

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genome polyprotein (Hepatitis C virus) | BDBM123407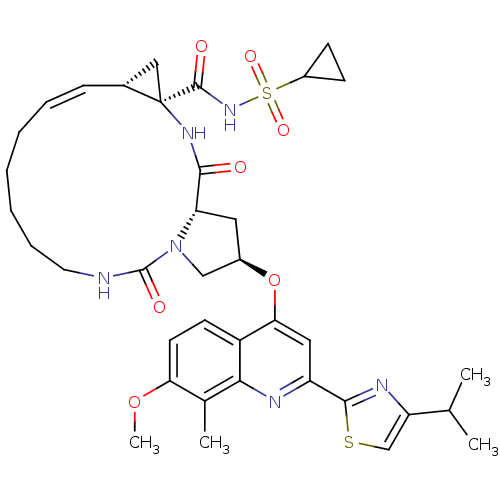 (US8741926, 91) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | -59.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123410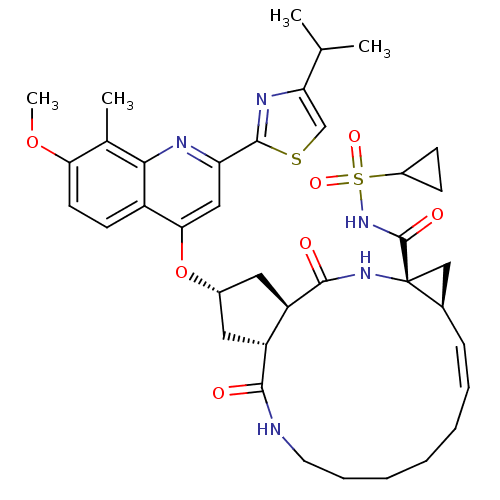 (US8741926, 82 | US8754106, 82 | US8754106, 91) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123415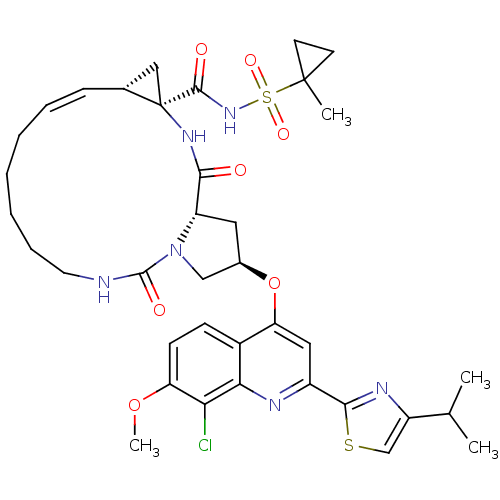 (US8741926, 95 | US8754106, 95) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123415 (US8741926, 95 | US8754106, 95) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123413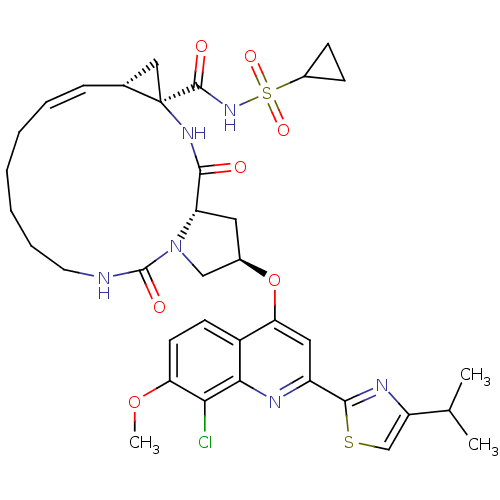 (US8741926, 94 | US8754106, 94) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123413 (US8741926, 94 | US8754106, 94) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124106 (US8754106, 56) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123411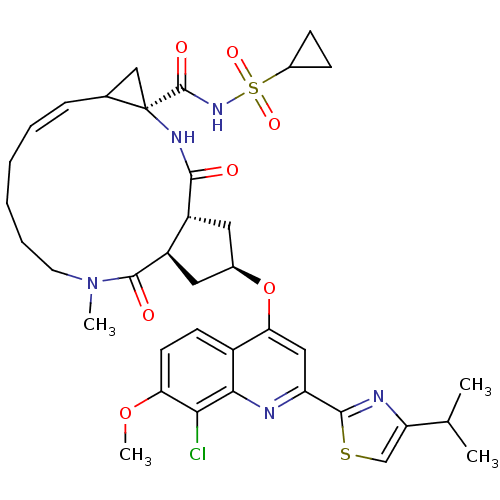 (US8741926, 56) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.100 | -58.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124107 (US8754105, 23) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.200 | -56.