 Found 898 hits with Last Name = 'schaber' and Initial = 'md'
Found 898 hits with Last Name = 'schaber' and Initial = 'md' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316183
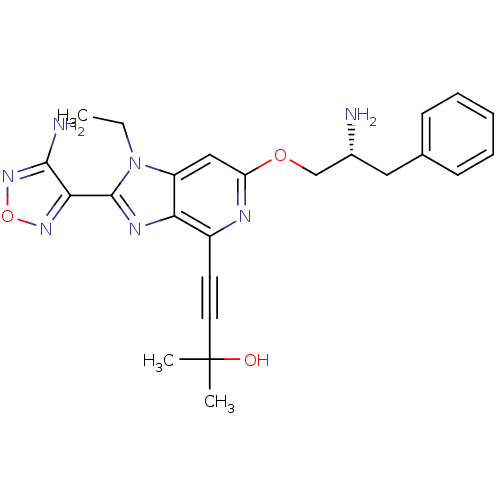
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(OC[C@H](N)Cc3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-18-13-19(33-14-16(25)12-15-8-6-5-7-9-15)27-17(10-11-24(2,3)32)20(18)28-23(31)21-22(26)30-34-29-21/h5-9,13,16,32H,4,12,14,25H2,1-3H3,(H2,26,30)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316184

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-3-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CCN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-17-14-19(33-18(11-13-25)15-8-6-5-7-9-15)27-16(10-12-24(2,3)32)20(17)28-23(31)21-22(26)30-34-29-21/h5-9,14,18,32H,4,11,13,25H2,1-3H3,(H2,26,30)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316192
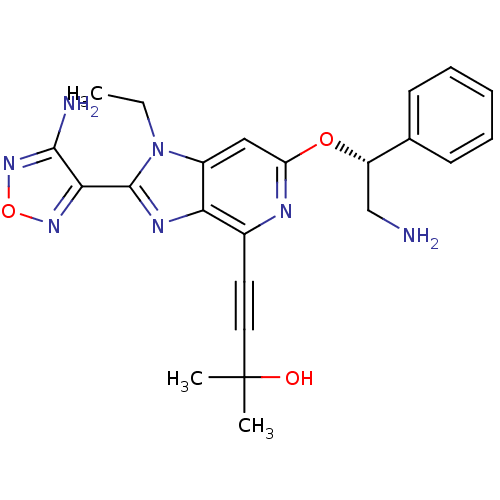
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C23H25N7O3/c1-4-30-16-12-18(32-17(13-24)14-8-6-5-7-9-14)26-15(10-11-23(2,3)31)19(16)27-22(30)20-21(25)29-33-28-20/h5-9,12,17,31H,4,13,24H2,1-3H3,(H2,25,29)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316184

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-3-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CCN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-17-14-19(33-18(11-13-25)15-8-6-5-7-9-15)27-16(10-12-24(2,3)32)20(17)28-23(31)21-22(26)30-34-29-21/h5-9,14,18,32H,4,11,13,25H2,1-3H3,(H2,26,30)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25013
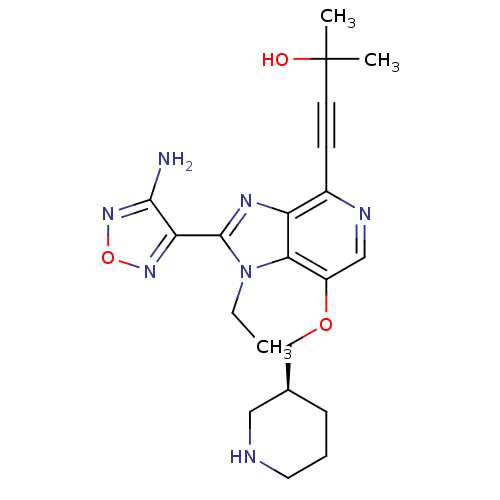
(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3S...)Show SMILES CCn1c(nc2c(ncc(OC[C@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316185
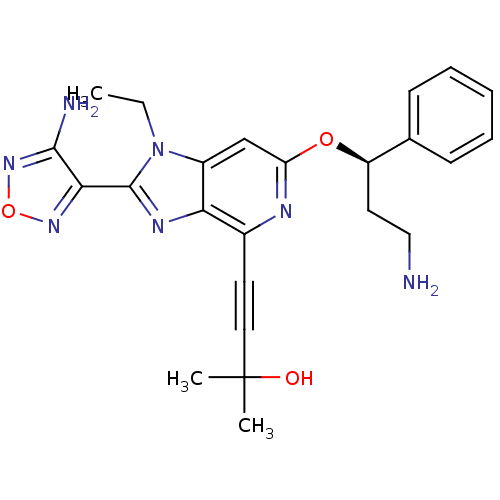
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-3-amino...)Show SMILES CCn1c(nc2c(nc(O[C@H](CCN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-17-14-19(33-18(11-13-25)15-8-6-5-7-9-15)27-16(10-12-24(2,3)32)20(17)28-23(31)21-22(26)30-34-29-21/h5-9,14,18,32H,4,11,13,25H2,1-3H3,(H2,26,30)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316183

