 Found 113 hits with Last Name = 'sangalang' and Initial = 'j'
Found 113 hits with Last Name = 'sangalang' and Initial = 'j' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for factor VIIa/TF |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.460 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards urokinase-type plasminogen activator (microPa) |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101882
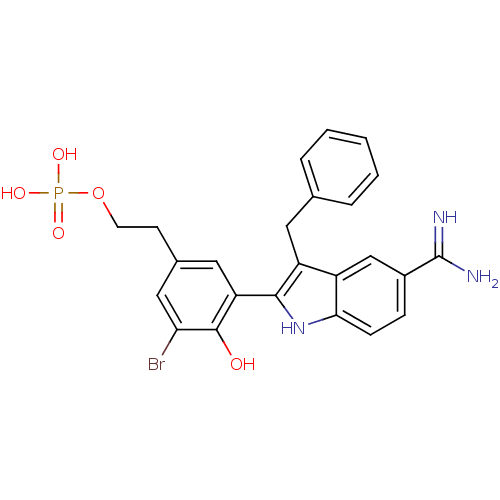
(CHEMBL53829 | Phosphoric acid mono-{2-[3-(3-benzyl...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CCOP(O)(O)=O)cc(Br)c1O Show InChI InChI=1S/C24H23BrN3O5P/c25-20-12-15(8-9-33-34(30,31)32)11-19(23(20)29)22-18(10-14-4-2-1-3-5-14)17-13-16(24(26)27)6-7-21(17)28-22/h1-7,11-13,28-29H,8-10H2,(H3,26,27)(H2,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101871

(3-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CCC(O)=O)cc(Br)c1O Show InChI InChI=1S/C25H22BrN3O3/c26-20-12-15(6-9-22(30)31)11-19(24(20)32)23-18(10-14-4-2-1-3-5-14)17-13-16(25(27)28)7-8-21(17)29-23/h1-5,7-8,11-13,29,32H,6,9-10H2,(H3,27,28)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Coagulation factor X |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101881
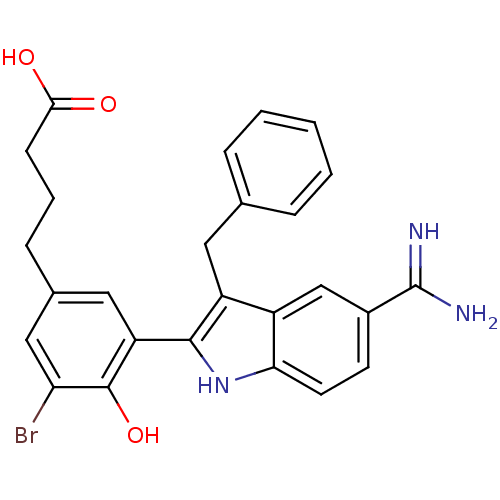
(4-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CCCC(O)=O)cc(Br)c1O Show InChI InChI=1S/C26H24BrN3O3/c27-21-13-16(7-4-8-23(31)32)12-20(25(21)33)24-19(11-15-5-2-1-3-6-15)18-14-17(26(28)29)9-10-22(18)30-24/h1-3,5-6,9-10,12-14,30,33H,4,7-8,11H2,(H3,28,29)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101883
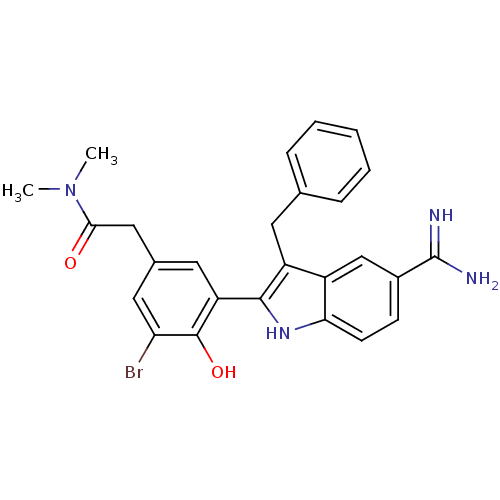
(2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES CN(C)C(=O)Cc1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C26H25BrN4O2/c1-31(2)23(32)13-16-11-20(25(33)21(27)12-16)24-19(10-15-6-4-3-5-7-15)18-14-17(26(28)29)8-9-22(18)30-24/h3-9,11-12,14,30,33H,10,13H2,1-2H3,(H3,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101885
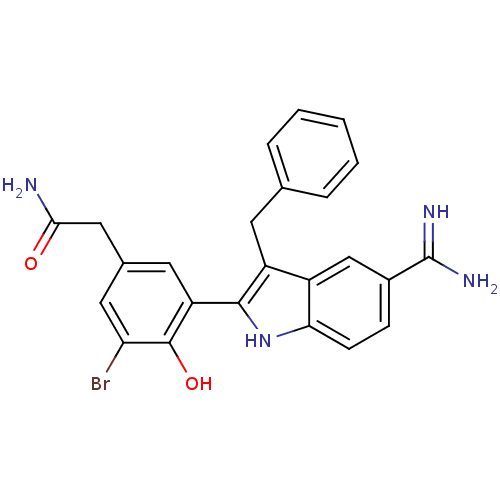
(2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES NC(=O)Cc1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C24H21BrN4O2/c25-19-10-14(11-21(26)30)9-18(23(19)31)22-17(8-13-4-2-1-3-5-13)16-12-15(24(27)28)6-7-20(16)29-22/h1-7,9-10,12,29,31H,8,11H2,(H2,26,30)(H3,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50103655
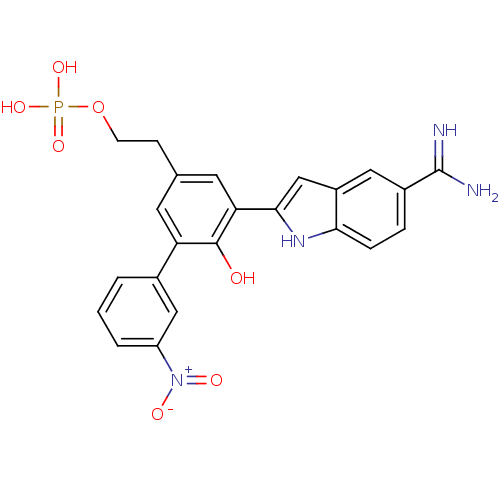
(CHEMBL72231 | Phosphoric acid mono-{2-[5-(5-carbam...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(CCO[P+](O)(O)[O-])cc(c1O)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C23H21N4O7P/c24-23(25)15-4-5-20-16(10-15)12-21(26-20)19-9-13(6-7-34-35(31,32)33)8-18(22(19)28)14-2-1-3-17(11-14)27(29)30/h1-5,8-12,26,28H,6-7H2,(H3,24,25)(H2,31,32,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for factor VIIa/TF |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM13778

