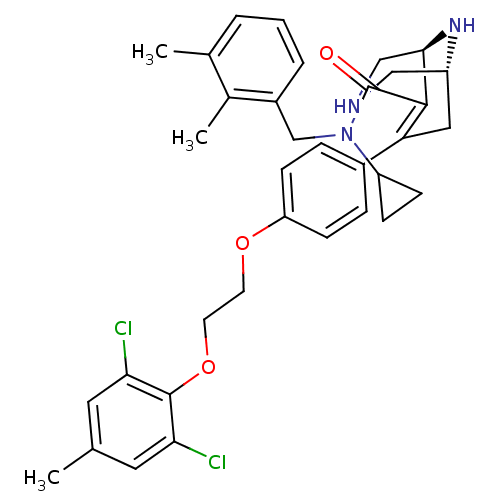
((1R,5S)-N-cyclopropyl-7-(4-(2-(2,6-dichloro-4-meth...)Show SMILES Cc1cc(Cl)c(OCCOc2ccc(cc2)C2=C([C@H]3CNC[C@@H](C2)N3)C(=O)N(Cc2cccc(C)c2C)C2CC2)c(Cl)c1 |r,t:17| Show InChI InChI=1S/C35H39Cl2N3O3/c1-21-15-30(36)34(31(37)16-21)43-14-13-42-28-11-7-24(8-12-28)29-17-26-18-38-19-32(39-26)33(29)35(41)40(27-9-10-27)20-25-6-4-5-22(2)23(25)3/h4-8,11-12,15-16,26-27,32,38-39H,9-10,13-14,17-20H2,1-3H3/t26-,32-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human renin |
Eur J Med Chem 46: 2469-76 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.035
BindingDB Entry DOI: 10.7270/Q2GM87NM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM17949
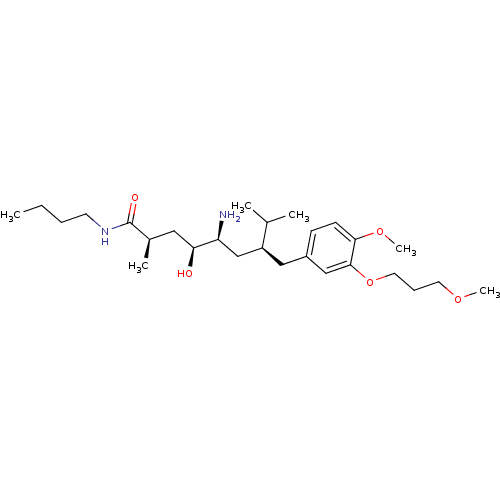
((2R,4S,5S,7S)-5-amino-N-butyl-4-hydroxy-7-{[4-meth...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@@H](N)C[C@H](Cc1ccc(OC)c(OCCCOC)c1)C(C)C |r| Show InChI InChI=1S/C27H48N2O5/c1-7-8-12-29-27(31)20(4)15-24(30)23(28)18-22(19(2)3)16-21-10-11-25(33-6)26(17-21)34-14-9-13-32-5/h10-11,17,19-20,22-24,30H,7-9,12-16,18,28H2,1-6H3,(H,29,31)/t20-,22+,23+,24+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human renin |
Eur J Med Chem 46: 2469-76 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.035
BindingDB Entry DOI: 10.7270/Q2GM87NM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM29949
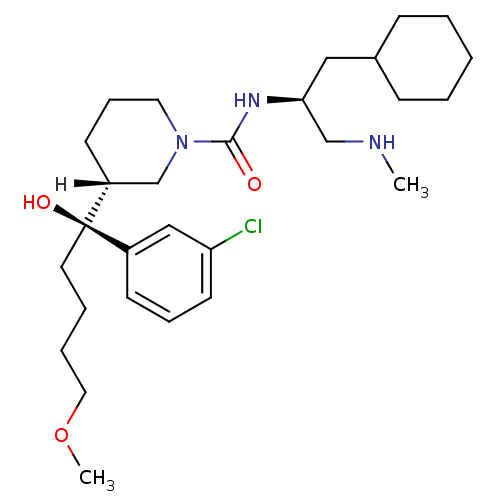
(piperidine-1-carboxamide, 21l)Show SMILES [H][C@]1(CCCN(C1)C(=O)N[C@H](CNC)CC1CCCCC1)[C@@](O)(CCCCOC)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C28H46ClN3O3/c1-30-20-26(18-22-10-4-3-5-11-22)31-27(33)32-16-9-13-24(21-32)28(34,15-6-7-17-35-2)23-12-8-14-25(29)19-23/h8,12,14,19,22,24,26,30,34H,3-7,9-11,13,15-18,20-21H2,1-2H3,(H,31,33)/t24-,26+,28-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human renin |
Eur J Med Chem 46: 2469-76 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.035
BindingDB Entry DOI: 10.7270/Q2GM87NM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116537
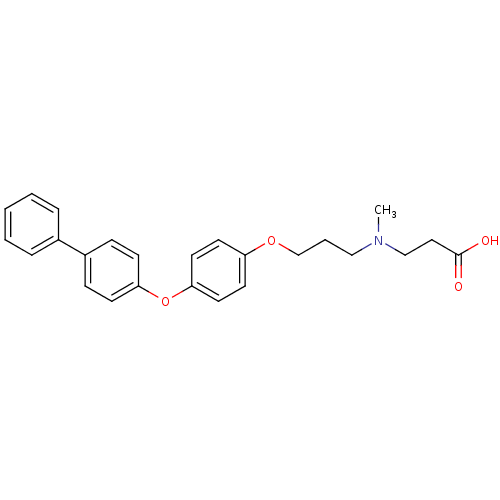
(3-({3-[4-(Biphenyl-4-yloxy)-phenoxy]-propyl}-methy...)Show SMILES CN(CCCOc1ccc(Oc2ccc(cc2)-c2ccccc2)cc1)CCC(O)=O Show InChI InChI=1S/C25H27NO4/c1-26(18-16-25(27)28)17-5-19-29-22-12-14-24(15-13-22)30-23-10-8-21(9-11-23)20-6-3-2-4-7-20/h2-4,6-15H,5,16-19H2,1H3,(H,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17950

((2S,4S,5S,7S)-5-amino-N-(2-carbamoyl-2,2-dimethyle...)Show SMILES COCCCOc1cc(C[C@@H](C[C@H](N)[C@@H](O)C[C@@H](C(C)C)C(=O)NCC(C)(C)C(N)=O)C(C)C)ccc1OC |r| Show InChI InChI=1S/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23-,24-,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human renin |
Eur J Med Chem 46: 2469-76 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.035
BindingDB Entry DOI: 10.7270/Q2GM87NM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50345192
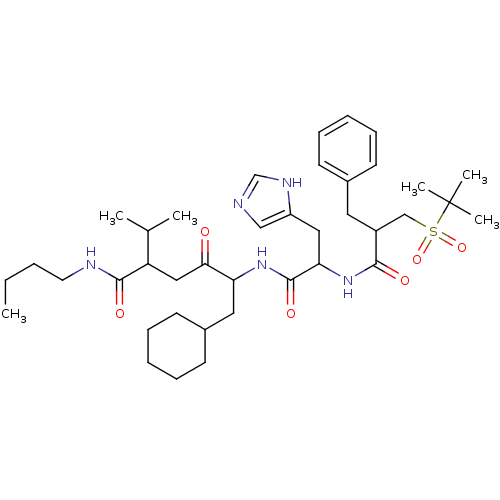