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(OC[C@H](N)Cc3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-18-13-19(33-14-16(25)12-15-8-6-5-7-9-15)27-17(10-11-24(2,3)32)20(18)28-23(31)21-22(26)30-34-29-21/h5-9,13,16,32H,4,12,14,25H2,1-3H3,(H2,26,30)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316182
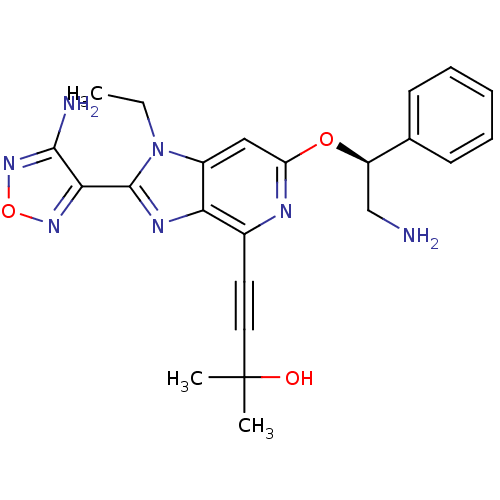
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-2-amino...)Show SMILES CCn1c(nc2c(nc(O[C@H](CN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C23H25N7O3/c1-4-30-16-12-18(32-17(13-24)14-8-6-5-7-9-14)26-15(10-11-23(2,3)31)19(16)27-22(30)20-21(25)29-33-28-20/h5-9,12,17,31H,4,13,24H2,1-3H3,(H2,25,29)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25013

(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3S...)Show SMILES CCn1c(nc2c(ncc(OC[C@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50059860
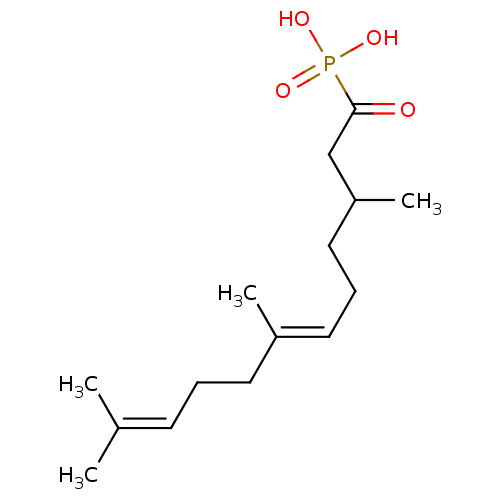
(((2E,6E)-1-Hydroxy-3,7,11-trimethyl-dodeca-2,6,10-...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]-[#6](=O)P([#8])([#8])=O Show InChI InChI=1S/C15H27O4P/c1-12(2)7-5-8-13(3)9-6-10-14(4)11-15(16)20(17,18)19/h7,9,14H,5-6,8,10-11H2,1-4H3,(H2,17,18,19)/b13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Assay using farnesyl-ras-CVLS as the protein acceptor substrate. |
Biochemistry 31: 3800-7 (1992)
Article DOI: 10.1021/bi00130a010
BindingDB Entry DOI: 10.7270/Q2MP51XH |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316192

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C23H25N7O3/c1-4-30-16-12-18(32-17(13-24)14-8-6-5-7-9-14)26-15(10-11-23(2,3)31)19(16)27-22(30)20-21(25)29-33-28-20/h5-9,12,17,31H,4,13,24H2,1-3H3,(H2,25,29)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50059860