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Medivir AB; Janssen R&D Ireland US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754105 (2014) BindingDB Entry DOI: 10.7270/Q2QC0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123414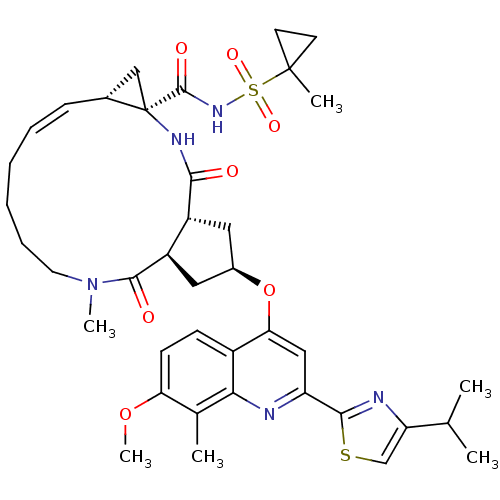 (US8741926, 48 | US8754106, 48) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123414 (US8741926, 48 | US8754106, 48) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.25 | -55.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123412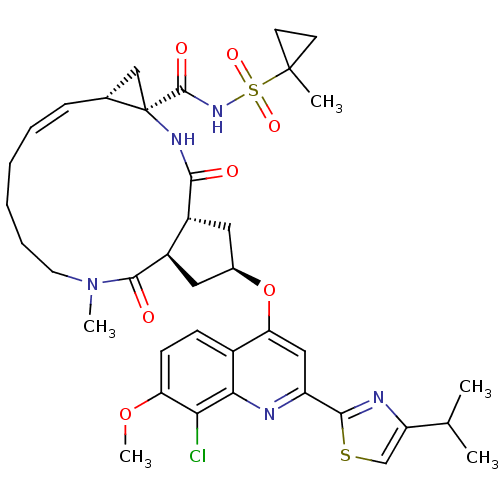 (US8741926, 57 | US8754106, 57) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123412 (US8741926, 57 | US8754106, 57) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 0.300 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485121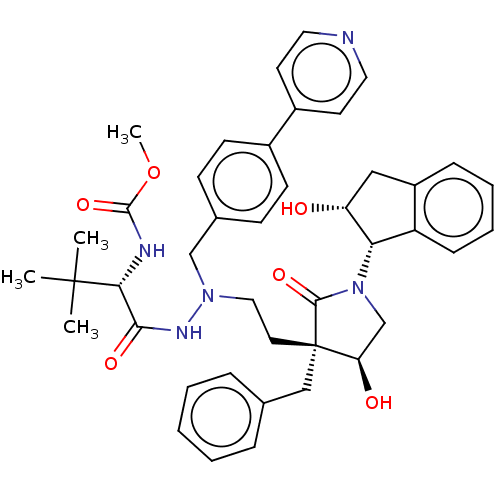 (CHEMBL2030954) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50336504 ((2R,3aR,10Z,11aS,12aR,14aR)-N-(cyclopropylsulfonyl...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | MMDB PDB US Patent | 0.5 | -54.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50336504 ((2R,3aR,10Z,11aS,12aR,14aR)-N-(cyclopropylsulfonyl...) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | Purchase CHEMBL MCE MMDB PC cid PC sid PDB UniChem Similars | US Patent | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485126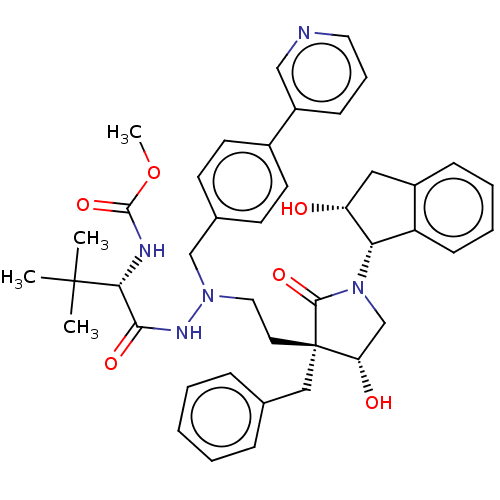 (CHEMBL2030953) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485120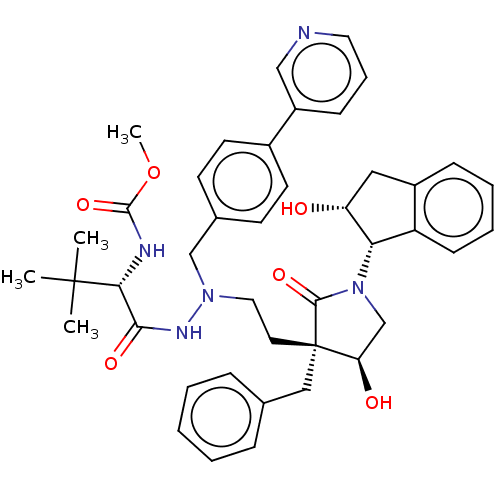 (CHEMBL2030952) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485130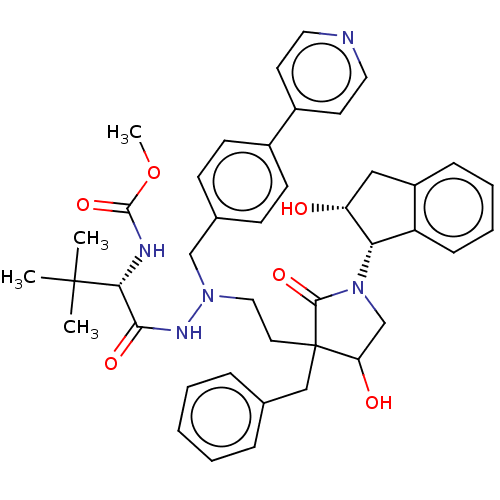 (CHEMBL2031103) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485118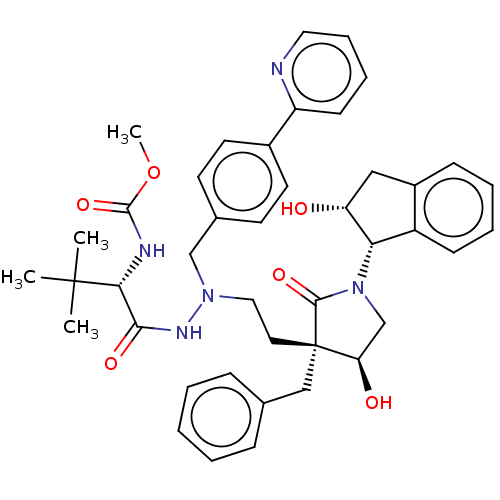 (CHEMBL2030950) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485122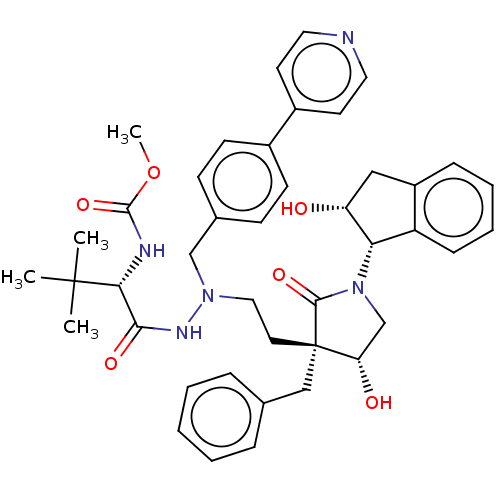 (CHEMBL2030955) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485119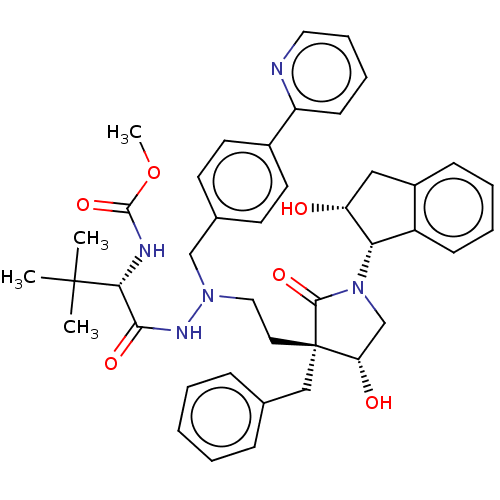 (CHEMBL2030951) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485116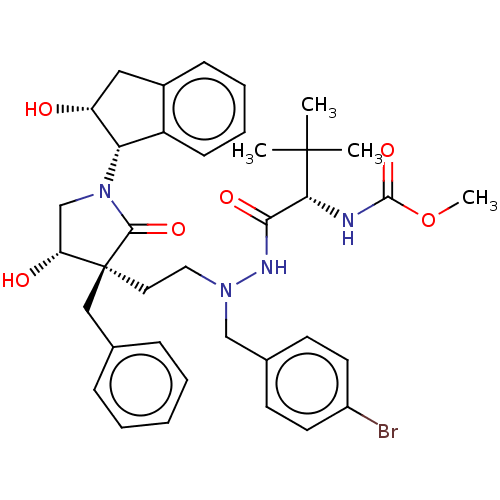 (CHEMBL2030946) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124112 (US8754105, 25) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.40 | -50.