(2-[3-(5-carbamimidoyl-1H-indol-2-yl)-4-hydroxy-5-(...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(CC(O)=O)cc(c1O)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C23H18N4O5/c24-23(25)14-4-5-19-15(9-14)11-20(26-19)18-7-12(8-21(28)29)6-17(22(18)30)13-2-1-3-16(10-13)27(31)32/h1-7,9-11,26,30H,8H2,(H3,24,25)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for factor VIIa/TF |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101879
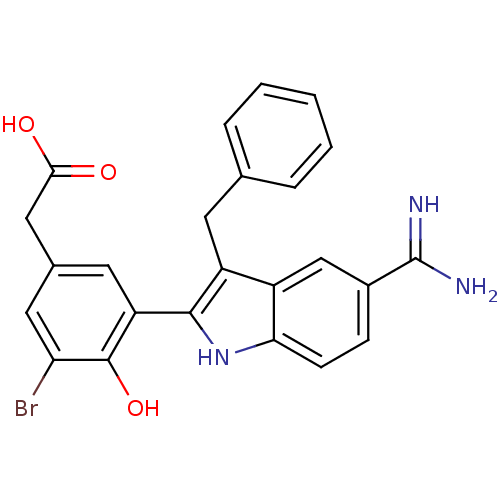
(CHEMBL50924 | [3-(3-Benzyl-5-carbamimidoyl-1H-indo...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CC(O)=O)cc(Br)c1O Show InChI InChI=1S/C24H20BrN3O3/c25-19-10-14(11-21(29)30)9-18(23(19)31)22-17(8-13-4-2-1-3-5-13)16-12-15(24(26)27)6-7-20(16)28-22/h1-7,9-10,12,28,31H,8,11H2,(H3,26,27)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101880
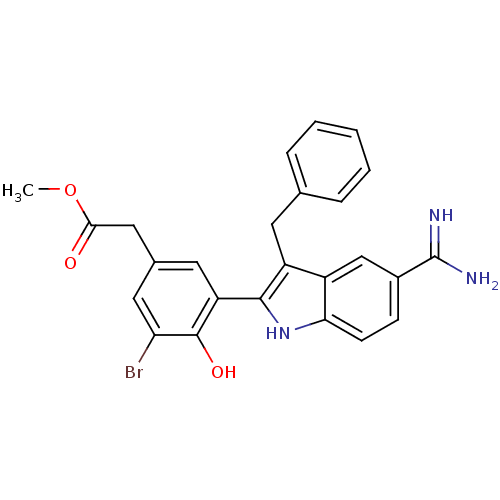
(CHEMBL416127 | [3-(3-Benzyl-5-carbamimidoyl-1H-ind...)Show SMILES COC(=O)Cc1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C25H22BrN3O3/c1-32-22(30)12-15-10-19(24(31)20(26)11-15)23-18(9-14-5-3-2-4-6-14)17-13-16(25(27)28)7-8-21(17)29-23/h2-8,10-11,13,29,31H,9,12H2,1H3,(H3,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Plasminogen
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Plasmin |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101870
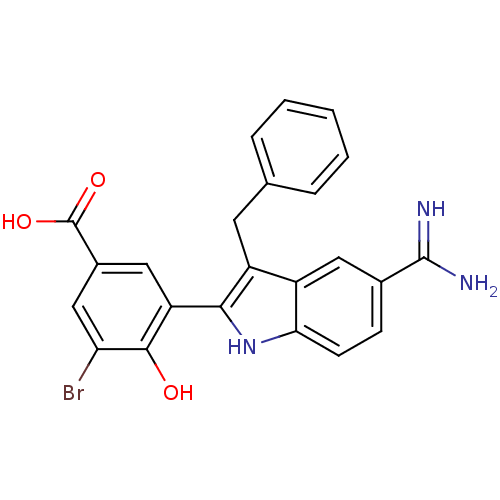
(3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-bromo...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(cc(Br)c1O)C(O)=O Show InChI InChI=1S/C23H18BrN3O3/c24-18-11-14(23(29)30)10-17(21(18)28)20-16(8-12-4-2-1-3-5-12)15-9-13(22(25)26)6-7-19(15)27-20/h1-7,9-11,27-28H,8H2,(H3,25,26)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Coagulation factor II |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Serine protease 1
(Homo sapiens (Human)) | BDBM50103651

