 Found 255 hits with Last Name = 'santi' and Initial = 's'
Found 255 hits with Last Name = 'santi' and Initial = 's' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50403485

(CHEMBL292857)Show SMILES C[N+]1(C)C2CCC1CC1(C2)OC(=O)C(O1)(c1ccccc1)c1ccccc1 |TLB:14:8:1:4.5,THB:10:8:1:4.5| Show InChI InChI=1S/C23H26NO3/c1-24(2)19-13-14-20(24)16-22(15-19)26-21(25)23(27-22,17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibiting [3H]pirenzepine Binding to Muscarinic receptor (M1) receptor in Rat Cortex Homogenates |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50403485

(CHEMBL292857)Show SMILES C[N+]1(C)C2CCC1CC1(C2)OC(=O)C(O1)(c1ccccc1)c1ccccc1 |TLB:14:8:1:4.5,THB:10:8:1:4.5| Show InChI InChI=1S/C23H26NO3/c1-24(2)19-13-14-20(24)16-22(15-19)26-21(25)23(27-22,17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 0.398 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibiting [3H]pirenzepine Binding to Muscarinic receptor (M1) receptor in Rat Cortex Homogenates |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50176065
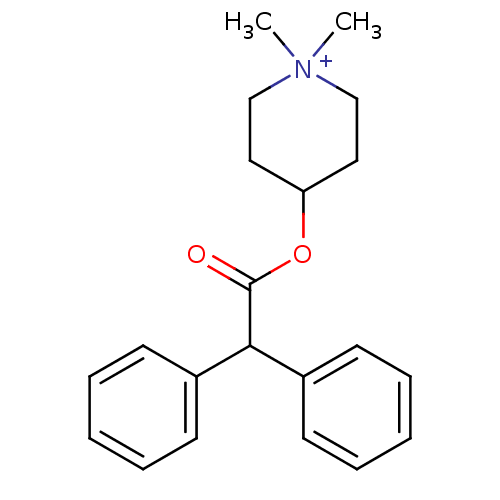
(4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...)Show InChI InChI=1S/C21H26NO2/c1-22(2)15-13-19(14-16-22)24-21(23)20(17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| 0.513 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluation of antagonistic affinity against muscarinic receptor (M2) in guinea pig left atria |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50176065

(4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...)Show InChI InChI=1S/C21H26NO2/c1-22(2)15-13-19(14-16-22)24-21(23)20(17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| 0.589 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibiting [3H]pirenzepine Binding to Muscarinic receptor (M1) receptor in Rat Cortex Homogenates |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50082769
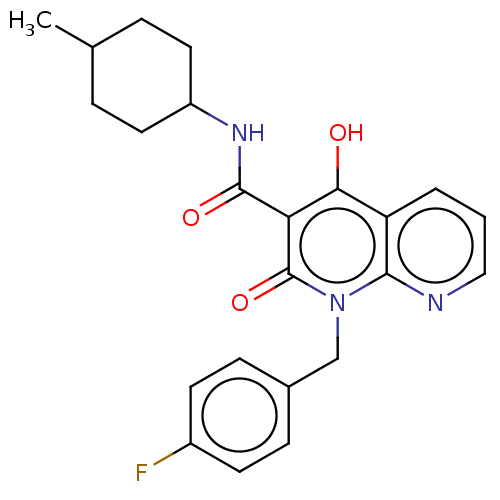
(CHEMBL3422790)Show SMILES CC1CCC(CC1)NC(=O)c1c(O)c2cccnc2n(Cc2ccc(F)cc2)c1=O |(9.06,6.01,;7.99,5.4,;6.66,6.17,;5.33,5.4,;5.33,3.86,;6.66,3.09,;7.99,3.86,;3.99,3.08,;3.99,1.54,;5.06,.93,;2.66,.77,;1.33,1.54,;1.33,2.77,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;1.33,-3.08,;2.67,-3.85,;2.68,-5.39,;4.01,-6.15,;5.34,-5.38,;6.41,-5.99,;5.34,-3.84,;4,-3.07,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C23H24FN3O3/c1-14-4-10-17(11-5-14)26-22(29)19-20(28)18-3-2-12-25-21(18)27(23(19)30)13-15-6-8-16(24)9-7-15/h2-3,6-9,12,14,17,28H,4-5,10-11,13H2,1H3,(H,26,29) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50082777
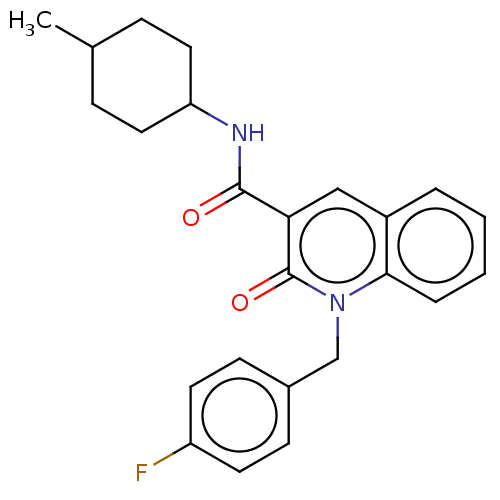
(CHEMBL3422784)Show SMILES CC1CCC(CC1)NC(=O)c1cc2ccccc2n(Cc2ccc(F)cc2)c1=O |(9.06,6.01,;7.99,5.4,;6.66,6.17,;5.33,5.4,;5.33,3.86,;6.66,3.09,;7.99,3.86,;3.99,3.08,;3.99,1.54,;5.06,.93,;2.66,.77,;1.33,1.54,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;1.33,-3.08,;2.67,-3.85,;2.68,-5.39,;4.01,-6.15,;5.34,-5.38,;6.41,-5.99,;5.34,-3.84,;4,-3.07,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C24H25FN2O2/c1-16-6-12-20(13-7-16)26-23(28)21-14-18-4-2-3-5-22(18)27(24(21)29)15-17-8-10-19(25)11-9-17/h2-5,8-11,14,16,20H,6-7,12-13,15H2,1H3,(H,26,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.75 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50176065