(5-(2-(2-benzyl-3-(tert-butylsulfonyl)propanamido)-...)Show SMILES CCCCNC(=O)C(CC(=O)C(CC1CCCCC1)NC(=O)C(Cc1cnc[nH]1)NC(=O)C(Cc1ccccc1)CS(=O)(=O)C(C)(C)C)C(C)C Show InChI InChI=1S/C39H61N5O6S/c1-7-8-19-41-37(47)32(27(2)3)23-35(45)33(21-29-17-13-10-14-18-29)43-38(48)34(22-31-24-40-26-42-31)44-36(46)30(20-28-15-11-9-12-16-28)25-51(49,50)39(4,5)6/h9,11-12,15-16,24,26-27,29-30,32-34H,7-8,10,13-14,17-23,25H2,1-6H3,(H,40,42)(H,41,47)(H,43,48)(H,44,46) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human renin |
Eur J Med Chem 46: 2469-76 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.035
BindingDB Entry DOI: 10.7270/Q2GM87NM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM17943
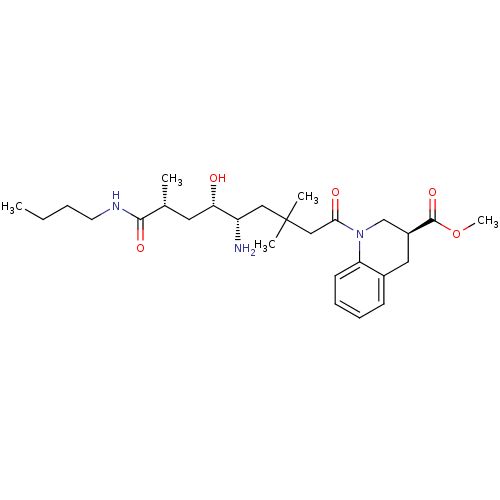
(Renin nonpeptide inhibitor, 3 | methyl (3S)-1-[(5S...)Show SMILES CCCCNC(=O)[C@H](C)C[C@H](O)[C@@H](N)CC(C)(C)CC(=O)N1C[C@H](Cc2ccccc12)C(=O)OC |r| Show InChI InChI=1S/C27H43N3O5/c1-6-7-12-29-25(33)18(2)13-23(31)21(28)15-27(3,4)16-24(32)30-17-20(26(34)35-5)14-19-10-8-9-11-22(19)30/h8-11,18,20-21,23,31H,6-7,12-17,28H2,1-5H3,(H,29,33)/t18-,20+,21+,23+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human renin |
Eur J Med Chem 46: 2469-76 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.035
BindingDB Entry DOI: 10.7270/Q2GM87NM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Renin
(Homo sapiens (Human)) | BDBM50345193
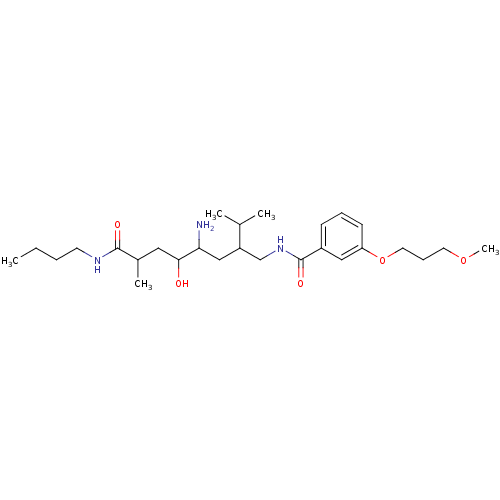
(CHEMBL1783186 | N-(4-amino-8-(butylamino)-5-hydrox...)Show SMILES CCCCNC(=O)C(C)CC(O)C(N)CC(CNC(=O)c1cccc(OCCCOC)c1)C(C)C Show InChI InChI=1S/C27H47N3O5/c1-6-7-12-29-26(32)20(4)15-25(31)24(28)17-22(19(2)3)18-30-27(33)21-10-8-11-23(16-21)35-14-9-13-34-5/h8,10-11,16,19-20,22,24-25,31H,6-7,9,12-15,17-18,28H2,1-5H3,(H,29,32)(H,30,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human renin |
Eur J Med Chem 46: 2469-76 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.035
BindingDB Entry DOI: 10.7270/Q2GM87NM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Renin
(Homo sapiens (Human)) | BDBM50077669

((S)-2-Benzyl-N-[(S)-1-((1S,2R,3S)-1-cyclohexylmeth...)Show SMILES CC(C)(C)S(=O)(=O)C[C@@H](Cc1ccccc1)C(=O)N[C@@H](Cc1cnc[nH]1)C(=O)N[C@@H](CC1CCCCC1)[C@@H](O)[C@@H](O)C1CC1 Show InChI InChI=1S/C33H50N4O6S/c1-33(2,3)44(42,43)20-25(16-22-10-6-4-7-11-22)31(40)37-28(18-26-19-34-21-35-26)32(41)36-27(17-23-12-8-5-9-13-23)30(39)29(38)24-14-15-24/h4,6-7,10-11,19,21,23-25,27-30,38-39H,5,8-9,12-18,20H2,1-3H3,(H,34,35)(H,36,41)(H,37,40)/t25-,27+,28+,29+,30-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human renin |
Eur J Med Chem 46: 2469-76 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.035
BindingDB Entry DOI: 10.7270/Q2GM87NM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116560

(3-{Methyl-[3-(4-thiophen-3-ylmethyl-phenoxy)-propy...)Show InChI InChI=1S/C18H23NO3S/c1-19(10-7-18(20)21)9-2-11-22-17-5-3-15(4-6-17)13-16-8-12-23-14-16/h3-6,8,12,14H,2,7,9-11,13H2,1H3,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM17946
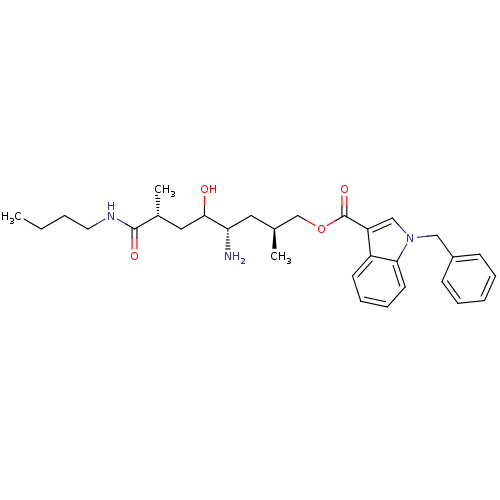