(2-(3'-Amino-5-chloro-2-hydroxy-biphenyl-3-yl)-1H-b...)Show SMILES NC(=N)c1ccc2nc([nH]c2c1)-c1cc(Cl)cc(-c2cccc(N)c2)c1O Show InChI InChI=1S/C20H16ClN5O/c21-12-8-14(10-2-1-3-13(22)6-10)18(27)15(9-12)20-25-16-5-4-11(19(23)24)7-17(16)26-20/h1-9,27H,22H2,(H3,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards Trypsin |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50103652
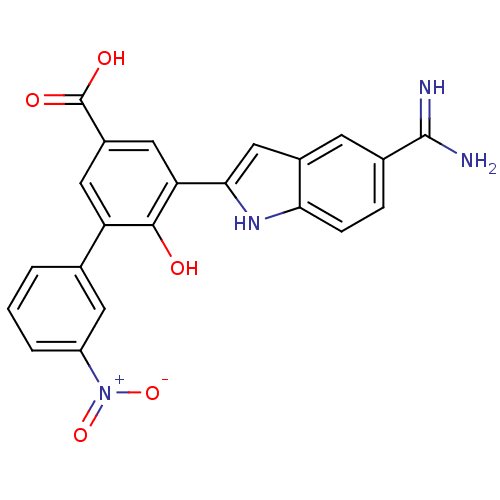
(5-(5-Carbamimidoyl-1H-indol-2-yl)-6-hydroxy-3'-nit...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(cc(c1O)-c1cccc(c1)[N+]([O-])=O)C(O)=O Show InChI InChI=1S/C22H16N4O5/c23-21(24)12-4-5-18-13(6-12)10-19(25-18)17-9-14(22(28)29)8-16(20(17)27)11-2-1-3-15(7-11)26(30)31/h1-10,25,27H,(H3,23,24)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for factor VIIa/TF |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101867

(3-Benzyl-2-[3-bromo-2-hydroxy-5-(2H-tetrazol-5-yl)...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(cc(Br)c1O)-c1nnn[nH]1 Show InChI InChI=1S/C23H18BrN7O/c24-18-11-14(23-28-30-31-29-23)10-17(21(18)32)20-16(8-12-4-2-1-3-5-12)15-9-13(22(25)26)6-7-19(15)27-20/h1-7,9-11,27,32H,8H2,(H3,25,26)(H,28,29,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101869