(4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...)Show InChI InChI=1S/C21H26NO2/c1-22(2)15-13-19(14-16-22)24-21(23)20(17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibiting [3H]N-Methyl-scopolamine Binding to Muscarinic receptor (M3) in Rat Submaxillary Gland |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50082768
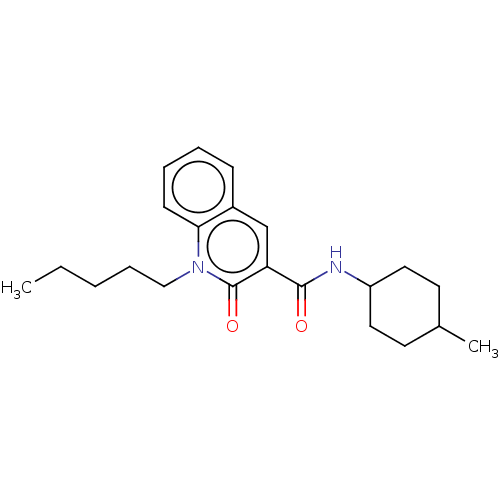
(CHEMBL3422788)Show SMILES CCCCCn1c2ccccc2cc(C(=O)NC2CCC(C)CC2)c1=O |(4.01,-7.39,;4.01,-6.16,;2.67,-5.39,;2.67,-3.85,;1.33,-3.08,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;3.99,1.54,;5.06,.93,;3.99,3.08,;5.33,3.86,;5.33,5.4,;6.66,6.17,;7.99,5.4,;9.06,6.01,;7.99,3.86,;6.66,3.09,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C22H30N2O2/c1-3-4-7-14-24-20-9-6-5-8-17(20)15-19(22(24)26)21(25)23-18-12-10-16(2)11-13-18/h5-6,8-9,15-16,18H,3-4,7,10-14H2,1-2H3,(H,23,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50403484
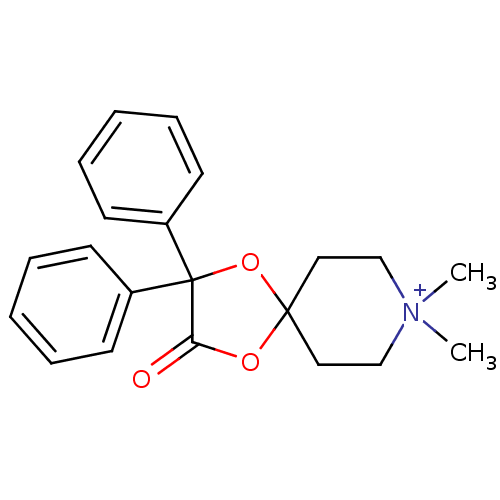
(CHEMBL306182)Show SMILES C[N+]1(C)CCC2(CC1)OC(=O)C(O2)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C21H24NO3/c1-22(2)15-13-20(14-16-22)24-19(23)21(25-20,17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibiting [3H]N-Methyl-scopolamine Binding to Muscarinic receptor (M2) in Rat Heart |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50180036
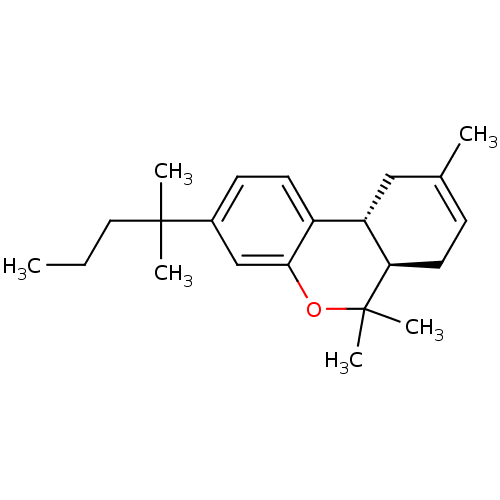
((6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetra...)Show SMILES CCCC(C)(C)c1ccc2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |r,c:13| Show InChI InChI=1S/C22H32O/c1-7-12-21(3,4)16-9-10-17-18-13-15(2)8-11-19(18)22(5,6)23-20(17)14-16/h8-10,14,18-19H,7,11-13H2,1-6H3/t18-,19+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50403484

(CHEMBL306182)Show SMILES C[N+]1(C)CCC2(CC1)OC(=O)C(O2)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C21H24NO3/c1-22(2)15-13-20(14-16-22)24-19(23)21(25-20,17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibiting [3H]N-Methyl-scopolamine Binding to Muscarinic receptor (M3) in Rat Submaxillary Gland |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50176065