((2S,4S,7R)-4-amino-7-(butylcarbamoyl)-5-hydroxy-2,...)Show SMILES CCCCNC(=O)[C@H](C)CC(O)[C@@H](N)C[C@H](C)COC(=O)c1cn(Cc2ccccc2)c2ccccc12 |r| Show InChI InChI=1S/C30H41N3O4/c1-4-5-15-32-29(35)22(3)17-28(34)26(31)16-21(2)20-37-30(36)25-19-33(18-23-11-7-6-8-12-23)27-14-10-9-13-24(25)27/h6-14,19,21-22,26,28,34H,4-5,15-18,20,31H2,1-3H3,(H,32,35)/t21-,22+,26-,28?/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human renin |
Eur J Med Chem 46: 2469-76 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.035
BindingDB Entry DOI: 10.7270/Q2GM87NM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50342776

(4-((3-(4-(biphenyl-4-yloxy)phenylamino)-6-azabicyc...)Show SMILES OC(=O)c1ccc(CN2CC3CC(Nc4ccc(Oc5ccc(cc5)-c5ccccc5)cc4)C2CCC3)cc1 |THB:7:8:12.11:34.35.36| Show InChI InChI=1S/C34H34N2O3/c37-34(38)28-11-9-24(10-12-28)22-36-23-25-5-4-8-33(36)32(21-25)35-29-15-19-31(20-16-29)39-30-17-13-27(14-18-30)26-6-2-1-3-7-26/h1-3,6-7,9-20,25,32-33,35H,4-5,8,21-23H2,(H,37,38) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251446
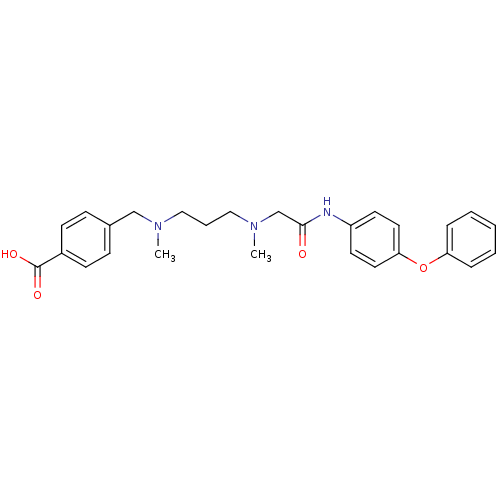
(4-((methyl(3-(methyl(2-oxo-2-(4-phenoxyphenylamino...)Show SMILES CN(CCCN(C)Cc1ccc(cc1)C(O)=O)CC(=O)Nc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C27H31N3O4/c1-29(19-21-9-11-22(12-10-21)27(32)33)17-6-18-30(2)20-26(31)28-23-13-15-25(16-14-23)34-24-7-4-3-5-8-24/h3-5,7-16H,6,17-20H2,1-2H3,(H,28,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251500
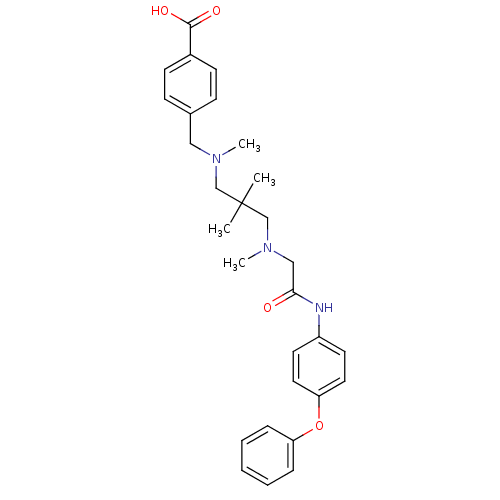
(4-(((2,2-dimethyl-3-(methyl(2-oxo-2-(4-phenoxyphen...)Show SMILES CN(CC(=O)Nc1ccc(Oc2ccccc2)cc1)CC(C)(C)CN(C)Cc1ccc(cc1)C(O)=O Show InChI InChI=1S/C29H35N3O4/c1-29(2,20-31(3)18-22-10-12-23(13-11-22)28(34)35)21-32(4)19-27(33)30-24-14-16-26(17-15-24)36-25-8-6-5-7-9-25/h5-17H,18-21H2,1-4H3,(H,30,33)(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50330347
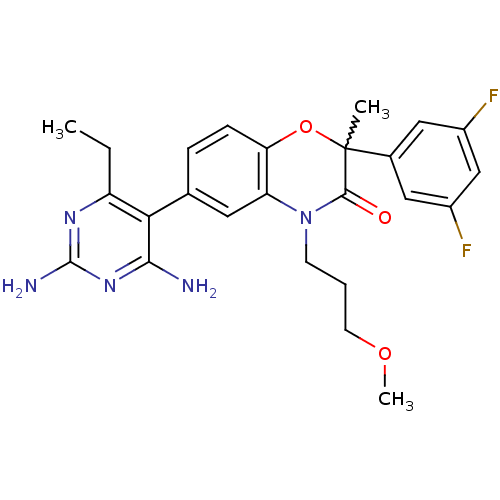
(6-(2,4-diamino-6-ethyl-pyrimidin-5-yl)-2-(3,5-difl...)Show SMILES CCc1nc(N)nc(N)c1-c1ccc2OC(C)(C(=O)N(CCCOC)c2c1)c1cc(F)cc(F)c1 |w:15.16| Show InChI InChI=1S/C25H27F2N5O3/c1-4-18-21(22(28)31-24(29)30-18)14-6-7-20-19(10-14)32(8-5-9-34-3)23(33)25(2,35-20)15-11-16(26)13-17(27)12-15/h6-7,10-13H,4-5,8-9H2,1-3H3,(H4,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human renin |
Eur J Med Chem 46: 2469-76 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.035
BindingDB Entry DOI: 10.7270/Q2GM87NM |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50342777
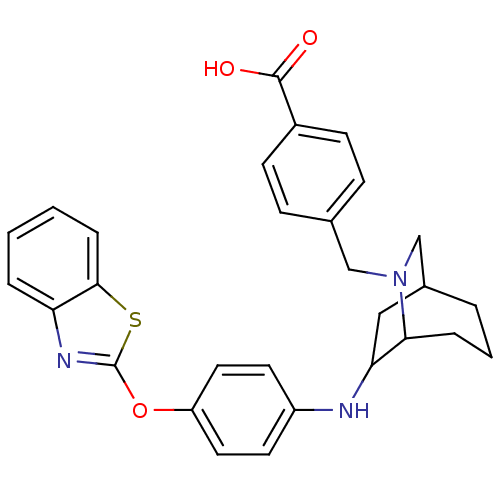
(4-((3-(4-(benzo[d]thiazol-2-yloxy)phenylamino)-6-a...)Show SMILES OC(=O)c1ccc(CN2CC3CC(Nc4ccc(Oc5nc6ccccc6s5)cc4)C2CCC3)cc1 |THB:7:8:12.11:31.32.33| Show InChI InChI=1S/C29H29N3O3S/c33-28(34)21-10-8-19(9-11-21)17-32-18-20-4-3-6-26(32)25(16-20)30-22-12-14-23(15-13-22)35-29-31-24-5-1-2-7-27(24)36-29/h1-2,5,7-15,20,25-26,30H,3-4,6,16-18H2,(H,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50125420

(5-[2-(4-Benzyl-phenoxy)-ethyl]-5H-imidazo[4,5-c]py...)Show InChI InChI=1S/C21H19N3O/c1-2-4-17(5-3-1)14-18-6-8-19(9-7-18)25-13-12-24-11-10-20-21(15-24)23-16-22-20/h1-11,15-16H,12-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251501