(2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES CC(C)(C(O)=O)c1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C26H24BrN3O3/c1-26(2,25(32)33)16-12-19(23(31)20(27)13-16)22-18(10-14-6-4-3-5-7-14)17-11-15(24(28)29)8-9-21(17)30-22/h3-9,11-13,30-31H,10H2,1-2H3,(H3,28,29)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50103662
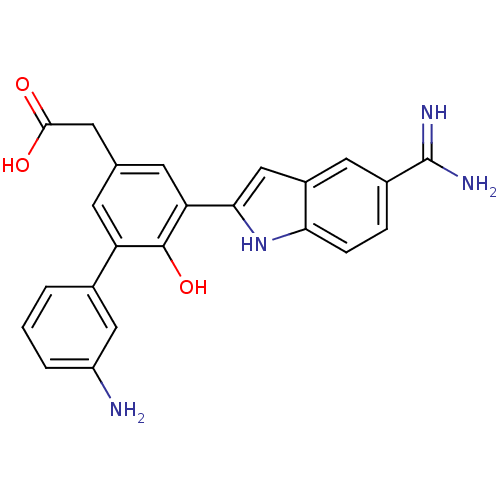
(CHEMBL73162 | [3'-Amino-5-(5-carbamimidoyl-1H-indo...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(CC(O)=O)cc(c1O)-c1cccc(N)c1 Show InChI InChI=1S/C23H20N4O3/c24-16-3-1-2-13(10-16)17-6-12(8-21(28)29)7-18(22(17)30)20-11-15-9-14(23(25)26)4-5-19(15)27-20/h1-7,9-11,27,30H,8,24H2,(H3,25,26)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for factor VIIa/TF |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101878
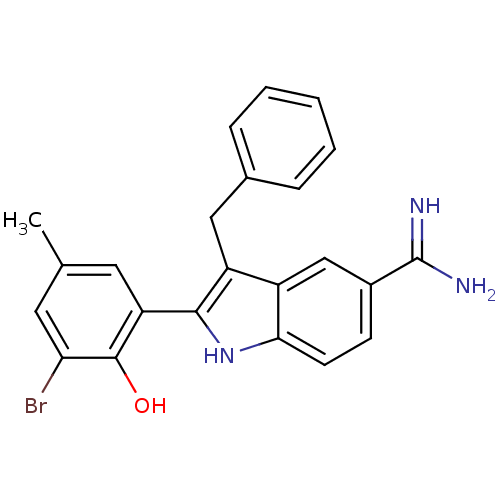
(3-Benzyl-2-(3-bromo-2-hydroxy-5-methyl-phenyl)-1H-...)Show SMILES Cc1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C23H20BrN3O/c1-13-9-18(22(28)19(24)10-13)21-17(11-14-5-3-2-4-6-14)16-12-15(23(25)26)7-8-20(16)27-21/h2-10,12,27-28H,11H2,1H3,(H3,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101868
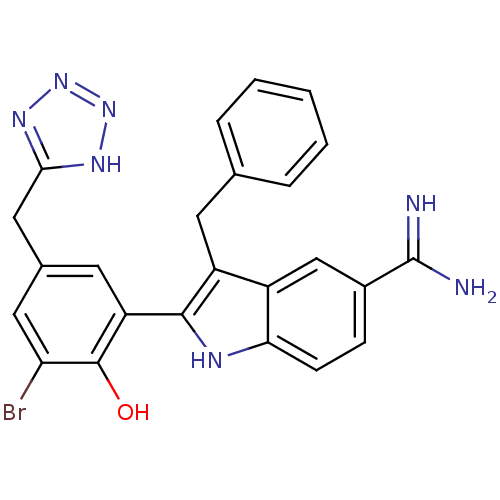
(3-Benzyl-2-[3-bromo-2-hydroxy-5-(2H-tetrazol-5-ylm...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(Cc2nnn[nH]2)cc(Br)c1O Show InChI InChI=1S/C24H20BrN7O/c25-19-10-14(11-21-29-31-32-30-21)9-18(23(19)33)22-17(8-13-4-2-1-3-5-13)16-12-15(24(26)27)6-7-20(16)28-22/h1-7,9-10,12,28,33H,8,11H2,(H3,26,27)(H,29,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50101885

(2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES NC(=O)Cc1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C24H21BrN4O2/c25-19-10-14(11-21(26)30)9-18(23(19)31)22-17(8-13-4-2-1-3-5-13)16-12-15(24(27)28)6-7-20(16)29-22/h1-7,9-10,12,29,31H,8,11H2,(H2,26,30)(H3,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin(fIIa) in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50103660
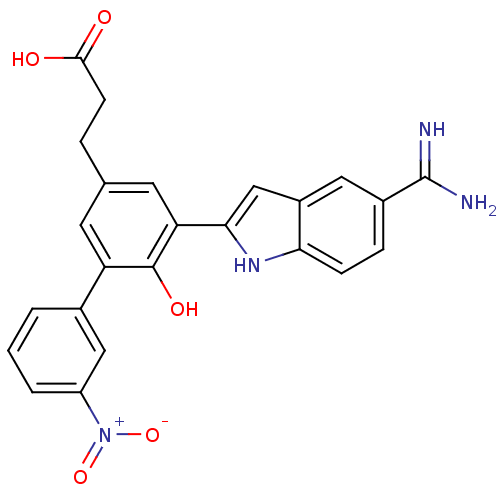
(3-[5-(5-Carbamimidoyl-1H-indol-2-yl)-6-hydroxy-3'-...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(CCC(O)=O)cc(c1O)-c1cccc(c1)[N+]([O-])=O Show InChI InChI=1S/C24H20N4O5/c25-24(26)15-5-6-20-16(10-15)12-21(27-20)19-9-13(4-7-22(29)30)8-18(23(19)31)14-2-1-3-17(11-14)28(32)33/h1-3,5-6,8-12,27,31H,4,7H2,(H3,25,26)(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for factor VIIa/TF |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50103654
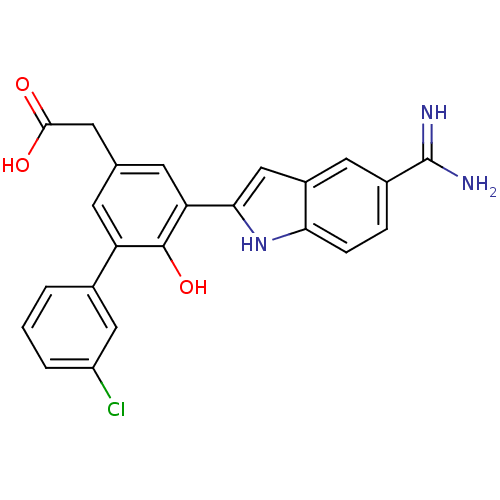
(CHEMBL310933 | [5-(5-Carbamimidoyl-1H-indol-2-yl)-...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(CC(O)=O)cc(c1O)-c1cccc(Cl)c1 Show InChI InChI=1S/C23H18ClN3O3/c24-16-3-1-2-13(10-16)17-6-12(8-21(28)29)7-18(22(17)30)20-11-15-9-14(23(25)26)4-5-19(15)27-20/h1-7,9-11,27,30H,8H2,(H3,25,26)(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for factor VIIa/TF |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50101881