(4-DAMP | 4-Diphenylacetoxy-1,1-dimethyl-piperidini...)Show InChI InChI=1S/C21H26NO2/c1-22(2)15-13-19(14-16-22)24-21(23)20(17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibiting [3H]N-Methyl-scopolamine Binding to Muscarinic receptor (M2) in Rat Heart |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50082776
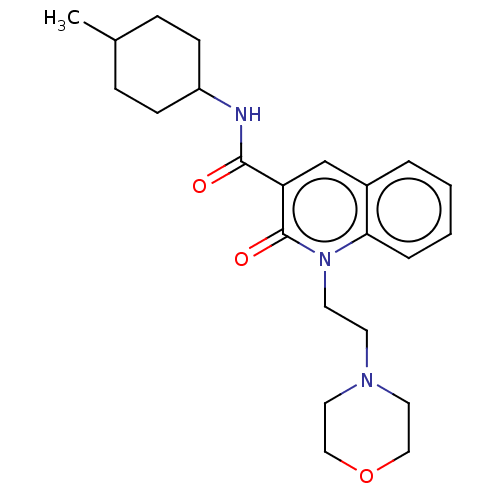
(CHEMBL3422785)Show SMILES CC1CCC(CC1)NC(=O)c1cc2ccccc2n(CCN2CCOCC2)c1=O |(9.06,6.01,;7.99,5.4,;6.66,6.17,;5.33,5.4,;5.33,3.86,;6.66,3.09,;7.99,3.86,;3.99,3.08,;3.99,1.54,;5.06,.93,;2.66,.77,;1.33,1.54,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;1.33,-3.08,;2.67,-3.85,;2.67,-5.39,;4.01,-6.16,;4.01,-7.7,;2.67,-8.47,;1.34,-7.7,;1.34,-6.16,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C23H31N3O3/c1-17-6-8-19(9-7-17)24-22(27)20-16-18-4-2-3-5-21(18)26(23(20)28)11-10-25-12-14-29-15-13-25/h2-5,16-17,19H,6-15H2,1H3,(H,24,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50403485

(CHEMBL292857)Show SMILES C[N+]1(C)C2CCC1CC1(C2)OC(=O)C(O1)(c1ccccc1)c1ccccc1 |TLB:14:8:1:4.5,THB:10:8:1:4.5| Show InChI InChI=1S/C23H26NO3/c1-24(2)19-13-14-20(24)16-22(15-19)26-21(25)23(27-22,17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against Muscarinic acetylcholine receptor M4 in NG 108-15 cell homogenates |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50403485

(CHEMBL292857)Show SMILES C[N+]1(C)C2CCC1CC1(C2)OC(=O)C(O1)(c1ccccc1)c1ccccc1 |TLB:14:8:1:4.5,THB:10:8:1:4.5| Show InChI InChI=1S/C23H26NO3/c1-24(2)19-13-14-20(24)16-22(15-19)26-21(25)23(27-22,17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for binding affinity against Muscarinic acetylcholine receptor M4 in NG 108-15 cell homogenates |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50403484

(CHEMBL306182)Show SMILES C[N+]1(C)CCC2(CC1)OC(=O)C(O2)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C21H24NO3/c1-22(2)15-13-20(14-16-22)24-19(23)21(25-20,17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12H,13-16H2,1-2H3/q+1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluation of antagonistic affinity against muscarinic receptor (M3) in guinea pig ileum |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM39341

(11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...)Show InChI InChI=1S/C19H21N5O2/c1-22-9-11-23(12-10-22)13-17(25)24-16-7-3-2-5-14(16)19(26)21-15-6-4-8-20-18(15)24/h2-8H,9-13H2,1H3,(H,21,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibiting [3H]pirenzepine Binding to Muscarinic receptor (M1) receptor in Rat Cortex Homogenates |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50082772
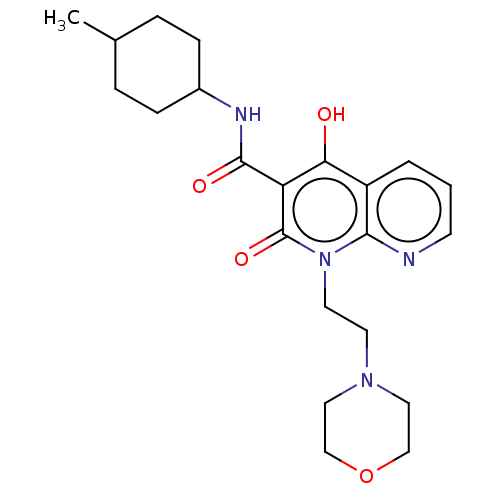
(CHEMBL3422791)Show SMILES CC1CCC(CC1)NC(=O)c1c(O)c2cccnc2n(CCN2CCOCC2)c1=O |(9.06,6.01,;7.99,5.4,;6.66,6.17,;5.33,5.4,;5.33,3.86,;6.66,3.09,;7.99,3.86,;3.99,3.08,;3.99,1.54,;5.06,.93,;2.66,.77,;1.33,1.54,;1.33,2.77,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;1.33,-3.08,;2.67,-3.85,;2.67,-5.39,;4.01,-6.16,;4.01,-7.7,;2.67,-8.47,;1.34,-7.7,;1.34,-6.16,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C22H30N4O4/c1-15-4-6-16(7-5-15)24-21(28)18-19(27)17-3-2-8-23-20(17)26(22(18)29)10-9-25-11-13-30-14-12-25/h2-3,8,15-16,27H,4-7,9-14H2,1H3,(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 7.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50403485

(CHEMBL292857)Show SMILES C[N+]1(C)C2CCC1CC1(C2)OC(=O)C(O1)(c1ccccc1)c1ccccc1 |TLB:14:8:1:4.5,THB:10:8:1:4.5| Show InChI InChI=1S/C23H26NO3/c1-24(2)19-13-14-20(24)16-22(15-19)26-21(25)23(27-22,17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibiting [3H]N-Methyl-scopolamine Binding to Muscarinic receptor (M3) in Rat Submaxillary Gland |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM50403485

(CHEMBL292857)Show SMILES C[N+]1(C)C2CCC1CC1(C2)OC(=O)C(O1)(c1ccccc1)c1ccccc1 |TLB:14:8:1:4.5,THB:10:8:1:4.5| Show InChI InChI=1S/C23H26NO3/c1-24(2)19-13-14-20(24)16-22(15-19)26-21(25)23(27-22,17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 10.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibiting [3H]N-Methyl-scopolamine Binding to Muscarinic receptor (M3) in Rat Submaxillary Gland |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM50403484