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Medivir AB; Janssen R&D Ireland US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754105 (2014) BindingDB Entry DOI: 10.7270/Q2QC0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM13934 (Atazanavir | BMS 232632 | CGP 73547 | CHEMBL1163 |...) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485131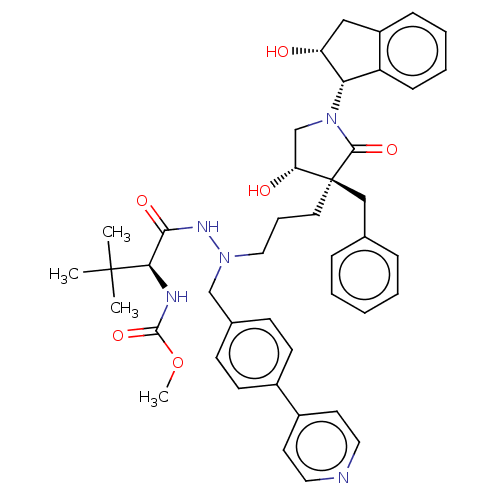 (CHEMBL2031101) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123408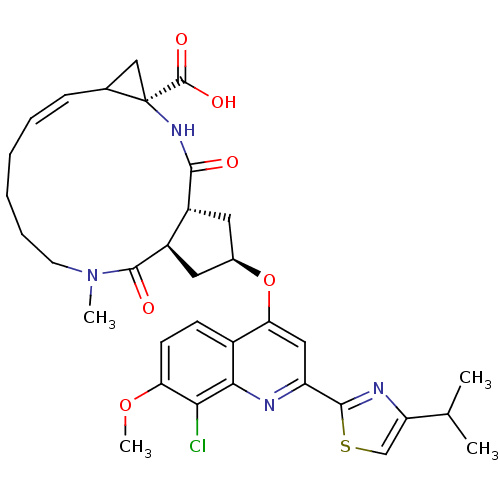 (US8741926, 55) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5 | -48.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124105 (US8754106, 55) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485124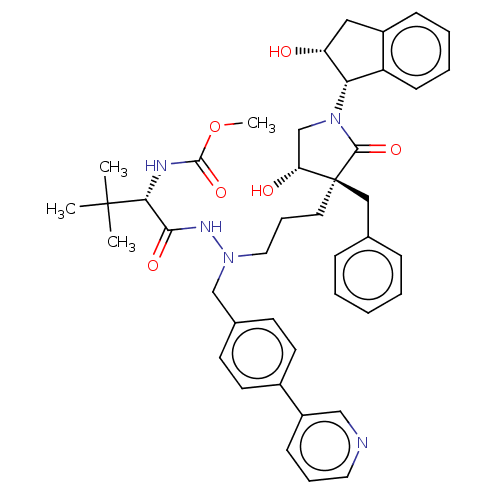 (CHEMBL2031100) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485114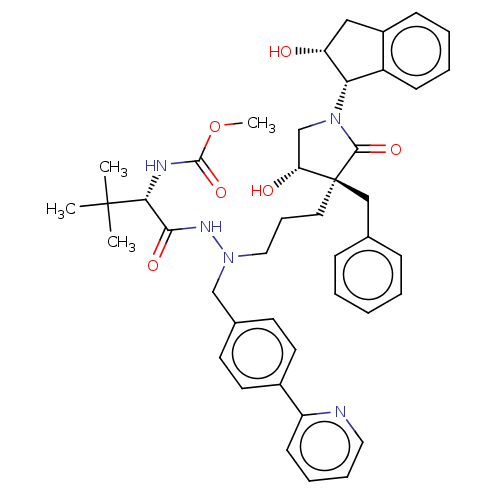 (CHEMBL2031102) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485129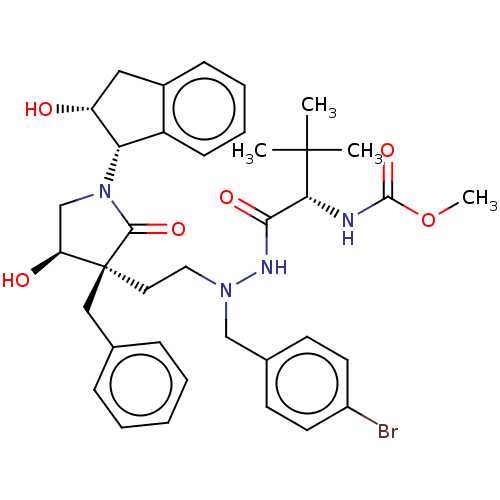 (CHEMBL2030949) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123410 (US8741926, 82 | US8754106, 82 | US8754106, 91) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754106 (2014) BindingDB Entry DOI: 10.7270/Q2V40SWX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123410 (US8741926, 82 | US8754106, 82 | US8754106, 91) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9.40 | -46.