

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50078646 (CHEMBL49322 | N-[4-(4,10-Dihydro-1-thia-9-aza-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]-AVP binding to human V2 receptor from murine fibroblast cell line (LV2) | Bioorg Med Chem Lett 9: 1733-6 (1999) BindingDB Entry DOI: 10.7270/Q2736Q34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50078656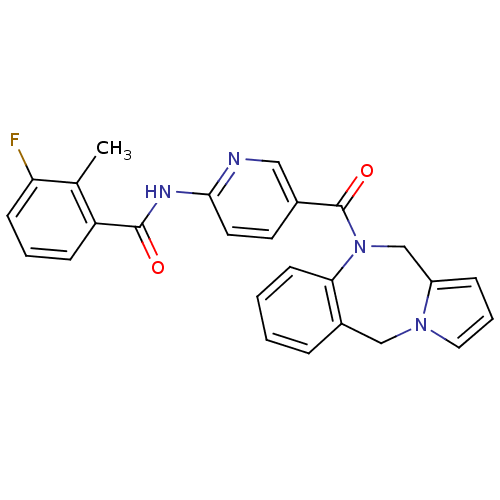 (CHEMBL46295 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to human V2 receptor | Bioorg Med Chem Lett 15: 5003-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.007 BindingDB Entry DOI: 10.7270/Q2C828WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50078652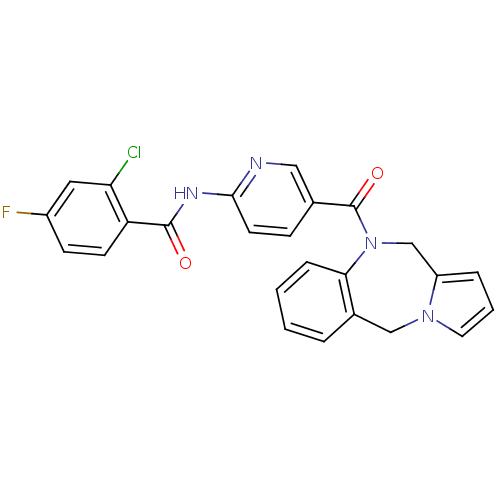 (CHEMBL301788 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]-AVP binding to human V2 receptor from murine fibroblast cell line (LV2) | Bioorg Med Chem Lett 9: 1733-6 (1999) BindingDB Entry DOI: 10.7270/Q2736Q34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065124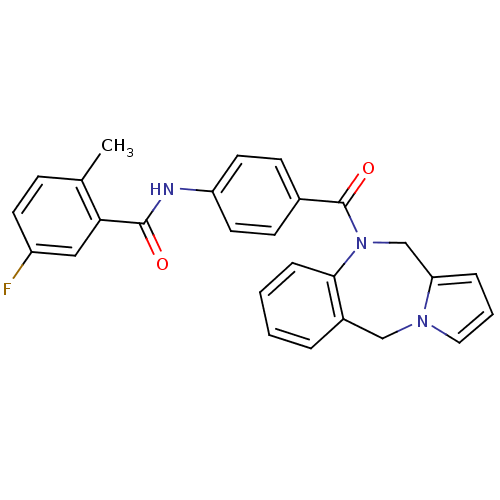 (CHEMBL68085 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50078660 (CHEMBL299532 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50078651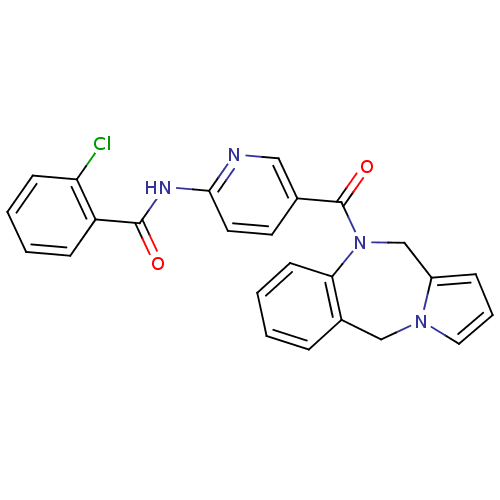 (CHEMBL300963 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50129464 (CHEMBL71355 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50173291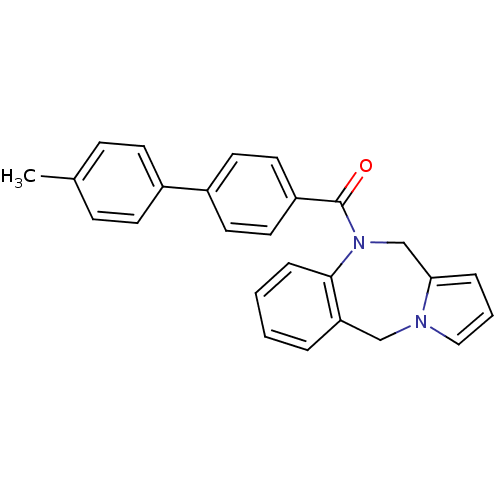 ((5H,11H-Benzo[e]pyrrolo[1,2-a][1,4]diazepin-10-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against [3H]-AVP binding to human