(CHEMBL306182)Show SMILES C[N+]1(C)CCC2(CC1)OC(=O)C(O2)(c1ccccc1)c1ccccc1 Show InChI InChI=1S/C21H24NO3/c1-22(2)15-13-20(14-16-22)24-19(23)21(25-20,17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibiting [3H]N-Methyl-scopolamine Binding to Muscarinic acetylcholine receptor M4 in NG 108-15 Cell |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50082771
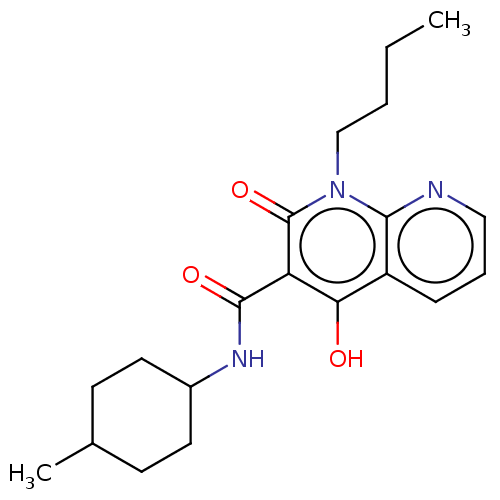
(CHEMBL3422792)Show SMILES CCCCn1c2ncccc2c(O)c(C(=O)NC2CCC(C)CC2)c1=O |(3.74,-6,;2.67,-5.39,;2.67,-3.85,;1.33,-3.08,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.33,2.77,;2.66,.77,;3.99,1.54,;5.06,.93,;3.99,3.08,;5.33,3.86,;5.33,5.4,;6.66,6.17,;7.99,5.4,;9.06,6.01,;7.99,3.86,;6.66,3.09,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C20H27N3O3/c1-3-4-12-23-18-15(6-5-11-21-18)17(24)16(20(23)26)19(25)22-14-9-7-13(2)8-10-14/h5-6,11,13-14,24H,3-4,7-10,12H2,1-2H3,(H,22,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50403485

(CHEMBL292857)Show SMILES C[N+]1(C)C2CCC1CC1(C2)OC(=O)C(O1)(c1ccccc1)c1ccccc1 |TLB:14:8:1:4.5,THB:10:8:1:4.5| Show InChI InChI=1S/C23H26NO3/c1-24(2)19-13-14-20(24)16-22(15-19)26-21(25)23(27-22,17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibiting [3H]N-Methyl-scopolamine Binding to Muscarinic receptor (M2) in Rat Heart |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50082777

(CHEMBL3422784)Show SMILES CC1CCC(CC1)NC(=O)c1cc2ccccc2n(Cc2ccc(F)cc2)c1=O |(9.06,6.01,;7.99,5.4,;6.66,6.17,;5.33,5.4,;5.33,3.86,;6.66,3.09,;7.99,3.86,;3.99,3.08,;3.99,1.54,;5.06,.93,;2.66,.77,;1.33,1.54,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;1.33,-3.08,;2.67,-3.85,;2.68,-5.39,;4.01,-6.15,;5.34,-5.38,;6.41,-5.99,;5.34,-3.84,;4,-3.07,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C24H25FN2O2/c1-16-6-12-20(13-7-16)26-23(28)21-14-18-4-2-3-5-22(18)27(24(21)29)15-17-8-10-19(25)11-9-17/h2-5,8-11,14,16,20H,6-7,12-13,15H2,1H3,(H,26,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB1 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50082774
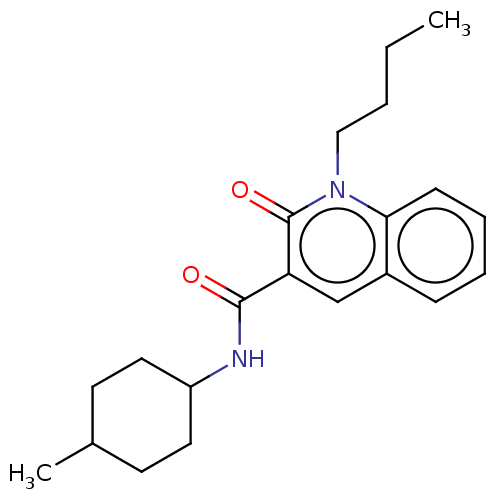
(CHEMBL3422787)Show SMILES CCCCn1c2ccccc2cc(C(=O)NC2CCC(C)CC2)c1=O |(3.74,-6,;2.67,-5.39,;2.67,-3.85,;1.33,-3.08,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;3.99,1.54,;5.06,.93,;3.99,3.08,;5.33,3.86,;5.33,5.4,;6.66,6.17,;7.99,5.4,;9.06,6.01,;7.99,3.86,;6.66,3.09,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C21H28N2O2/c1-3-4-13-23-19-8-6-5-7-16(19)14-18(21(23)25)20(24)22-17-11-9-15(2)10-12-17/h5-8,14-15,17H,3-4,9-13H2,1-2H3,(H,22,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50082775
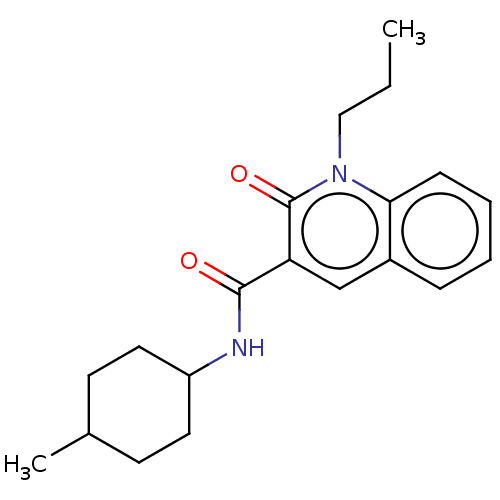
(CHEMBL3422786)Show SMILES CCCn1c2ccccc2cc(C(=O)NC2CCC(C)CC2)c1=O |(2.67,-5.08,;2.67,-3.85,;1.33,-3.08,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;3.99,1.54,;5.06,.93,;3.99,3.08,;5.33,3.86,;5.33,5.4,;6.66,6.17,;7.99,5.4,;9.06,6.01,;7.99,3.86,;6.66,3.09,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C20H26N2O2/c1-3-12-22-18-7-5-4-6-15(18)13-17(20(22)24)19(23)21-16-10-8-14(2)9-11-16/h4-7,13-14,16H,3,8-12H2,1-2H3,(H,21,23) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM50403485