(4-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CCCC(O)=O)cc(Br)c1O Show InChI InChI=1S/C26H24BrN3O3/c27-21-13-16(7-4-8-23(31)32)12-20(25(21)33)24-19(11-15-5-2-1-3-6-15)18-14-17(26(28)29)9-10-22(18)30-24/h1-3,5-6,9-10,12-14,30,33H,4,7-8,11H2,(H3,28,29)(H,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin(fIIa) in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50103656
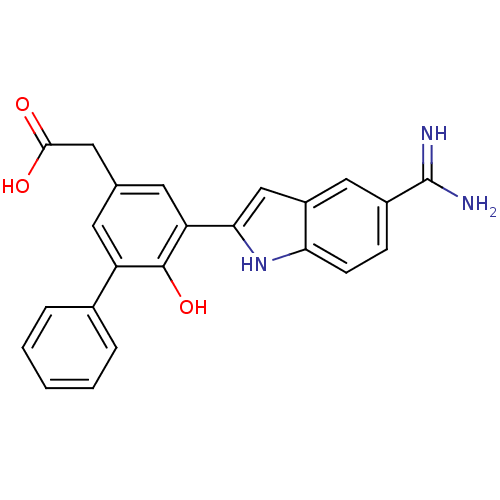
(CHEMBL73411 | [5-(5-Carbamimidoyl-1H-indol-2-yl)-6...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(CC(O)=O)cc(c1O)-c1ccccc1 Show InChI InChI=1S/C23H19N3O3/c24-23(25)15-6-7-19-16(11-15)12-20(26-19)18-9-13(10-21(27)28)8-17(22(18)29)14-4-2-1-3-5-14/h1-9,11-12,26,29H,10H2,(H3,24,25)(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for factor VIIa/TF |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50101883

(2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES CN(C)C(=O)Cc1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C26H25BrN4O2/c1-31(2)23(32)13-16-11-20(25(33)21(27)12-16)24-19(10-15-6-4-3-5-7-15)18-14-17(26(28)29)8-9-22(18)30-24/h3-9,11-12,14,30,33H,10,13H2,1-2H3,(H3,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 43 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin(fIIa) in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101877