(((2E,6E)-1-Hydroxy-3,7,11-trimethyl-dodeca-2,6,10-...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]-[#6](=O)P([#8])([#8])=O Show InChI InChI=1S/C15H27O4P/c1-12(2)7-5-8-13(3)9-6-10-14(4)11-15(16)20(17,18)19/h7,9,14H,5-6,8,10-11H2,1-4H3,(H2,17,18,19)/b13-9+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
FPTase activity was assayed in the biosynthetically forward direction at 30C. |
Biochemistry 31: 3800-7 (1992)
Article DOI: 10.1021/bi00130a010
BindingDB Entry DOI: 10.7270/Q2MP51XH |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316189
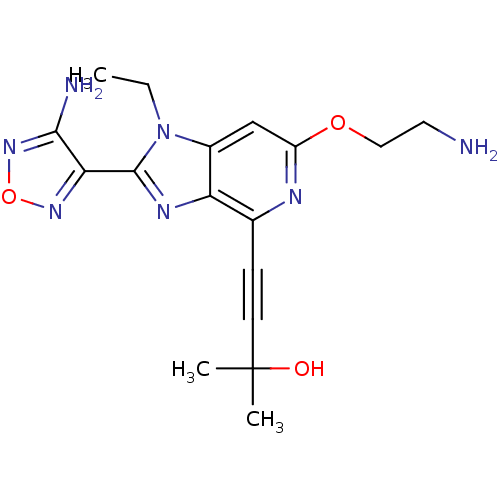
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-(2-aminoetho...)Show SMILES CCn1c(nc2c(nc(OCCN)cc12)C#CC(C)(C)O)-c1nonc1N Show InChI InChI=1S/C17H21N7O3/c1-4-24-11-9-12(26-8-7-18)20-10(5-6-17(2,3)25)13(11)21-16(24)14-15(19)23-27-22-14/h9,25H,4,7-8,18H2,1-3H3,(H2,19,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316182

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-2-amino...)Show SMILES CCn1c(nc2c(nc(O[C@H](CN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C23H25N7O3/c1-4-30-16-12-18(32-17(13-24)14-8-6-5-7-9-14)26-15(10-11-23(2,3)31)19(16)27-22(30)20-21(25)29-33-28-20/h5-9,12,17,31H,4,13,24H2,1-3H3,(H2,25,29)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316189

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-(2-aminoetho...)Show SMILES CCn1c(nc2c(nc(OCCN)cc12)C#CC(C)(C)O)-c1nonc1N Show InChI InChI=1S/C17H21N7O3/c1-4-24-11-9-12(26-8-7-18)20-10(5-6-17(2,3)25)13(11)21-16(24)14-15(19)23-27-22-14/h9,25H,4,7-8,18H2,1-3H3,(H2,19,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 39 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316185

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-3-amino...)Show SMILES CCn1c(nc2c(nc(O[C@H](CCN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-17-14-19(33-18(11-13-25)15-8-6-5-7-9-15)27-16(10-12-24(2,3)32)20(17)28-23(31)21-22(26)30-34-29-21/h5-9,14,18,32H,4,11,13,25H2,1-3H3,(H2,26,30)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 105 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316197

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-(3-aminoprop...)Show SMILES CCn1c(nc2c(nc(OCCCN)cc12)C#CC(C)(C)O)-c1nonc1N Show InChI InChI=1S/C18H23N7O3/c1-4-25-12-10-13(27-9-5-8-19)21-11(6-7-18(2,3)26)14(12)22-17(25)15-16(20)24-28-23-15/h10,26H,4-5,8-9,19H2,1-3H3,(H2,20,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 115 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316197