(CHEMBL292857)Show SMILES C[N+]1(C)C2CCC1CC1(C2)OC(=O)C(O1)(c1ccccc1)c1ccccc1 |TLB:14:8:1:4.5,THB:10:8:1:4.5| Show InChI InChI=1S/C23H26NO3/c1-24(2)19-13-14-20(24)16-22(15-19)26-21(25)23(27-22,17-9-5-3-6-10-17)18-11-7-4-8-12-18/h3-12,19-20H,13-16H2,1-2H3/q+1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 33 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibiting [3H]N-Methyl-scopolamine Binding to Muscarinic receptor (M2) in Rat Heart |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M4
(Homo sapiens (Human)) | BDBM39341

(11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...)Show InChI InChI=1S/C19H21N5O2/c1-22-9-11-23(12-10-22)13-17(25)24-16-7-3-2-5-14(16)19(26)21-15-6-4-8-20-18(15)24/h2-8H,9-13H2,1H3,(H,21,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluation of antagonistic affinity against muscarinic receptor (M3) in guinea pig ileum |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50082773
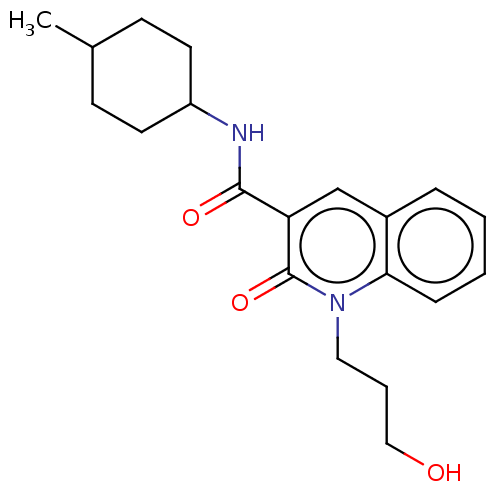
(CHEMBL3422789)Show SMILES CC1CCC(CC1)NC(=O)c1cc2ccccc2n(CCCO)c1=O |(9.06,6.01,;7.99,5.4,;6.66,6.17,;5.33,5.4,;5.33,3.86,;6.66,3.09,;7.99,3.86,;3.99,3.08,;3.99,1.54,;5.06,.93,;2.66,.77,;1.33,1.54,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;1.33,-3.08,;2.67,-3.85,;2.67,-5.39,;3.74,-6,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C20H26N2O3/c1-14-7-9-16(10-8-14)21-19(24)17-13-15-5-2-3-6-18(15)22(20(17)25)11-4-12-23/h2-3,5-6,13-14,16,23H,4,7-12H2,1H3,(H,21,24) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50082769

(CHEMBL3422790)Show SMILES CC1CCC(CC1)NC(=O)c1c(O)c2cccnc2n(Cc2ccc(F)cc2)c1=O |(9.06,6.01,;7.99,5.4,;6.66,6.17,;5.33,5.4,;5.33,3.86,;6.66,3.09,;7.99,3.86,;3.99,3.08,;3.99,1.54,;5.06,.93,;2.66,.77,;1.33,1.54,;1.33,2.77,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;1.33,-3.08,;2.67,-3.85,;2.68,-5.39,;4.01,-6.15,;5.34,-5.38,;6.41,-5.99,;5.34,-3.84,;4,-3.07,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C23H24FN3O3/c1-14-4-10-17(11-5-14)26-22(29)19-20(28)18-3-2-12-25-21(18)27(23(19)30)13-15-6-8-16(24)9-7-15/h2-3,6-9,12,14,17,28H,4-5,10-11,13H2,1H3,(H,26,29) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 139 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB1 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M3
(RAT) | BDBM39341

(11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...)Show InChI InChI=1S/C19H21N5O2/c1-22-9-11-23(12-10-22)13-17(25)24-16-7-3-2-5-14(16)19(26)21-15-6-4-8-20-18(15)24/h2-8H,9-13H2,1H3,(H,21,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 174 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibiting [3H]N-Methyl-scopolamine Binding to Muscarinic receptor (M3) in Rat Submaxillary Gland |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 2
(Homo sapiens (Human)) | BDBM50082770
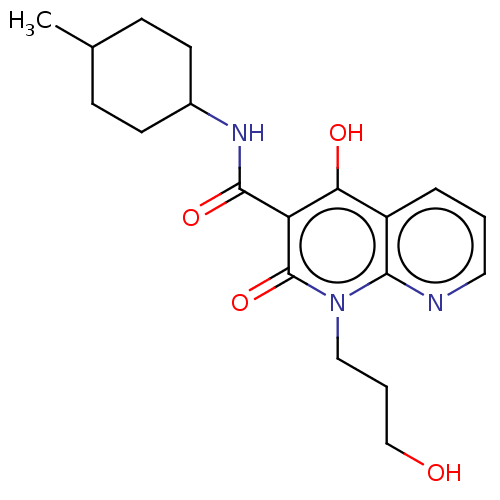
(CHEMBL3422793)Show SMILES CC1CCC(CC1)NC(=O)c1c(O)c2cccnc2n(CCCO)c1=O |(9.06,6.01,;7.99,5.4,;6.66,6.17,;5.33,5.4,;5.33,3.86,;6.66,3.09,;7.99,3.86,;3.99,3.08,;3.99,1.54,;5.06,.93,;2.66,.77,;1.33,1.54,;1.33,2.77,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;1.33,-3.08,;2.67,-3.85,;2.67,-5.39,;3.74,-6,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C19H25N3O4/c1-12-5-7-13(8-6-12)21-18(25)15-16(24)14-4-2-9-20-17(14)22(19(15)26)10-3-11-23/h2,4,9,12-13,23-24H,3,5-8,10-11H2,1H3,(H,21,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 215 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB2 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50082768