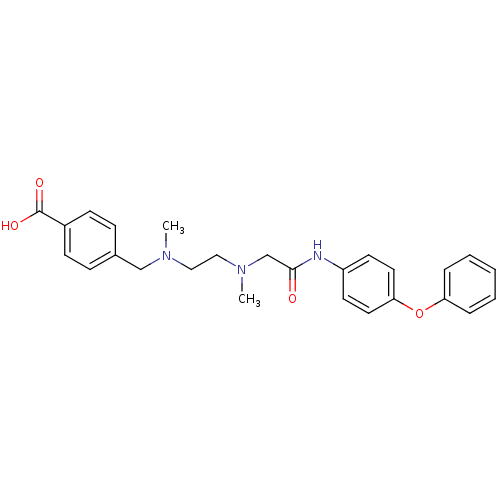
(4-((methyl(2-(methyl(2-oxo-2-(4-phenoxyphenylamino...)Show SMILES CN(CCN(C)Cc1ccc(cc1)C(O)=O)CC(=O)Nc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C26H29N3O4/c1-28(18-20-8-10-21(11-9-20)26(31)32)16-17-29(2)19-25(30)27-22-12-14-24(15-13-22)33-23-6-4-3-5-7-23/h3-15H,16-19H2,1-2H3,(H,27,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116531

(3-[4-(4-Benzyl-phenoxy)-butylamino]-propionic acid...)Show InChI InChI=1S/C20H25NO3/c22-20(23)12-14-21-13-4-5-15-24-19-10-8-18(9-11-19)16-17-6-2-1-3-7-17/h1-3,6-11,21H,4-5,12-16H2,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251825

(4-((methyl(3-(methyl(2-oxo-2-(4-phenoxyphenylamino...)Show SMILES CN(CCCN(C)CC(=O)Nc1ccc(Oc2ccccc2)cc1)CC1CCC(CC1)C(O)=O |(7.49,-15.66,;7.49,-14.12,;6.16,-13.35,;4.83,-14.12,;3.49,-13.35,;2.16,-14.12,;2.16,-15.66,;.83,-13.35,;-.51,-14.11,;-.51,-15.65,;-1.84,-13.34,;-3.18,-14.11,;-4.52,-13.34,;-5.85,-14.11,;-5.85,-15.66,;-7.18,-16.43,;-8.51,-15.65,;-9.85,-16.43,;-11.18,-15.66,;-11.18,-14.12,;-9.83,-13.35,;-8.51,-14.12,;-4.52,-16.43,;-3.18,-15.66,;8.83,-13.35,;10.16,-14.12,;10.15,-15.66,;11.48,-16.43,;12.81,-15.67,;12.82,-14.13,;11.49,-13.35,;14.15,-16.45,;14.14,-17.99,;15.48,-15.68,)| Show InChI InChI=1S/C27H37N3O4/c1-29(19-21-9-11-22(12-10-21)27(32)33)17-6-18-30(2)20-26(31)28-23-13-15-25(16-14-23)34-24-7-4-3-5-8-24/h3-5,7-8,13-16,21-22H,6,9-12,17-20H2,1-2H3,(H,28,31)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM18041
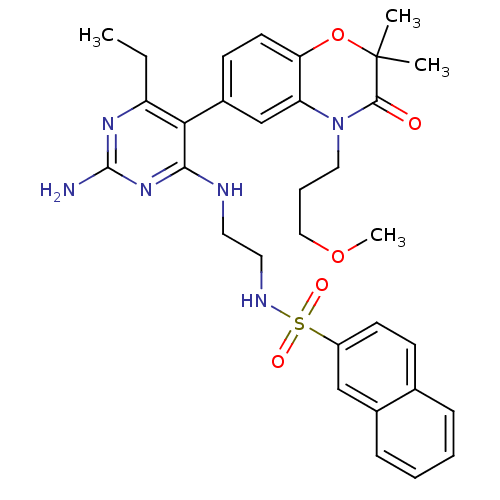
(N-[2-({2-amino-6-ethyl-5-[4-(3-methoxypropyl)-2,2-...)Show SMILES CCc1nc(N)nc(NCCNS(=O)(=O)c2ccc3ccccc3c2)c1-c1ccc2OC(C)(C)C(=O)N(CCCOC)c2c1 Show InChI InChI=1S/C32H38N6O5S/c1-5-25-28(23-12-14-27-26(20-23)38(17-8-18-42-4)30(39)32(2,3)43-27)29(37-31(33)36-25)34-15-16-35-44(40,41)24-13-11-21-9-6-7-10-22(21)19-24/h6-7,9-14,19-20,35H,5,8,15-18H2,1-4H3,(H3,33,34,36,37) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human renin |
Eur J Med Chem 46: 2469-76 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.035
BindingDB Entry DOI: 10.7270/Q2GM87NM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50121023

(1-[2-(4-Benzyl-phenoxy)-ethyl]-pyrrolidin-3-ol | C...)Show InChI InChI=1S/C19H23NO2/c21-18-10-11-20(15-18)12-13-22-19-8-6-17(7-9-19)14-16-4-2-1-3-5-16/h1-9,18,21H,10-15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50251387
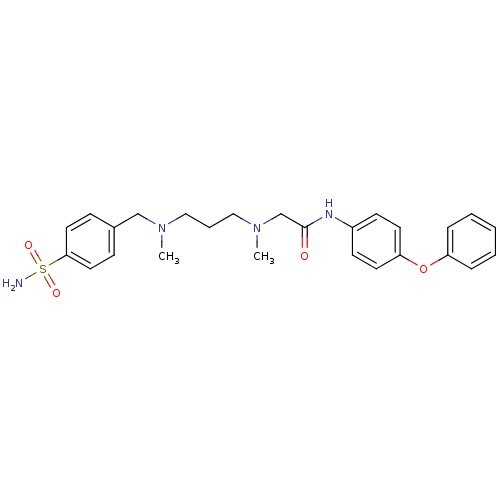
(2-(Methyl-{3-[methyl-(4-sulfamoyl-benzyl)-amino]-p...)Show SMILES CN(CCCN(C)Cc1ccc(cc1)S(N)(=O)=O)CC(=O)Nc1ccc(Oc2ccccc2)cc1 Show InChI InChI=1S/C26H32N4O4S/c1-29(19-21-9-15-25(16-10-21)35(27,32)33)17-6-18-30(2)20-26(31)28-22-11-13-24(14-12-22)34-23-7-4-3-5-8-23/h3-5,7-16H,6,17-20H2,1-2H3,(H,28,31)(H2,27,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 33 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Renin
(Homo sapiens (Human)) | BDBM50345194
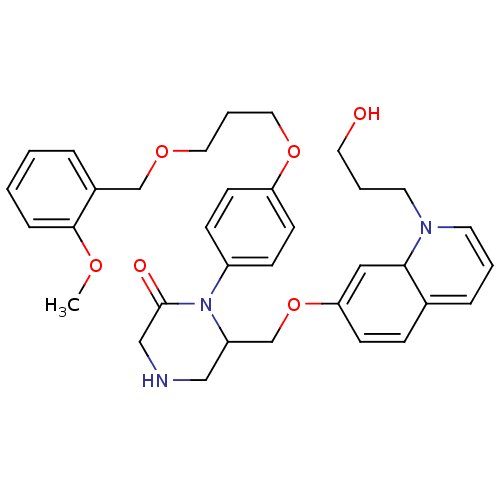