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485123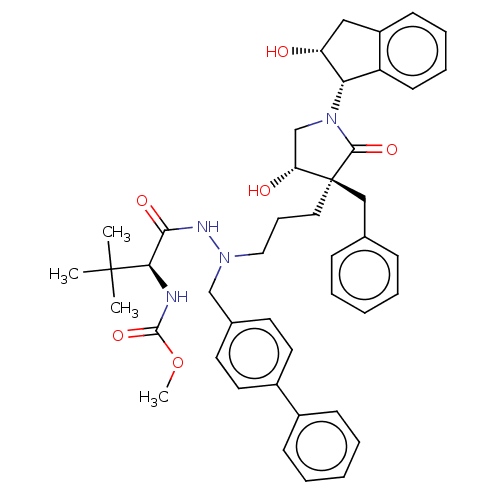 (CHEMBL2031099) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid PDB UniChem | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485128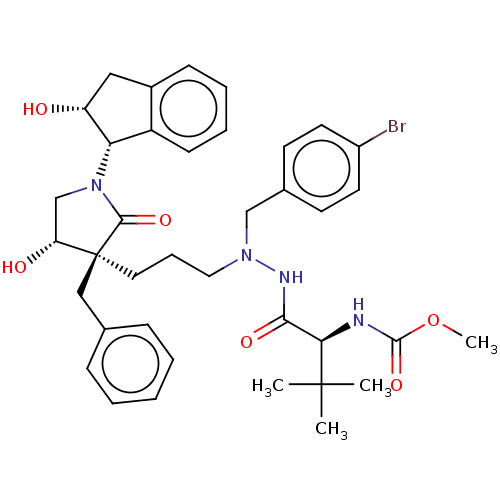 (CHEMBL2030956) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124111 (US8754105, 24) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 40 | -42.9 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Medivir AB; Janssen R&D Ireland US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754105 (2014) BindingDB Entry DOI: 10.7270/Q2QC0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124110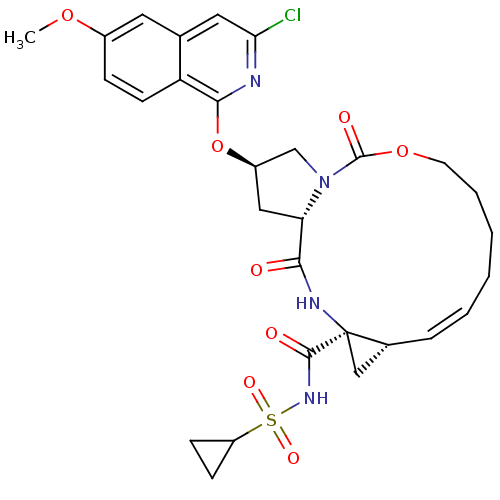 (US8754105, 17) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 43 | -42.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Medivir AB; Janssen R&D Ireland US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754105 (2014) BindingDB Entry DOI: 10.7270/Q2QC0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485125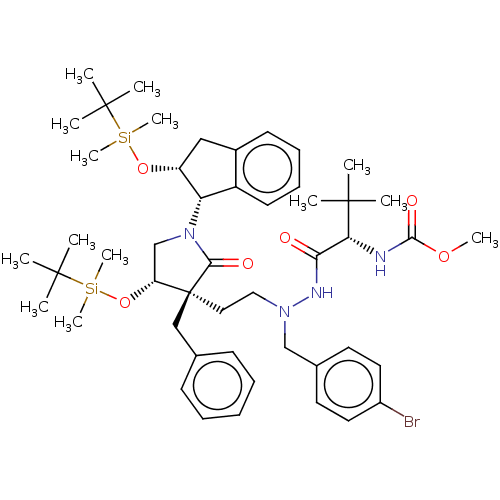 (CHEMBL2030944) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 760 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485115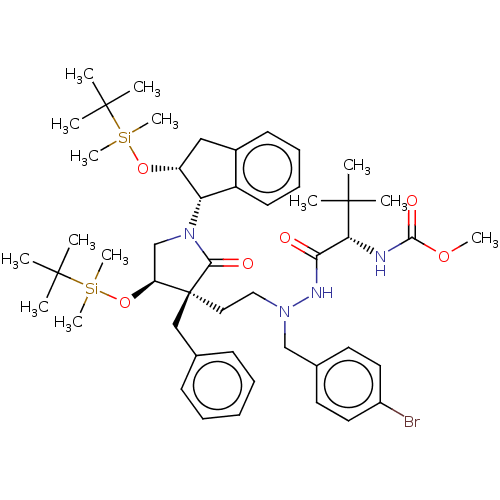 (CHEMBL2030945) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485127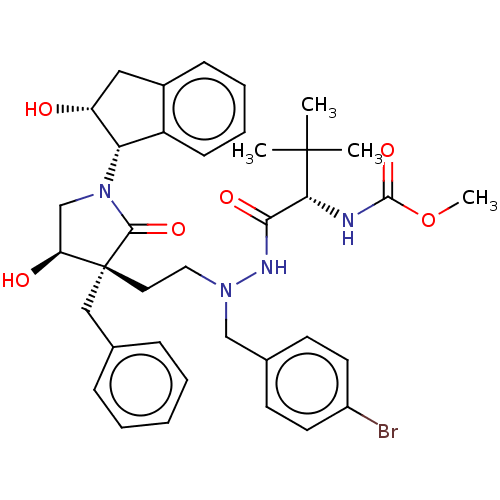 (CHEMBL2030948) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 940 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM123409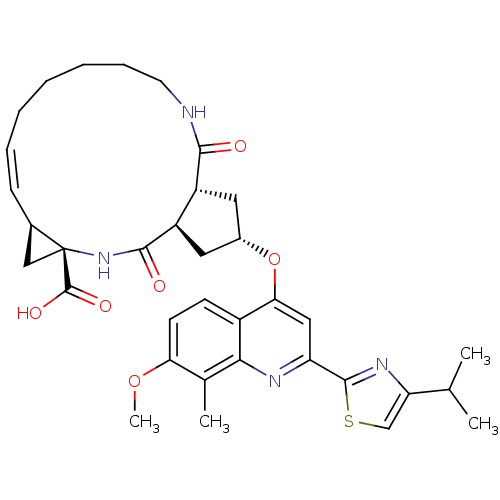 (US8741926, 81) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.00E+3 | -34.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Janssen R&D Ireland; Medivir AB US Patent | Assay Description The inhibition of full-length hepatitis C NS3 protease enzyme was measured essentially as described in Poliakov, 2002 Prot Expression & Purification ... | US Patent US8741926 (2014) BindingDB Entry DOI: 10.7270/Q2Z31XBC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124109 (US8754105, 16) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 1.00E+3 | -34.