(CHEMBL3422788)Show SMILES CCCCCn1c2ccccc2cc(C(=O)NC2CCC(C)CC2)c1=O |(4.01,-7.39,;4.01,-6.16,;2.67,-5.39,;2.67,-3.85,;1.33,-3.08,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;3.99,1.54,;5.06,.93,;3.99,3.08,;5.33,3.86,;5.33,5.4,;6.66,6.17,;7.99,5.4,;9.06,6.01,;7.99,3.86,;6.66,3.09,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C22H30N2O2/c1-3-4-7-14-24-20-9-6-5-8-17(20)15-19(22(24)26)21(25)23-18-12-10-16(2)11-13-18/h5-6,8-9,15-16,18H,3-4,7,10-14H2,1-2H3,(H,23,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 227 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB1 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50187669

(CHEMBL207208 | N-(4-methoxy-phenyl)-2-cyclopentyl-...)Show SMILES COc1ccc(Nc2nc3ccccc3c3nc([nH]c23)C2CCCC2)cc1 Show InChI InChI=1S/C22H22N4O/c1-27-16-12-10-15(11-13-16)23-22-20-19(17-8-4-5-9-18(17)24-22)25-21(26-20)14-6-2-3-7-14/h4-5,8-14H,2-3,6-7H2,1H3,(H,23,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 410 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human adenosine A3 receptor in CHO membrane |
J Med Chem 49: 3354-61 (2006)
Article DOI: 10.1021/jm060086s
BindingDB Entry DOI: 10.7270/Q2K073V0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50082774

(CHEMBL3422787)Show SMILES CCCCn1c2ccccc2cc(C(=O)NC2CCC(C)CC2)c1=O |(3.74,-6,;2.67,-5.39,;2.67,-3.85,;1.33,-3.08,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;3.99,1.54,;5.06,.93,;3.99,3.08,;5.33,3.86,;5.33,5.4,;6.66,6.17,;7.99,5.4,;9.06,6.01,;7.99,3.86,;6.66,3.09,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C21H28N2O2/c1-3-4-13-23-19-8-6-5-7-16(19)14-18(21(23)25)20(24)22-17-11-9-15(2)10-12-17/h5-8,14-15,17H,3-4,9-13H2,1-2H3,(H,22,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 551 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB1 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50082772

(CHEMBL3422791)Show SMILES CC1CCC(CC1)NC(=O)c1c(O)c2cccnc2n(CCN2CCOCC2)c1=O |(9.06,6.01,;7.99,5.4,;6.66,6.17,;5.33,5.4,;5.33,3.86,;6.66,3.09,;7.99,3.86,;3.99,3.08,;3.99,1.54,;5.06,.93,;2.66,.77,;1.33,1.54,;1.33,2.77,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;1.33,-3.08,;2.67,-3.85,;2.67,-5.39,;4.01,-6.16,;4.01,-7.7,;2.67,-8.47,;1.34,-7.7,;1.34,-6.16,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C22H30N4O4/c1-15-4-6-16(7-5-15)24-21(28)18-19(27)17-3-2-8-23-20(17)26(22(18)29)10-9-25-11-13-30-14-12-25/h2-3,8,15-16,27H,4-7,9-14H2,1H3,(H,24,28) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB1 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50180036

((6aR,10aR)-3-(1,1-dimethylbutyl)-6a,7,10,10a-tetra...)Show SMILES CCCC(C)(C)c1ccc2[C@@H]3CC(C)=CC[C@H]3C(C)(C)Oc2c1 |r,c:13| Show InChI InChI=1S/C22H32O/c1-7-12-21(3,4)16-9-10-17-18-13-15(2)8-11-19(18)22(5,6)23-20(17)14-16/h8-10,14,18-19H,7,11-13H2,1-6H3/t18-,19+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 677 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB1 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50082771

(CHEMBL3422792)Show SMILES CCCCn1c2ncccc2c(O)c(C(=O)NC2CCC(C)CC2)c1=O |(3.74,-6,;2.67,-5.39,;2.67,-3.85,;1.33,-3.08,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;1.33,2.77,;2.66,.77,;3.99,1.54,;5.06,.93,;3.99,3.08,;5.33,3.86,;5.33,5.4,;6.66,6.17,;7.99,5.4,;9.06,6.01,;7.99,3.86,;6.66,3.09,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C20H27N3O3/c1-3-4-12-23-18-15(6-5-11-21-18)17(24)16(20(23)26)19(25)22-14-9-7-13(2)8-10-14/h5-6,11,13-14,24H,3-4,7-10,12H2,1-2H3,(H,22,25) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 702 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB1 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50011596

((2-Cyclopentyl-1H-imidazo[4,5-c]quinolin-4-yl)-phe...)Show SMILES C1CCC(C1)c1nc2c([nH]1)c(Nc1ccccc1)nc1ccccc21 Show InChI InChI=1S/C21H20N4/c1-2-10-15(11-3-1)22-21-19-18(16-12-6-7-13-17(16)23-21)24-20(25-19)14-8-4-5-9-14/h1-3,6-7,10-14H,4-5,8-9H2,(H,22,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 786 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human adenosine A3 receptor in CHO membrane |
J Med Chem 49: 3354-61 (2006)
Article DOI: 10.1021/jm060086s
BindingDB Entry DOI: 10.7270/Q2K073V0 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M2
(RAT) | BDBM39341