(6-((1-(3-hydroxypropyl)-1,8a-dihydroquinolin-7-ylo...)Show SMILES COc1ccccc1COCCCOc1ccc(cc1)N1C(COC2=CC3N(CCCO)C=CC=C3C=C2)CNCC1=O |c:34,36,39,t:26| Show InChI InChI=1S/C34H41N3O6/c1-40-33-9-3-2-7-27(33)24-41-19-6-20-42-30-14-11-28(12-15-30)37-29(22-35-23-34(37)39)25-43-31-13-10-26-8-4-16-36(17-5-18-38)32(26)21-31/h2-4,7-16,21,29,32,35,38H,5-6,17-20,22-25H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human renin |
Eur J Med Chem 46: 2469-76 (2011)
Article DOI: 10.1016/j.ejmech.2011.03.035
BindingDB Entry DOI: 10.7270/Q2GM87NM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085255
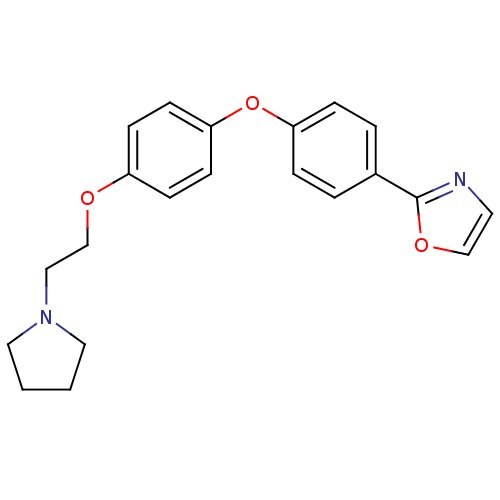
(2-{4-[4-(2-Pyrrolidin-1-yl-ethoxy)-phenoxy]-phenyl...)Show InChI InChI=1S/C21H22N2O3/c1-2-13-23(12-1)14-16-24-18-7-9-20(10-8-18)26-19-5-3-17(4-6-19)21-22-11-15-25-21/h3-11,15H,1-2,12-14,16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50121008
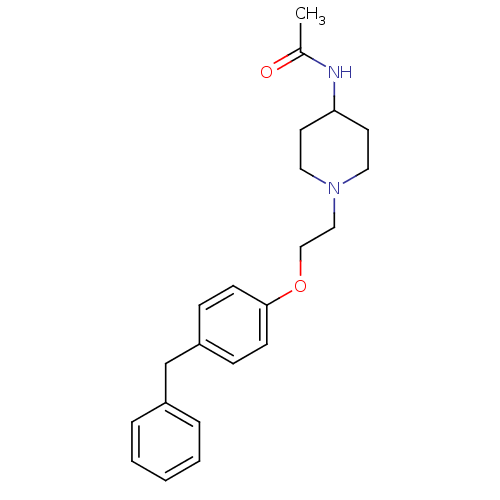
(CHEMBL114563 | N-{1-[2-(4-Benzyl-phenoxy)-ethyl]-p...)Show InChI InChI=1S/C22H28N2O2/c1-18(25)23-21-11-13-24(14-12-21)15-16-26-22-9-7-20(8-10-22)17-19-5-3-2-4-6-19/h2-10,21H,11-17H2,1H3,(H,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM24797
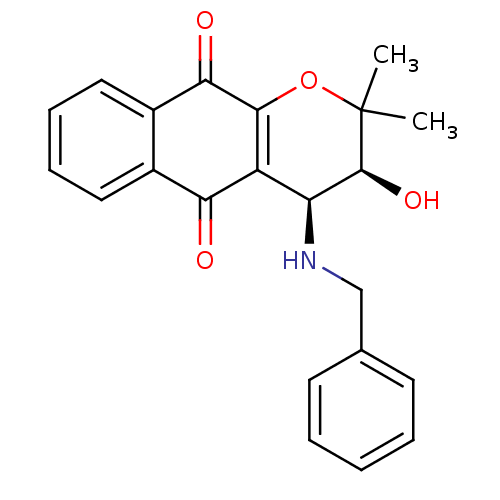
((3S,4S)-4-(benzylamino)-3-hydroxy-2,2-dimethyl-2H,...)Show SMILES CC1(C)OC2=C([C@H](NCc3ccccc3)[C@@H]1O)C(=O)c1ccccc1C2=O |r,c:4| Show InChI InChI=1S/C22H21NO4/c1-22(2)21(26)17(23-12-13-8-4-3-5-9-13)16-18(24)14-10-6-7-11-15(14)19(25)20(16)27-22/h3-11,17,21,23,26H,12H2,1-2H3/t17-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 55 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University
Curated by ChEMBL
| Assay Description
Inhibition of 2,3 dioxygenase |
Eur J Med Chem 45: 4004-12 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.057
BindingDB Entry DOI: 10.7270/Q2HX1CW5 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50121005
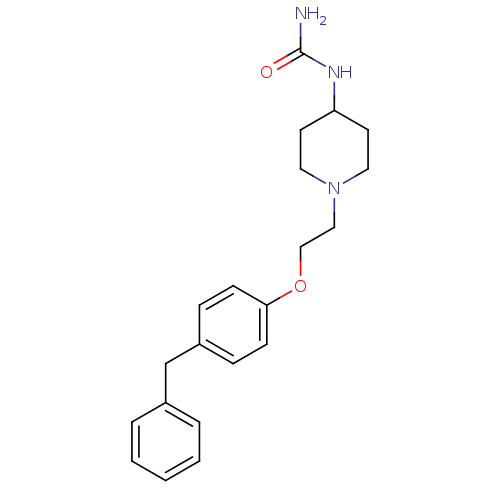
(CHEMBL115227 | {1-[2-(4-Benzyl-phenoxy)-ethyl]-pip...)Show InChI InChI=1S/C21H27N3O2/c22-21(25)23-19-10-12-24(13-11-19)14-15-26-20-8-6-18(7-9-20)16-17-4-2-1-3-5-17/h1-9,19H,10-16H2,(H3,22,23,25) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116557
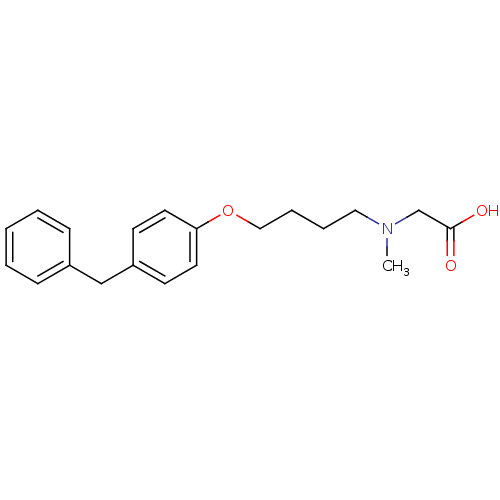
(CHEMBL117455 | {[4-(4-Benzyl-phenoxy)-butyl]-methy...)Show InChI InChI=1S/C20H25NO3/c1-21(16-20(22)23)13-5-6-14-24-19-11-9-18(10-12-19)15-17-7-3-2-4-8-17/h2-4,7-12H,5-6,13-16H2,1H3,(H,22,23) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM24801
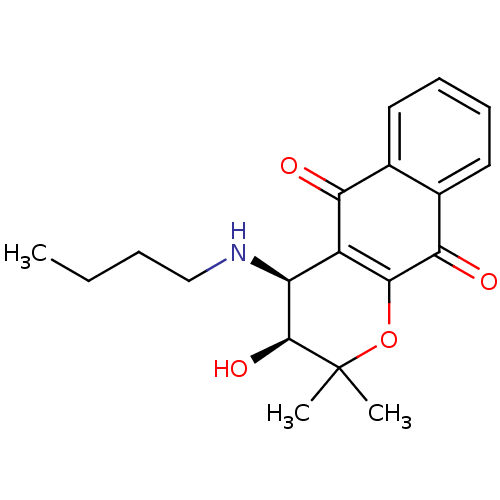
((3S,4S)-4-(butylamino)-3-hydroxy-2,2-dimethyl-2H,3...)Show SMILES CCCCN[C@@H]1[C@H](O)C(C)(C)OC2=C1C(=O)c1ccccc1C2=O |r,c:12| Show InChI InChI=1S/C19H23NO4/c1-4-5-10-20-14-13-15(21)11-8-6-7-9-12(11)16(22)17(13)24-19(2,3)18(14)23/h6-9,14,18,20,23H,4-5,10H2,1-3H3/t14-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University
Curated by ChEMBL
| Assay Description
Inhibition of 2,3 dioxygenase |
Eur J Med Chem 45: 4004-12 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.057
BindingDB Entry DOI: 10.7270/Q2HX1CW5 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116540