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Medivir AB; Janssen R&D Ireland US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754105 (2014) BindingDB Entry DOI: 10.7270/Q2QC0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM124108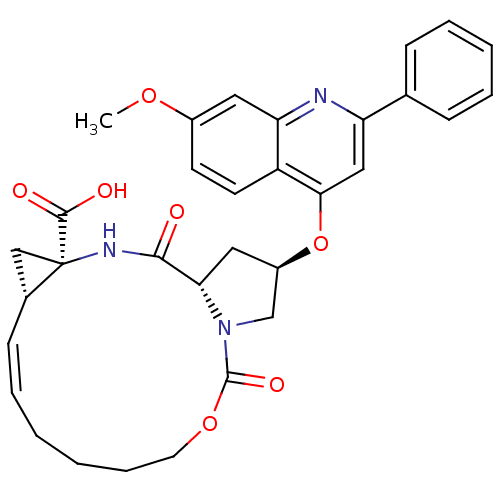 (US8754105, 22) | PDB UniProtKB/TrEMBL GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.07E+3 | -34.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 30 |
Medivir AB; Janssen R&D Ireland US Patent | Assay Description The aim of this in vitro assay was to measure the inhibition of HCV NS3/4A protease complexes by the compounds of the present invention. This assay p... | US Patent US8754105 (2014) BindingDB Entry DOI: 10.7270/Q2QC0250 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Protease (Human immunodeficiency virus 1 (HIV-1)) | BDBM50485117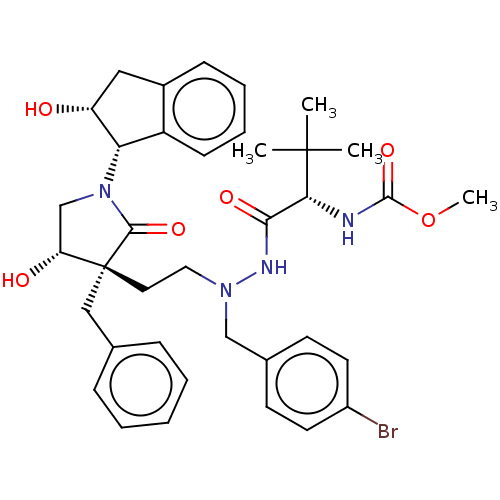 (CHEMBL2030947) | PDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Uppsala University Curated by ChEMBL | Assay Description Inhibition of HIV1 protease expressed in Escherichia coli using DABCYL-Abu-Ser-Gln-ASN-Tyr-Pro-Ile-Val-Gln-EDANS as substrate preincubated for 20 min... | J Med Chem 55: 2724-36 (2012) Article DOI: 10.1021/jm201620t BindingDB Entry DOI: 10.7270/Q2C25086 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||