(3-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES COc1c(Br)cc(CCC(O)=O)cc1-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C26H24BrN3O3/c1-33-25-20(12-16(13-21(25)27)7-10-23(31)32)24-19(11-15-5-3-2-4-6-15)18-14-17(26(28)29)8-9-22(18)30-24/h2-6,8-9,12-14,30H,7,10-11H2,1H3,(H3,28,29)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50103653
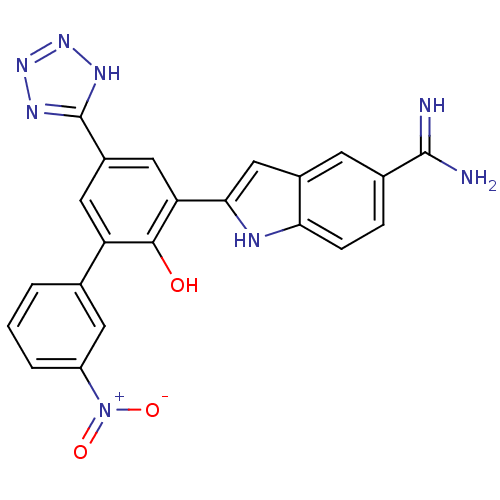
(2-[2-Hydroxy-3'-nitro-5-(2H-tetrazol-5-yl)-bipheny...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(cc(c1O)-c1cccc(c1)[N+]([O-])=O)-c1nnn[nH]1 Show InChI InChI=1S/C22H16N8O3/c23-21(24)12-4-5-18-13(6-12)10-19(25-18)17-9-14(22-26-28-29-27-22)8-16(20(17)31)11-2-1-3-15(7-11)30(32)33/h1-10,25,31H,(H3,23,24)(H,26,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards factor VIIa/TF was determined |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII
(Homo sapiens (Human)) | BDBM50103658
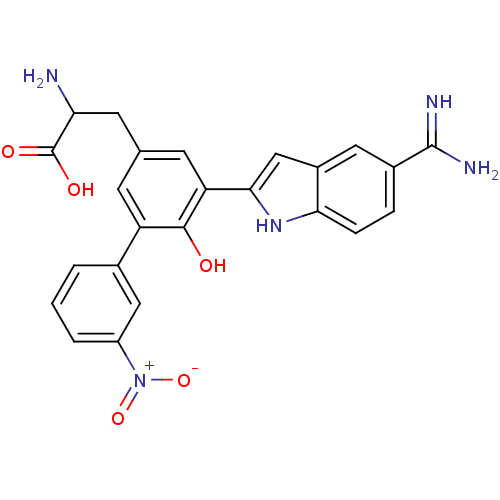
(2-Amino-3-[5-(5-carbamimidoyl-1H-indol-2-yl)-6-hyd...)Show SMILES NC(Cc1cc(-c2cc3cc(ccc3[nH]2)C(N)=N)c(O)c(c1)-c1cccc(c1)[N+]([O-])=O)C(O)=O Show InChI InChI=1S/C24H21N5O5/c25-19(24(31)32)8-12-6-17(13-2-1-3-16(10-13)29(33)34)22(30)18(7-12)21-11-15-9-14(23(26)27)4-5-20(15)28-21/h1-7,9-11,19,28,30H,8,25H2,(H3,26,27)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity of the compound towards factor VIIa/TF was determined |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50101880

(CHEMBL416127 | [3-(3-Benzyl-5-carbamimidoyl-1H-ind...)Show SMILES COC(=O)Cc1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C25H22BrN3O3/c1-32-22(30)12-15-10-19(24(31)20(26)11-15)23-18(9-14-5-3-2-4-6-14)17-13-16(25(27)28)7-8-21(17)29-23/h2-8,10-11,13,29,31H,9,12H2,1H3,(H3,27,28) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin(fIIa) in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101873
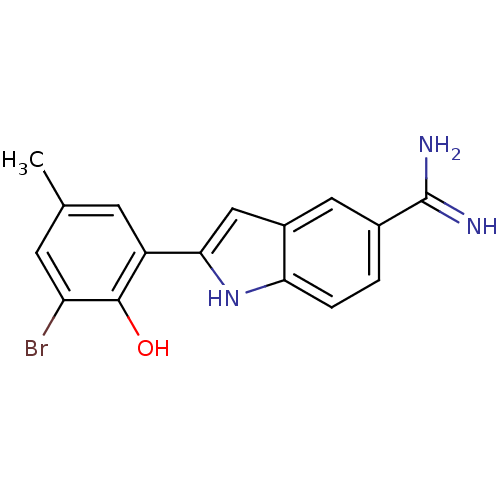
(2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-1H-indole-5-...)Show SMILES Cc1cc(Br)c(O)c(c1)-c1cc2cc(ccc2[nH]1)C(N)=N Show InChI InChI=1S/C16H14BrN3O/c1-8-4-11(15(21)12(17)5-8)14-7-10-6-9(16(18)19)2-3-13(10)20-14/h2-7,20-21H,1H3,(H3,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50101873

(2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-1H-indole-5-...)Show SMILES Cc1cc(Br)c(O)c(c1)-c1cc2cc(ccc2[nH]1)C(N)=N Show InChI InChI=1S/C16H14BrN3O/c1-8-4-11(15(21)12(17)5-8)14-7-10-6-9(16(18)19)2-3-13(10)20-14/h2-7,20-21H,1H3,(H3,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50101866
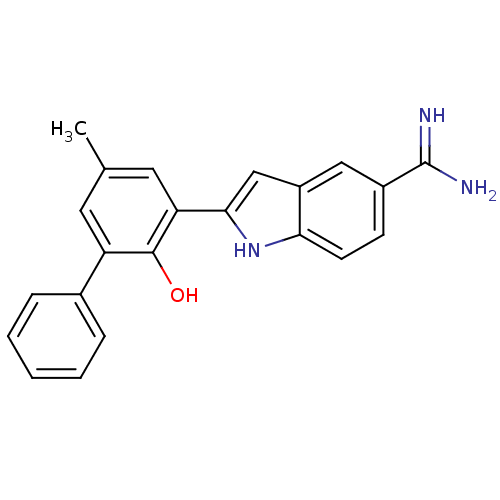
(2-(2-Hydroxy-5-methyl-biphenyl-3-yl)-1H-indole-5-c...)Show SMILES Cc1cc(-c2cc3cc(ccc3[nH]2)C(N)=N)c(O)c(c1)-c1ccccc1 Show InChI InChI=1S/C22H19N3O/c1-13-9-17(14-5-3-2-4-6-14)21(26)18(10-13)20-12-16-11-15(22(23)24)7-8-19(16)25-20/h2-12,25-26H,1H3,(H3,23,24) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50101871