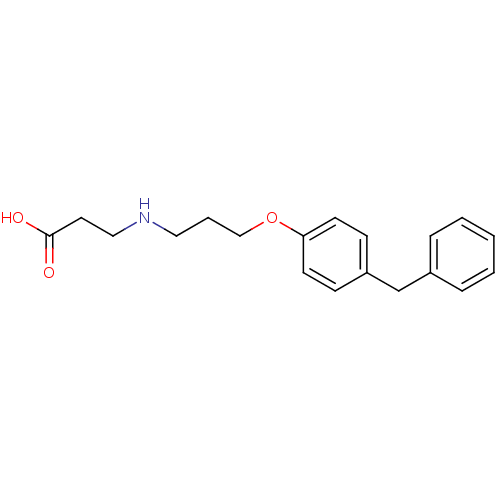
(3-[3-(4-Benzyl-phenoxy)-propylamino]-propionic aci...)Show InChI InChI=1S/C19H23NO3/c21-19(22)11-13-20-12-4-14-23-18-9-7-17(8-10-18)15-16-5-2-1-3-6-16/h1-3,5-10,20H,4,11-15H2,(H,21,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085266

(4-[4-(2-Pyrrolidin-1-yl-ethoxy)-benzyl]-thiazole |...)Show InChI InChI=1S/C16H20N2OS/c1-2-8-18(7-1)9-10-19-16-5-3-14(4-6-16)11-15-12-20-13-17-15/h3-6,12-13H,1-2,7-11H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM24788
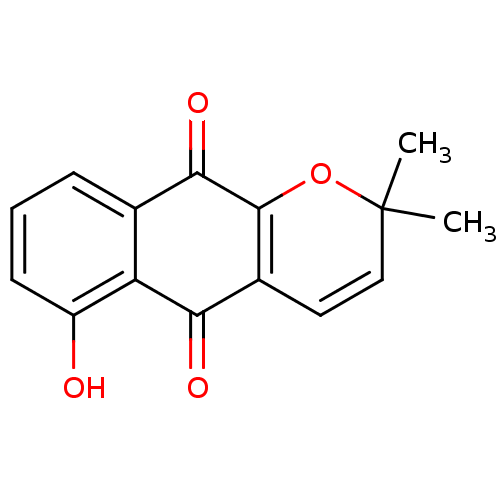
(6-hydroxy-2,2-dimethyl-2H,5H,10H-benzo[g]chromene-...)Show SMILES CC1(C)OC2=C(C=C1)C(=O)c1c(O)cccc1C2=O |c:4,6| Show InChI InChI=1S/C15H12O4/c1-15(2)7-6-9-12(17)11-8(4-3-5-10(11)16)13(18)14(9)19-15/h3-7,16H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University
Curated by ChEMBL
| Assay Description
Inhibition of 2,3 dioxygenase |
Eur J Med Chem 45: 4004-12 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.057
BindingDB Entry DOI: 10.7270/Q2HX1CW5 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116547
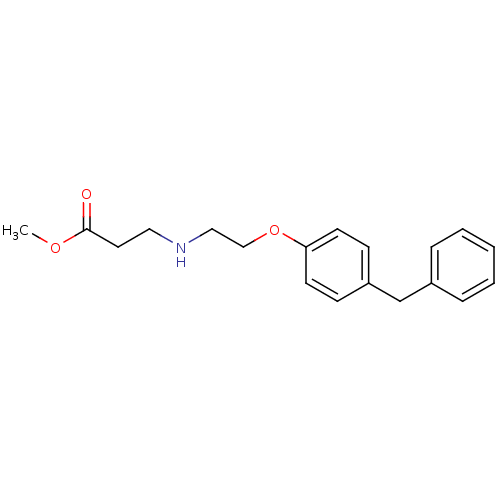
(3-[2-(4-Benzyl-phenoxy)-ethylamino]-propionic acid...)Show InChI InChI=1S/C19H23NO3/c1-22-19(21)11-12-20-13-14-23-18-9-7-17(8-10-18)15-16-5-3-2-4-6-16/h2-10,20H,11-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
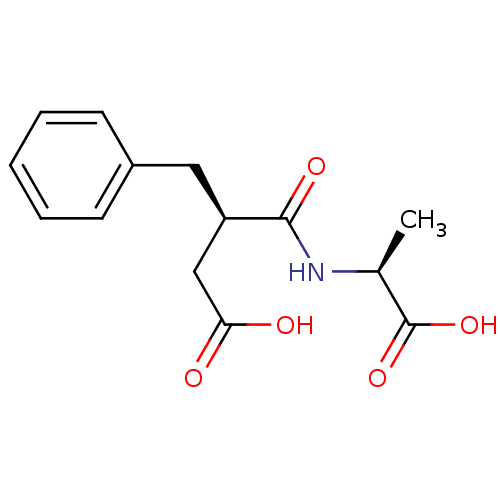
(Homo sapiens (Human)) | BDBM50285633
((R)-3-Benzyl-N-((S)-1-carboxy-ethyl)-succinamic ac...)Show SMILES C[C@H](NC(=O)[C@@H](CC(O)=O)Cc1ccccc1)C(O)=O Show InChI InChI=1S/C14H17NO5/c1-9(14(19)20)15-13(18)11(8-12(16)17)7-10-5-3-2-4-6-10/h2-6,9,11H,7-8H2,1H3,(H,15,18)(H,16,17)(H,19,20)/t9-,11+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
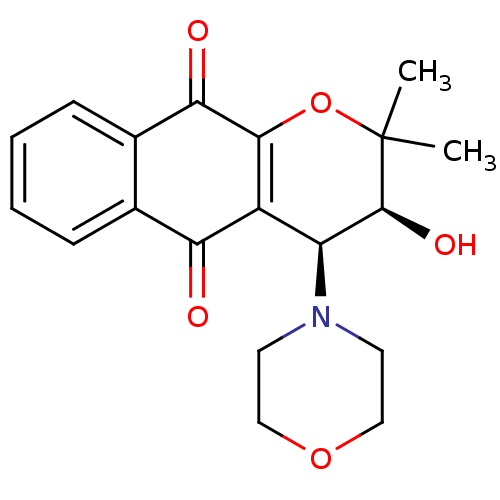
(Homo sapiens (Human)) | BDBM24803
((3S,4S)-3-hydroxy-2,2-dimethyl-4-(morpholin-4-yl)-...)Show SMILES CC1(C)OC2=C([C@@H]([C@@H]1O)N1CCOCC1)C(=O)c1ccccc1C2=O |r,c:4| Show InChI InChI=1S/C19H21NO5/c1-19(2)18(23)14(20-7-9-24-10-8-20)13-15(21)11-5-3-4-6-12(11)16(22)17(13)25-19/h3-6,14,18,23H,7-10H2,1-2H3/t14-,18-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University
Curated by ChEMBL
| Assay Description
Inhibition of 2,3 dioxygenase |
Eur J Med Chem 45: 4004-12 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.057
BindingDB Entry DOI: 10.7270/Q2HX1CW5 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50121013

((S)-1-[2-(4-Benzyl-phenoxy)-ethyl]-pyrrolidine-2-c...)Show InChI InChI=1S/C21H25NO3/c1-24-21(23)20-8-5-13-22(20)14-15-25-19-11-9-18(10-12-19)16-17-6-3-2-4-7-17/h2-4,6-7,9-12,20H,5,8,13-16H2,1H3/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
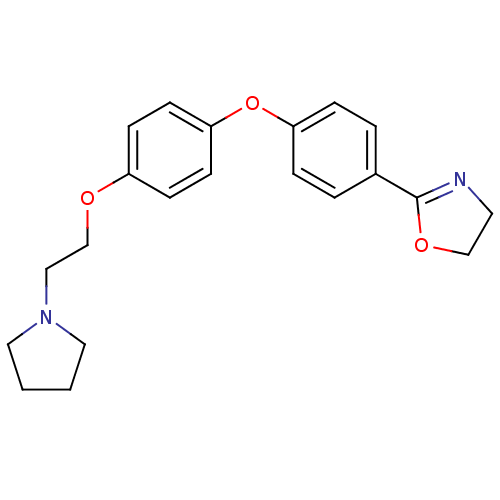
(Homo sapiens (Human)) | BDBM50085258
(2-{4-[4-(2-Pyrrolidin-1-yl-ethoxy)-phenoxy]-phenyl...)Show SMILES C(CN1CCCC1)Oc1ccc(Oc2ccc(cc2)C2=NCCO2)cc1 |t:21| Show InChI InChI=1S/C21H24N2O3/c1-2-13-23(12-1)14-16-24-18-7-9-20(10-8-18)26-19-5-3-17(4-6-19)21-22-11-15-25-21/h3-10H,1-2,11-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50116543