vasopressin V1a receptor | Bioorg Med Chem Lett 15: 5003-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.007 BindingDB Entry DOI: 10.7270/Q2C828WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065115 (3-chloro-4-(10,11-dihydro-5H-benzo[e]pyrrolo[1,2-a...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50173297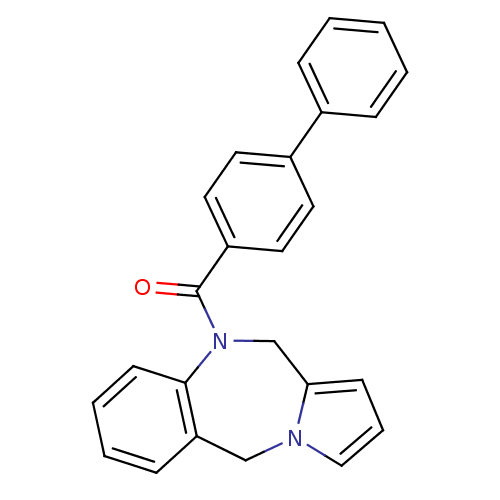 ((5H,11H-Benzo[e]pyrrolo[1,2-a][1,4]diazepin-10-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against [3H]-AVP binding to human vasopressin V1a receptor | Bioorg Med Chem Lett 15: 5003-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.007 BindingDB Entry DOI: 10.7270/Q2C828WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065110 (CHEMBL418890 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50078654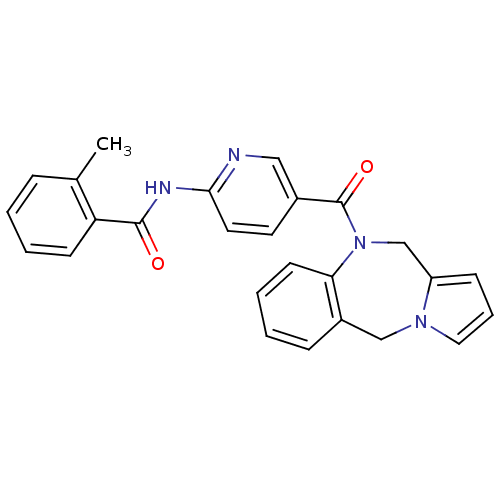 (CHEMBL297990 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of (P[3H]-AVP binding towards isolated rat kidney medullary Vasopre ssin V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50078655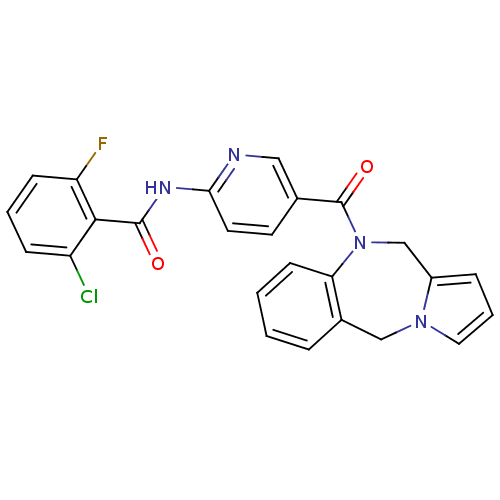 (CHEMBL49824 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065119 (CHEMBL70981 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065122 (CHEMBL306970 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065127 (CHEMBL71797 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50078663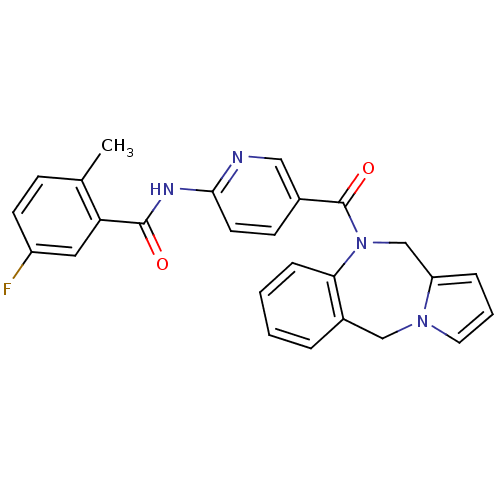 (CHEMBL300946 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of (P[3H]-AVP binding to isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50078665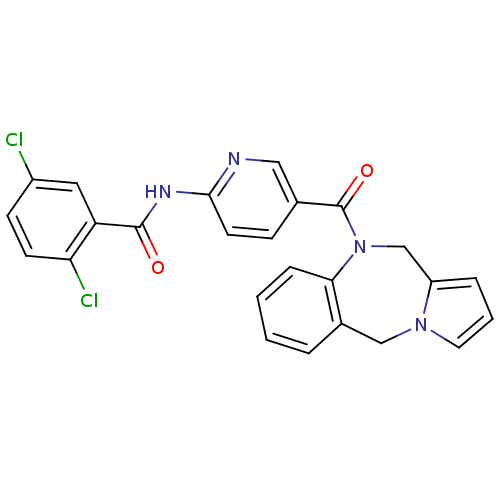 (CHEMBL298911 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065120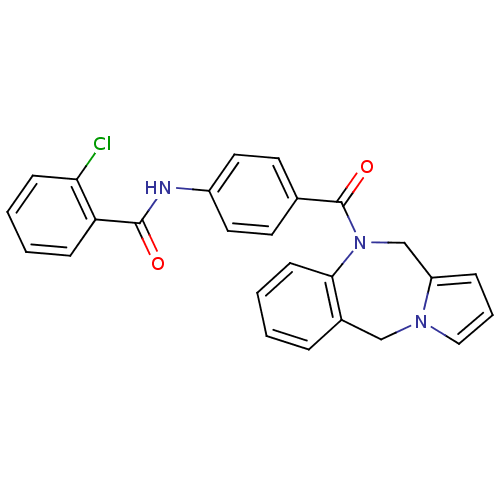 (CHEMBL302709 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50078652 (CHEMBL301788 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5 [3H]-) AVP from isolated rat hepatic V1a receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50078649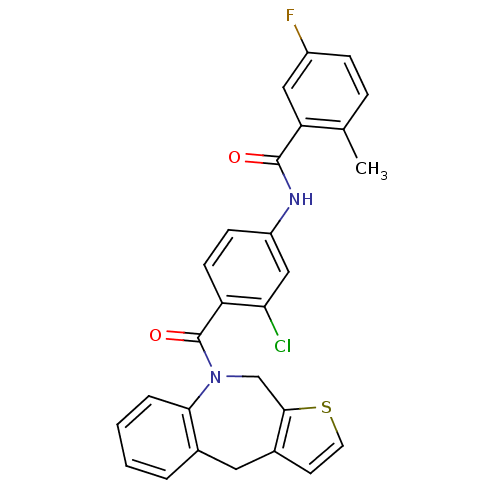 (CHEMBL48867 | N-[3-Chloro-4-(4,10-dihydro-1-thia-9...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]-AVP binding to human V2 receptor from murine fibroblast cell line (LV2) | Bioorg Med Chem Lett 9: 1733-6 (1999) BindingDB Entry DOI: 10.7270/Q2736Q34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065122 (CHEMBL306970 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50078660 (CHEMBL299532 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5 [3H]-) AVP from isolated rat hepatic V1a receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50129464 (CHEMBL71355 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50078661 (CHEMBL300433 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50078659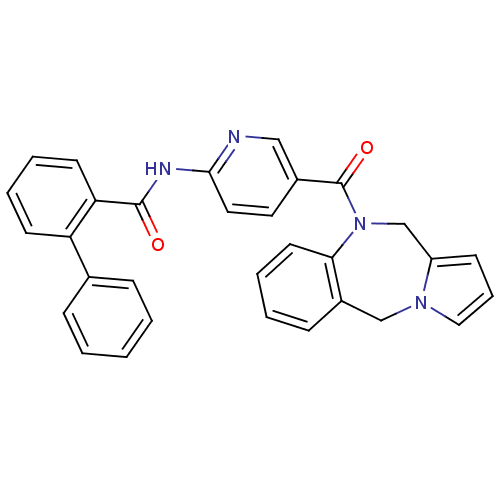 (Biphenyl-2-carboxylic acid [5-(5H,11H-benzo[e]pyrr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of P[3H]-AVP from isolated rat kidney medullary V2 receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50078646 (CHEMBL49322 | N-[4-(4,10-Dihydro-1-thia-9-aza-benz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]- AVP binding to V1a receptor from human platelet membrane | Bioorg Med Chem Lett 9: 1733-6 (1999) BindingDB Entry DOI: 10.7270/Q2736Q34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50078647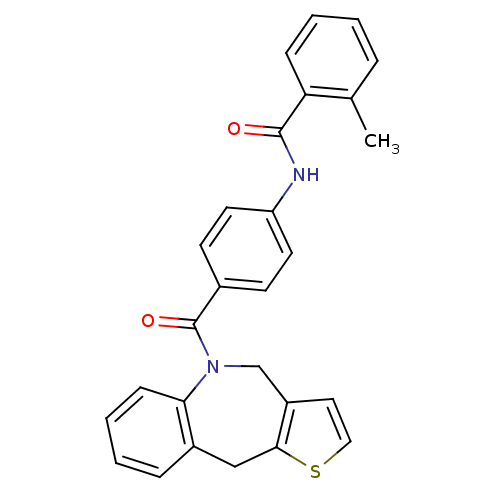 (CHEMBL295014 | N-[4-(4,10-Dihydro-3-thia-9-aza-ben...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]-AVP binding to human V2 receptor from murine fibroblast cell line (LV2) | Bioorg Med Chem Lett 9: 1733-6 (1999) BindingDB Entry DOI: 10.7270/Q2736Q34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50129469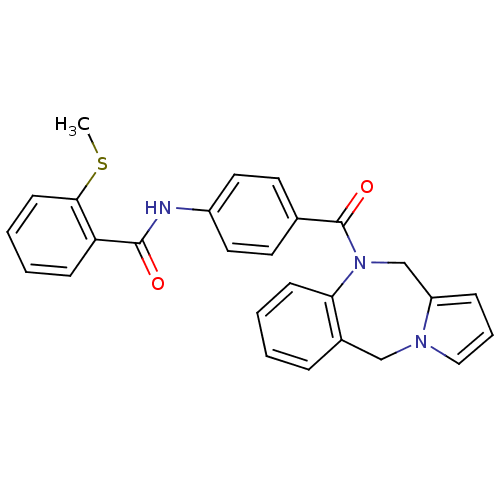 (CHEMBL304060 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50078656 (CHEMBL46295 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5 [3H]-) AVP from isolated rat hepatic V1a receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065120 (CHEMBL302709 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50078651 (CHEMBL300963 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description Displacement of (Phe-3,4,5 [3H]-) AVP from isolated rat hepatic V1a receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50173296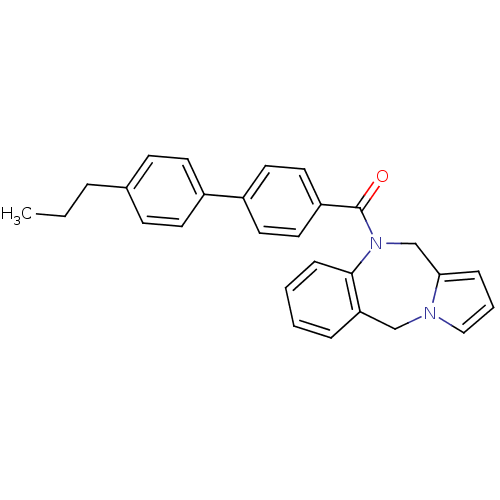 ((5H,11H-Benzo[e]pyrrolo[1,2-a][1,4]diazepin-10-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against [3H]-AVP binding to human vasopressin V1a receptor | Bioorg Med Chem Lett 15: 5003-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.007 BindingDB Entry DOI: 10.7270/Q2C828WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50129469 (CHEMBL304060 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50173294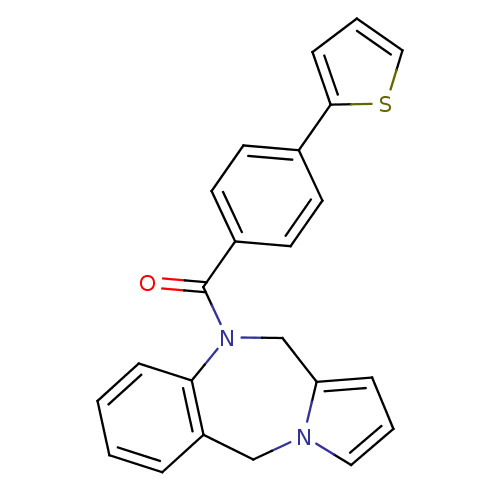 ((5H,11H-Benzo[e]pyrrolo[1,2-a][1,4]diazepin-10-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against [3H]-AVP binding to human vasopressin V1a receptor | Bioorg Med Chem Lett 15: 5003-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.007 BindingDB Entry DOI: 10.7270/Q2C828WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50173298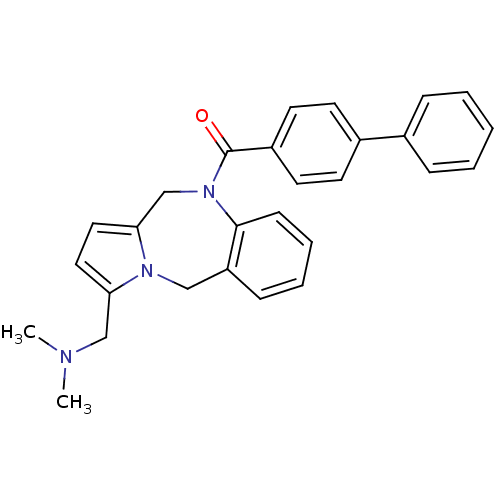 (Biphenyl-4-yl-(3-dimethylaminomethyl-5H,11H-benzo[...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against [3H]-AVP binding to human vasopressin V1a receptor | Bioorg Med Chem Lett 15: 5003-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.007 BindingDB Entry DOI: 10.7270/Q2C828WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065117 (CHEMBL304956 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065126 (CHEMBL73286 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50129475 (CHEMBL68948 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50129468 (CHEMBL302955 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50129472 (CHEMBL70949 | N-[4-(5,11-Dihydro-dibenzo[b,e][1,4]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Homo sapiens (Human)) | BDBM50078648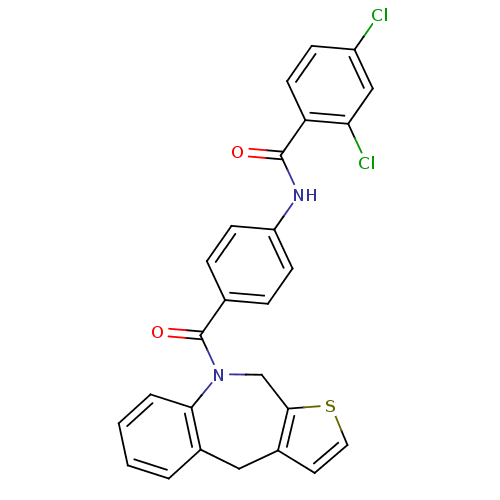 (2,4-Dichloro-N-[4-(4,10-dihydro-1-thia-9-aza-benzo...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of [3H]-AVP binding to human V2 receptor from murine fibroblast cell line (LV2) | Bioorg Med Chem Lett 9: 1733-6 (1999) BindingDB Entry DOI: 10.7270/Q2736Q34 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065124 (CHEMBL68085 