(11-[(4-methylpiperazin-1-yl)acetyl]-5,11-dihydro-6...)Show InChI InChI=1S/C19H21N5O2/c1-22-9-11-23(12-10-22)13-17(25)24-16-7-3-2-5-14(16)19(26)21-15-6-4-8-20-18(15)24/h2-8H,9-13H2,1H3,(H,21,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 794 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Evaluation of antagonistic affinity against muscarinic receptor (M3) in guinea pig ileum |
Bioorg Med Chem Lett 5: 2325-2330 (1995)
Article DOI: 10.1016/0960-894X(95)00403-G
BindingDB Entry DOI: 10.7270/Q28G8MW2 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50082776

(CHEMBL3422785)Show SMILES CC1CCC(CC1)NC(=O)c1cc2ccccc2n(CCN2CCOCC2)c1=O |(9.06,6.01,;7.99,5.4,;6.66,6.17,;5.33,5.4,;5.33,3.86,;6.66,3.09,;7.99,3.86,;3.99,3.08,;3.99,1.54,;5.06,.93,;2.66,.77,;1.33,1.54,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;1.33,-3.08,;2.67,-3.85,;2.67,-5.39,;4.01,-6.16,;4.01,-7.7,;2.67,-8.47,;1.34,-7.7,;1.34,-6.16,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C23H31N3O3/c1-17-6-8-19(9-7-17)24-22(27)20-16-18-4-2-3-5-21(18)26(23(20)28)11-10-25-12-14-29-15-13-25/h2-5,16-17,19H,6-15H2,1H3,(H,24,27) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB1 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50187670
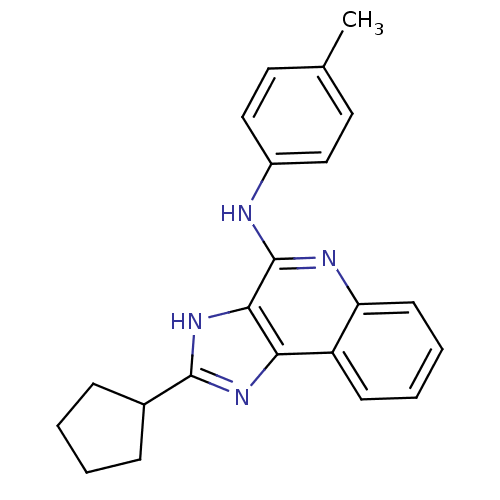
(CHEMBL210380 | N-(4-methyl-phenyl)-2-cyclopentyl-1...)Show SMILES Cc1ccc(Nc2nc3ccccc3c3nc([nH]c23)C2CCCC2)cc1 Show InChI InChI=1S/C22H22N4/c1-14-10-12-16(13-11-14)23-22-20-19(17-8-4-5-9-18(17)24-22)25-21(26-20)15-6-2-3-7-15/h4-5,8-13,15H,2-3,6-7H2,1H3,(H,23,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human adenosine A3 receptor in CHO membrane |
J Med Chem 49: 3354-61 (2006)
Article DOI: 10.1021/jm060086s
BindingDB Entry DOI: 10.7270/Q2K073V0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50187671

(CHEMBL378047 | N-(4-chloro-phenyl)-2-cyclopentyl-1...)Show SMILES Clc1ccc(Nc2nc3ccccc3c3nc([nH]c23)C2CCCC2)cc1 Show InChI InChI=1S/C21H19ClN4/c22-14-9-11-15(12-10-14)23-21-19-18(16-7-3-4-8-17(16)24-21)25-20(26-19)13-5-1-2-6-13/h3-4,7-13H,1-2,5-6H2,(H,23,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human adenosine A3 receptor in CHO membrane |
J Med Chem 49: 3354-61 (2006)
Article DOI: 10.1021/jm060086s
BindingDB Entry DOI: 10.7270/Q2K073V0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50082775

(CHEMBL3422786)Show SMILES CCCn1c2ccccc2cc(C(=O)NC2CCC(C)CC2)c1=O |(2.67,-5.08,;2.67,-3.85,;1.33,-3.08,;1.33,-1.54,;,-.77,;-1.33,-1.54,;-2.68,-.77,;-2.68,.77,;-1.33,1.54,;,.77,;1.33,1.54,;2.66,.77,;3.99,1.54,;5.06,.93,;3.99,3.08,;5.33,3.86,;5.33,5.4,;6.66,6.17,;7.99,5.4,;9.06,6.01,;7.99,3.86,;6.66,3.09,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C20H26N2O2/c1-3-12-22-18-7-5-4-6-15(18)13-17(20(22)24)19(23)21-16-10-8-14(2)9-11-16/h4-7,13-14,16H,3,8-12H2,1-2H3,(H,21,23) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.61E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB1 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A2a
(Homo sapiens (Human)) | BDBM50011596

((2-Cyclopentyl-1H-imidazo[4,5-c]quinolin-4-yl)-phe...)Show SMILES C1CCC(C1)c1nc2c([nH]1)c(Nc1ccccc1)nc1ccccc21 Show InChI InChI=1S/C21H20N4/c1-2-10-15(11-3-1)22-21-19-18(16-12-6-7-13-17(16)23-21)24-20(25-19)14-8-4-5-9-14/h1-3,6-7,10-14H,4-5,8-9H2,(H,22,23)(H,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-PIA from human adenosine A2A receptor in HEK293 cells |
J Med Chem 49: 3354-61 (2006)
Article DOI: 10.1021/jm060086s
BindingDB Entry DOI: 10.7270/Q2K073V0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50011596