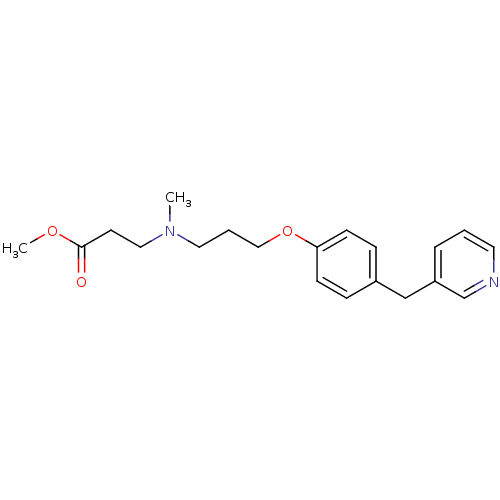
(3-{Methyl-[3-(4-pyridin-3-ylmethyl-phenoxy)-propyl...)Show InChI InChI=1S/C20H26N2O3/c1-22(13-10-20(23)24-2)12-4-14-25-19-8-6-17(7-9-19)15-18-5-3-11-21-16-18/h3,5-9,11,16H,4,10,12-15H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM50326130

(2-amino-5-(1-(carboxymethylamino)-3-(8-hydroxy-3-m...)Show SMILES CC1=C(SCC(NC(=O)CCC(N)C(O)=O)C(=O)NCC(O)=O)C(=O)c2c(O)cccc2C1=O |c:1| Show InChI InChI=1S/C21H23N3O9S/c1-9-17(29)10-3-2-4-13(25)16(10)18(30)19(9)34-8-12(20(31)23-7-15(27)28)24-14(26)6-5-11(22)21(32)33/h2-4,11-12,25H,5-8,22H2,1H3,(H,23,31)(H,24,26)(H,27,28)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 880 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University
Curated by ChEMBL
| Assay Description
Inhibition of 2,3 dioxygenase |
Eur J Med Chem 45: 4004-12 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.057
BindingDB Entry DOI: 10.7270/Q2HX1CW5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM24776
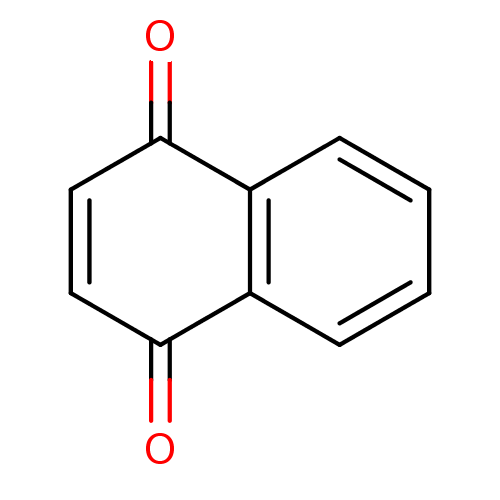
(1,4-Naphthoquinone | 1,4-Naphthoquinone (5a) | 1,4...)Show InChI InChI=1S/C10H6O2/c11-9-5-6-10(12)8-4-2-1-3-7(8)9/h1-6H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 990 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University
Curated by ChEMBL
| Assay Description
Inhibition of 2,3 dioxygenase |
Eur J Med Chem 45: 4004-12 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.057
BindingDB Entry DOI: 10.7270/Q2HX1CW5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM24778

(2-methyl-1,4-dihydronaphthalene-1,4-dione | 2-meth...)Show InChI InChI=1S/C11H8O2/c1-7-6-10(12)8-4-2-3-5-9(8)11(7)13/h2-6H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University
Curated by ChEMBL
| Assay Description
Inhibition of 2,3 dioxygenase |
Eur J Med Chem 45: 4004-12 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.057
BindingDB Entry DOI: 10.7270/Q2HX1CW5 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085254
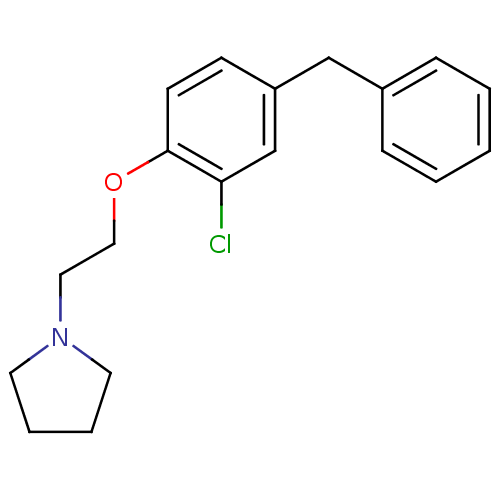
(1-[2-(4-Benzyl-2-chloro-phenoxy)-ethyl]-pyrrolidin...)Show InChI InChI=1S/C19H22ClNO/c20-18-15-17(14-16-6-2-1-3-7-16)8-9-19(18)22-13-12-21-10-4-5-11-21/h1-3,6-9,15H,4-5,10-14H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50285634

((R)-1-((R)-2-Benzyl-3-hydroxycarbamoyl-propionyl)-...)Show SMILES ONC(=O)C[C@@H](Cc1ccccc1)C(=O)N1CCCC[C@@H]1C(O)=O Show InChI InChI=1S/C17H22N2O5/c20-15(18-24)11-13(10-12-6-2-1-3-7-12)16(21)19-9-5-4-8-14(19)17(22)23/h1-3,6-7,13-14,24H,4-5,8-11H2,(H,18,20)(H,22,23)/t13-,14-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM24792
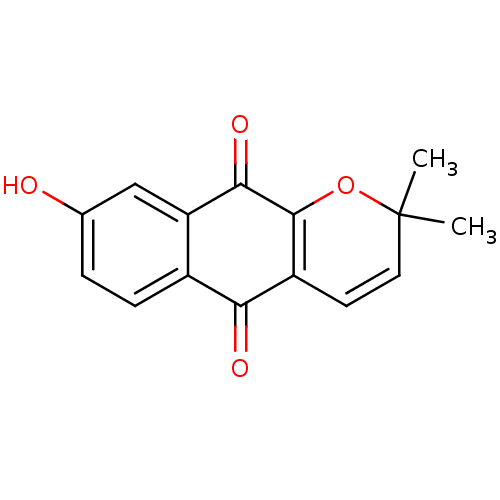
(8-hydroxy-2,2-dimethyl-2H,5H,10H-benzo[g]chromene-...)Show SMILES CC1(C)OC2=C(C=C1)C(=O)c1ccc(O)cc1C2=O |c:4,6| Show InChI InChI=1S/C15H12O4/c1-15(2)6-5-10-12(17)9-4-3-8(16)7-11(9)13(18)14(10)19-15/h3-7,16H,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University
Curated by ChEMBL
| Assay Description
Inhibition of 2,3 dioxygenase |
Eur J Med Chem 45: 4004-12 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.057