(3-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CCC(O)=O)cc(Br)c1O Show InChI InChI=1S/C25H22BrN3O3/c26-20-12-15(6-9-22(30)31)11-19(24(20)32)23-18(10-14-4-2-1-3-5-14)17-13-16(25(27)28)7-8-21(17)29-23/h1-5,7-8,11-13,29,32H,6,9-10H2,(H3,27,28)(H,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin(fIIa) in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101876
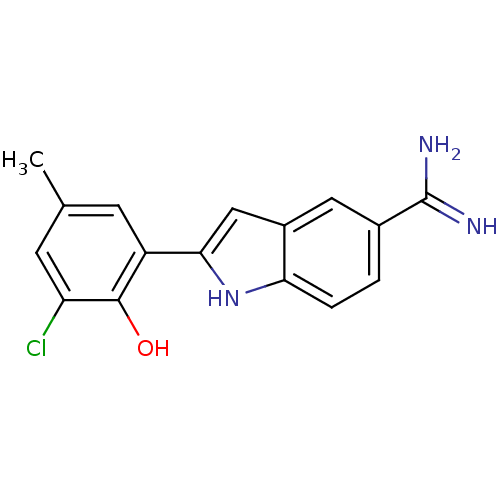
(2-(3-Chloro-2-hydroxy-5-methyl-phenyl)-1H-indole-5...)Show SMILES Cc1cc(Cl)c(O)c(c1)-c1cc2cc(ccc2[nH]1)C(N)=N Show InChI InChI=1S/C16H14ClN3O/c1-8-4-11(15(21)12(17)5-8)14-7-10-6-9(16(18)19)2-3-13(10)20-14/h2-7,20-21H,1H3,(H3,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50101876

(2-(3-Chloro-2-hydroxy-5-methyl-phenyl)-1H-indole-5...)Show SMILES Cc1cc(Cl)c(O)c(c1)-c1cc2cc(ccc2[nH]1)C(N)=N Show InChI InChI=1S/C16H14ClN3O/c1-8-4-11(15(21)12(17)5-8)14-7-10-6-9(16(18)19)2-3-13(10)20-14/h2-7,20-21H,1H3,(H3,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50101878

(3-Benzyl-2-(3-bromo-2-hydroxy-5-methyl-phenyl)-1H-...)Show SMILES Cc1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C23H20BrN3O/c1-13-9-18(22(28)19(24)10-13)21-17(11-14-5-3-2-4-6-14)16-12-15(23(25)26)7-8-20(16)27-21/h2-10,12,27-28H,11H2,1H3,(H3,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin(fIIa) in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50101881

(4-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CCCC(O)=O)cc(Br)c1O Show InChI InChI=1S/C26H24BrN3O3/c27-21-13-16(7-4-8-23(31)32)12-20(25(21)33)24-19(11-15-5-2-1-3-6-15)18-14-17(26(28)29)9-10-22(18)30-24/h1-3,5-6,9-10,12-14,30,33H,4,7-8,11H2,(H3,28,29)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50101871

(3-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CCC(O)=O)cc(Br)c1O Show InChI InChI=1S/C25H22BrN3O3/c26-20-12-15(6-9-22(30)31)11-19(24(20)32)23-18(10-14-4-2-1-3-5-14)17-13-16(25(27)28)7-8-21(17)29-23/h1-5,7-8,11-13,29,32H,6,9-10H2,(H3,27,28)(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| 280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50101870

(3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-bromo...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(cc(Br)c1O)C(O)=O Show InChI InChI=1S/C23H18BrN3O3/c24-18-11-14(23(29)30)10-17(21(18)28)20-16(8-12-4-2-1-3-5-12)15-9-13(22(25)26)6-7-19(15)27-20/h1-7,9-11,27-28H,8H2,(H3,25,26)(H,29,30) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50103663

(3'-(5-Carbamimidoyl-1H-indol-2-yl)-5'-carboxymethy...)Show SMILES NC(=N)c1ccc2[nH]c(cc2c1)-c1cc(CC(O)=O)cc(c1O)-c1cccc(c1)C(O)=O Show InChI InChI=1S/C24H19N3O5/c25-23(26)14-4-5-19-16(10-14)11-20(27-19)18-7-12(8-21(28)29)6-17(22(18)30)13-2-1-3-15(9-13)24(31)32/h1-7,9-11,27,30H,8H2,(H3,25,26)(H,28,29)(H,31,32) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Binding affinity for factor VIIa/TF |
Bioorg Med Chem Lett 11: 2253-6 (2001)
BindingDB Entry DOI: 10.7270/Q2XG9QD9 |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50101870