((2-Cyclopentyl-1H-imidazo[4,5-c]quinolin-4-yl)-phe...)Show SMILES C1CCC(C1)c1nc2c([nH]1)c(Nc1ccccc1)nc1ccccc21 Show InChI InChI=1S/C21H20N4/c1-2-10-15(11-3-1)22-21-19-18(16-12-6-7-13-17(16)23-21)24-20(25-19)14-8-4-5-9-14/h1-3,6-7,10-14H,4-5,8-9H2,(H,22,23)(H,24,25) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-PIA from human adenosine A1 receptor in CHO membrane |
J Med Chem 49: 3354-61 (2006)
Article DOI: 10.1021/jm060086s
BindingDB Entry DOI: 10.7270/Q2K073V0 |
More data for this
Ligand-Target Pair | |
Cannabinoid receptor 1
(Homo sapiens (Human)) | BDBM50082773

(CHEMBL3422789)Show SMILES CC1CCC(CC1)NC(=O)c1cc2ccccc2n(CCCO)c1=O |(9.06,6.01,;7.99,5.4,;6.66,6.17,;5.33,5.4,;5.33,3.86,;6.66,3.09,;7.99,3.86,;3.99,3.08,;3.99,1.54,;5.06,.93,;2.66,.77,;1.33,1.54,;,.77,;-1.33,1.54,;-2.68,.77,;-2.68,-.77,;-1.33,-1.54,;,-.77,;1.33,-1.54,;1.33,-3.08,;2.67,-3.85,;2.67,-5.39,;3.74,-6,;2.66,-.77,;3.73,-1.38,)| Show InChI InChI=1S/C20H26N2O3/c1-14-7-9-16(10-8-14)21-19(24)17-13-15-5-2-3-6-18(15)22(20(17)25)11-4-12-23/h2-3,5-6,13-14,16,23H,4,7-12H2,1H3,(H,21,24) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Pisa
Curated by ChEMBL
| Assay Description
Displacement of [3H]-CP-55940 from recombinant human CB1 receptor overexpressed in HEK293 cell membranes after 90 mins |
Eur J Med Chem 97: 10-8 (2015)
Article DOI: 10.1016/j.ejmech.2015.04.034
BindingDB Entry DOI: 10.7270/Q2VQ34DS |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50187670

(CHEMBL210380 | N-(4-methyl-phenyl)-2-cyclopentyl-1...)Show SMILES Cc1ccc(Nc2nc3ccccc3c3nc([nH]c23)C2CCCC2)cc1 Show InChI InChI=1S/C22H22N4/c1-14-10-12-16(13-11-14)23-22-20-19(17-8-4-5-9-18(17)24-22)25-21(26-20)15-6-2-3-7-15/h4-5,8-13,15H,2-3,6-7H2,1H3,(H,23,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.85E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-PIA from human adenosine A1 receptor in CHO membrane |
J Med Chem 49: 3354-61 (2006)
Article DOI: 10.1021/jm060086s
BindingDB Entry DOI: 10.7270/Q2K073V0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A1
(Homo sapiens (Human)) | BDBM50187669

(CHEMBL207208 | N-(4-methoxy-phenyl)-2-cyclopentyl-...)Show SMILES COc1ccc(Nc2nc3ccccc3c3nc([nH]c23)C2CCCC2)cc1 Show InChI InChI=1S/C22H22N4O/c1-27-16-12-10-15(11-13-16)23-22-20-19(17-8-4-5-9-18(17)24-22)25-21(26-20)14-6-2-3-7-14/h4-5,8-14H,2-3,6-7H2,1H3,(H,23,24)(H,25,26) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.17E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [3H]R-PIA from human adenosine A1 receptor in CHO membrane |
J Med Chem 49: 3354-61 (2006)
Article DOI: 10.1021/jm060086s
BindingDB Entry DOI: 10.7270/Q2K073V0 |
More data for this
Ligand-Target Pair | |
Adenosine receptor A3
(Homo sapiens (Human)) | BDBM50187672
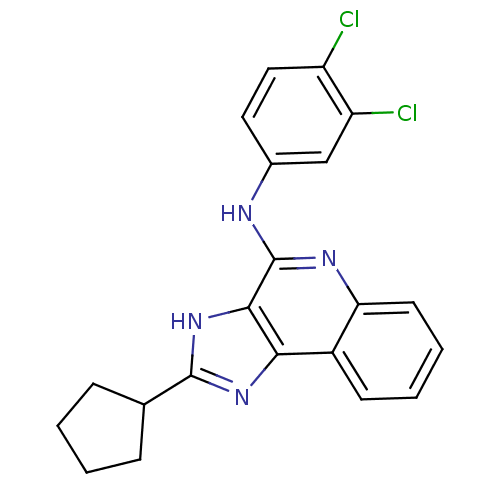
(CHEMBL207209 | N-(3,4-dichloro-phenyl)-2-cyclopent...)Show SMILES Clc1ccc(Nc2nc3ccccc3c3nc([nH]c23)C2CCCC2)cc1Cl Show InChI InChI=1S/C21H18Cl2N4/c22-15-10-9-13(11-16(15)23)24-21-19-18(14-7-3-4-8-17(14)25-21)26-20(27-19)12-5-1-2-6-12/h3-4,7-12H,1-2,5-6H2,(H,24,25)(H,26,27) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Leiden University
Curated by ChEMBL
| Assay Description
Displacement of [125I]I-AB-MECA from human adenosine A3 receptor in CHO membrane |
J Med Chem 49: 3354-61 (2006)
Article DOI: 10.1021/jm060086s
BindingDB Entry DOI: 10.7270/Q2K073V0 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data