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50078654 (CHEMBL297990 | N-[5-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth-Ayerst Research Curated by ChEMBL | Assay Description In vitro inhibition of (Phe-3,4,5 [3H]-) AVP binding towards isolated rat hepatic Vasopressin V1a receptor | Bioorg Med Chem Lett 9: 1737-40 (1999) BindingDB Entry DOI: 10.7270/Q23B5ZBZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (Homo sapiens (Human)) | BDBM50173293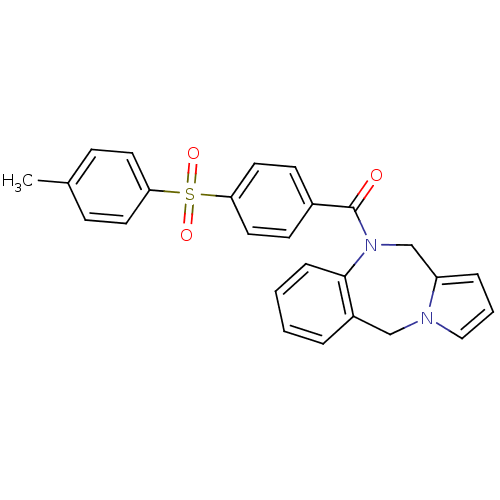 ((5H,11H-Benzo[e]pyrrolo[1,2-a][1,4]diazepin-10-yl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibitory concentration against [3H]-AVP binding to human vasopressin V1a receptor | Bioorg Med Chem Lett 15: 5003-6 (2005) Article DOI: 10.1016/j.bmcl.2005.08.007 BindingDB Entry DOI: 10.7270/Q2C828WH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V2 receptor (Rattus norvegicus (Rat)) | BDBM50065130 (CHEMBL71305 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a][...) | PDB Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat kidney medullary vasopressin V2 receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1a receptor (RAT) | BDBM50065110 (CHEMBL418890 | N-[4-(5H,11H-Benzo[e]pyrrolo[1,2-a]...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Wyeth Research Curated by ChEMBL | Assay Description Inhibition of [3H]-AVP binding to Dawley rat hepatic vasopressin V1a receptor. | Bioorg Med Chem Lett 13: 2195-8 (2003) BindingDB Entry DOI: 10.7270/Q2SF2VJH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 120 total ) | Next | Last >> |