(3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-bromo...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(cc(Br)c1O)C(O)=O Show InChI InChI=1S/C23H18BrN3O3/c24-18-11-14(23(29)30)10-17(21(18)28)20-16(8-12-4-2-1-3-5-12)15-9-13(22(25)26)6-7-19(15)27-20/h1-7,9-11,27-28H,8H2,(H3,25,26)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin(fIIa) in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50101882

(CHEMBL53829 | Phosphoric acid mono-{2-[3-(3-benzyl...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(CCOP(O)(O)=O)cc(Br)c1O Show InChI InChI=1S/C24H23BrN3O5P/c25-20-12-15(8-9-33-34(30,31)32)11-19(23(20)29)22-18(10-14-4-2-1-3-5-14)17-13-16(24(26)27)6-7-21(17)28-22/h1-7,11-13,28-29H,8-10H2,(H3,26,27)(H2,30,31,32) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin(fIIa) in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Coagulation factor X
(Homo sapiens (Human)) | BDBM50101874
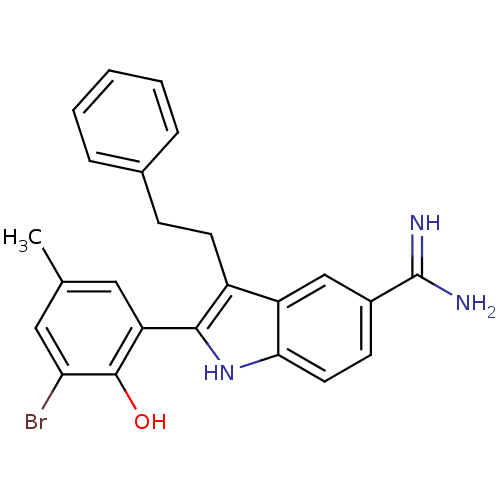
(2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-3-phenethyl-...)Show SMILES Cc1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1CCc1ccccc1)C(N)=N Show InChI InChI=1S/C24H22BrN3O/c1-14-11-19(23(29)20(25)12-14)22-17(9-7-15-5-3-2-4-6-15)18-13-16(24(26)27)8-10-21(18)28-22/h2-6,8,10-13,28-29H,7,9H2,1H3,(H3,26,27) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Coagulation factor X in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50101869

(2-[3-(3-Benzyl-5-carbamimidoyl-1H-indol-2-yl)-5-br...)Show SMILES CC(C)(C(O)=O)c1cc(Br)c(O)c(c1)-c1[nH]c2ccc(cc2c1Cc1ccccc1)C(N)=N Show InChI InChI=1S/C26H24BrN3O3/c1-26(2,25(32)33)16-12-19(23(31)20(27)13-16)22-18(10-14-6-4-3-5-7-14)17-11-15(24(28)29)8-9-21(17)30-22/h3-9,11-13,30-31H,10H2,1-2H3,(H3,28,29)(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50101867

(3-Benzyl-2-[3-bromo-2-hydroxy-5-(2H-tetrazol-5-yl)...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(cc(Br)c1O)-c1nnn[nH]1 Show InChI InChI=1S/C23H18BrN7O/c24-18-11-14(23-28-30-31-29-23)10-17(21(18)32)20-16(8-12-4-2-1-3-5-12)15-9-13(22(25)26)6-7-19(15)27-20/h1-7,9-11,27,32H,8H2,(H3,25,26)(H,28,29,30,31) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin(fIIa) in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Urokinase-type plasminogen activator
(Homo sapiens (Human)) | BDBM50101868

(3-Benzyl-2-[3-bromo-2-hydroxy-5-(2H-tetrazol-5-ylm...)Show SMILES NC(=N)c1ccc2[nH]c(c(Cc3ccccc3)c2c1)-c1cc(Cc2nnn[nH]2)cc(Br)c1O Show InChI InChI=1S/C24H20BrN7O/c25-19-10-14(11-21-29-31-32-30-21)9-18(23(19)33)22-17(8-13-4-2-1-3-5-13)16-12-15(24(26)27)6-7-20(16)28-22/h1-7,9-10,12,28,33H,8,11H2,(H3,26,27)(H,29,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against urokinase-type plasminogen activator (microPa) in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |
Prothrombin
(Homo sapiens (Human)) | BDBM50101873

(2-(3-Bromo-2-hydroxy-5-methyl-phenyl)-1H-indole-5-...)Show SMILES Cc1cc(Br)c(O)c(c1)-c1cc2cc(ccc2[nH]1)C(N)=N Show InChI InChI=1S/C16H14BrN3O/c1-8-4-11(15(21)12(17)5-8)14-7-10-6-9(16(18)19)2-3-13(10)20-14/h2-7,20-21H,1H3,(H3,18,19) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Axys Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibitory activity against thrombin(fIIa) in human plasma |
Bioorg Med Chem Lett 11: 1797-800 (2001)
BindingDB Entry DOI: 10.7270/Q20K27TW |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data