BindingDB Entry DOI: 10.7270/Q2HX1CW5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM24795
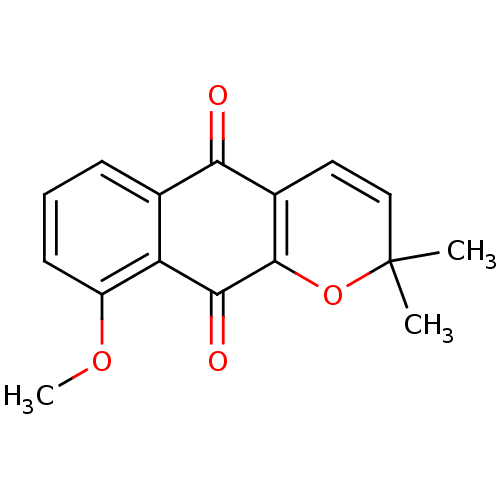
(9-methoxy-2,2-dimethyl-2H,5H,10H-benzo[g]chromene-...)Show SMILES COc1cccc2C(=O)C3=C(OC(C)(C)C=C3)C(=O)c12 |c:15,t:9| Show InChI InChI=1S/C16H14O4/c1-16(2)8-7-10-13(17)9-5-4-6-11(19-3)12(9)14(18)15(10)20-16/h4-8H,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University
Curated by ChEMBL
| Assay Description
Inhibition of 2,3 dioxygenase |
Eur J Med Chem 45: 4004-12 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.057
BindingDB Entry DOI: 10.7270/Q2HX1CW5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM24805
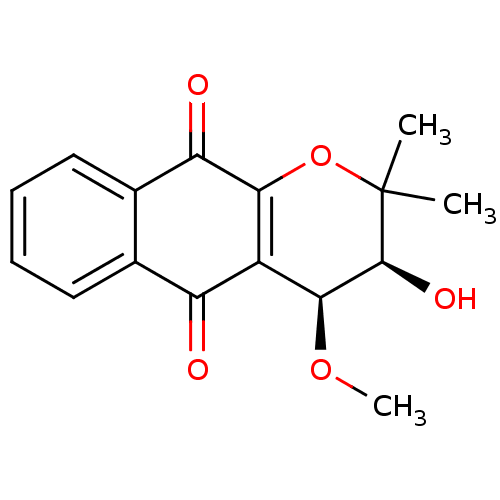
((3S,4S)-3-hydroxy-4-methoxy-2,2-dimethyl-2H,3H,4H,...)Show SMILES CO[C@@H]1[C@H](O)C(C)(C)OC2=C1C(=O)c1ccccc1C2=O |r,c:9| Show InChI InChI=1S/C16H16O5/c1-16(2)15(19)14(20-3)10-11(17)8-6-4-5-7-9(8)12(18)13(10)21-16/h4-7,14-15,19H,1-3H3/t14-,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.96E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University
Curated by ChEMBL
| Assay Description
Inhibition of 2,3 dioxygenase |
Eur J Med Chem 45: 4004-12 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.057
BindingDB Entry DOI: 10.7270/Q2HX1CW5 |
More data for this
Ligand-Target Pair | |
Leukotriene A-4 hydrolase
(Homo sapiens (Human)) | BDBM50085257
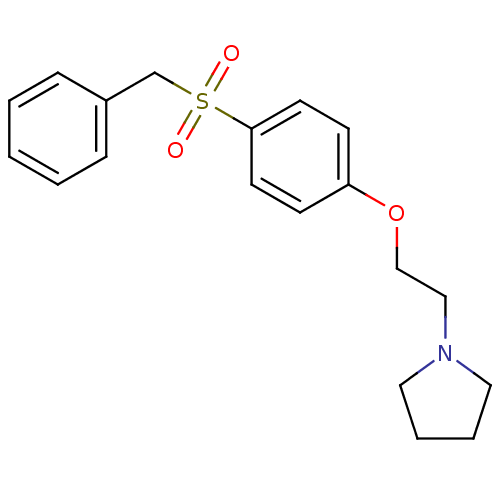
(1-[2-(4-Phenylmethanesulfonyl-phenoxy)-ethyl]-pyrr...)Show InChI InChI=1S/C19H23NO3S/c21-24(22,16-17-6-2-1-3-7-17)19-10-8-18(9-11-19)23-15-14-20-12-4-5-13-20/h1-3,6-11H,4-5,12-16H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University (GNU)
Curated by ChEMBL
| Assay Description
Inhibition of human leukotriene A4 hydrolase |
Eur J Med Chem 46: 1593-603 (2011)
Article DOI: 10.1016/j.ejmech.2011.02.007
BindingDB Entry DOI: 10.7270/Q2K64JDG |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM24796
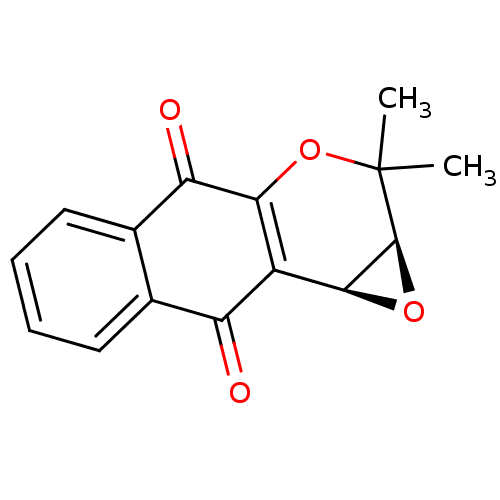
((11S,13S)-14,14-dimethyl-12,15-dioxatetracyclo[8.5...)Show SMILES CC1(C)OC2=C([C@@H]3O[C@H]13)C(=O)c1ccccc1C2=O |r,c:4| Show InChI InChI=1S/C15H12O4/c1-15(2)14-13(18-14)9-10(16)7-5-3-4-6-8(7)11(17)12(9)19-15/h3-6,13-14H,1-2H3/t13-,14-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University
Curated by ChEMBL
| Assay Description
Inhibition of 2,3 dioxygenase |
Eur J Med Chem 45: 4004-12 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.057
BindingDB Entry DOI: 10.7270/Q2HX1CW5 |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM24678
(4-fluoro-2-(1H-pyrazol-3-yl)phenol | pyrazole, 31)Show InChI InChI=1S/C9H7FN2O/c10-6-1-2-9(13)7(5-6)8-3-4-11-12-8/h1-5,13H,(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Gyeongsang National University
Curated by ChEMBL
| Assay Description
Inhibition of 2,3 dioxygenase |
Eur J Med Chem 45: 4004-12 (2010)
Article DOI: 10.1016/j.ejmech.2010.05.057
BindingDB Entry DOI: 10.7270/Q2HX1CW5 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data