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-(3-aminoprop...)Show SMILES CCn1c(nc2c(nc(OCCCN)cc12)C#CC(C)(C)O)-c1nonc1N Show InChI InChI=1S/C18H23N7O3/c1-4-25-12-10-13(27-9-5-8-19)21-11(6-7-18(2,3)26)14(12)22-17(25)15-16(20)24-28-23-15/h10,26H,4-5,8-9,19H2,1-3H3,(H2,20,24) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 646 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50059865
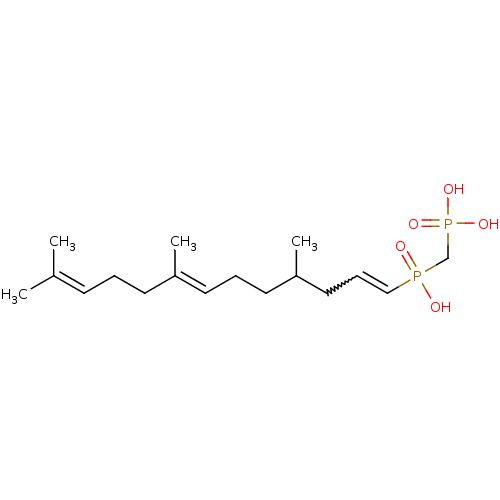
(4,8,12-trimethyl-(3E,7E)-3,7,11-tridecatrienylhydr...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]-[#6]=[#6]P([#8])(=O)[#6]P([#8])([#8])=O |w:14.13| Show InChI InChI=1S/C17H32O5P2/c1-15(2)8-5-9-16(3)10-6-11-17(4)12-7-13-23(18,19)14-24(20,21)22/h7-8,10,13,17H,5-6,9,11-12,14H2,1-4H3,(H,18,19)(H2,20,21,22)/b13-7?,16-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 830 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
FPTase activity was assayed in the biosynthetically forward direction at 30C. |
Biochemistry 31: 3800-7 (1992)
Article DOI: 10.1021/bi00130a010
BindingDB Entry DOI: 10.7270/Q2MP51XH |
More data for this
Ligand-Target Pair | |
Farnesyl pyrophosphate synthase
(Homo sapiens (Human)) | BDBM50059865

(4,8,12-trimethyl-(3E,7E)-3,7,11-tridecatrienylhydr...)Show SMILES [#6]-[#6](-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6])-[#6]-[#6]=[#6]P([#8])(=O)[#6]P([#8])([#8])=O |w:14.13| Show InChI InChI=1S/C17H32O5P2/c1-15(2)8-5-9-16(3)10-6-11-17(4)12-7-13-23(18,19)14-24(20,21)22/h7-8,10,13,17H,5-6,9,11-12,14H2,1-4H3,(H,18,19)(H2,20,21,22)/b13-7?,16-10+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
| Assay Description
Assay using farnesyl-ras-CVLS as the protein acceptor substrate. |
Biochemistry 31: 3800-7 (1992)
Article DOI: 10.1021/bi00130a010
BindingDB Entry DOI: 10.7270/Q2MP51XH |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420316
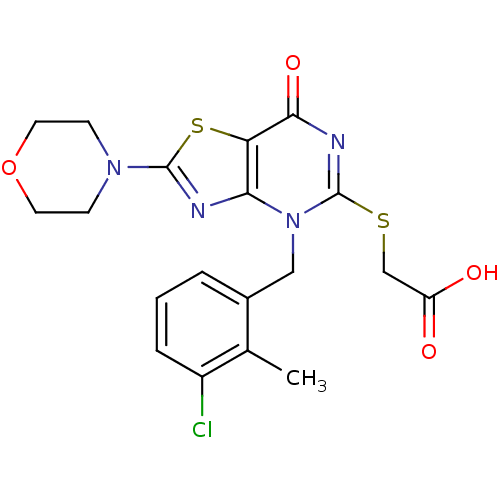
(CHEMBL2089119)Show SMILES Cc1c(Cl)cccc1Cn1c(SCC(O)=O)nc(=O)c2sc(nc12)N1CCOCC1 Show InChI InChI=1S/C19H19ClN4O4S2/c1-11-12(3-2-4-13(11)20)9-24-16-15(17(27)22-19(24)29-10-14(25)26)30-18(21-16)23-5-7-28-8-6-23/h2-4H,5-10H2,1H3,(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420311
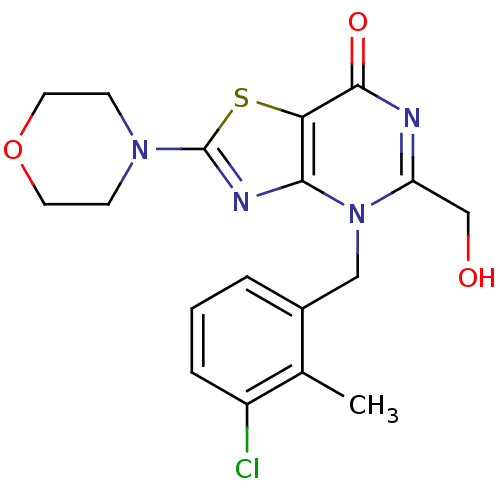
(CHEMBL2089114)Show SMILES Cc1c(Cl)cccc1Cn1c(CO)nc(=O)c2sc(nc12)N1CCOCC1 Show InChI InChI=1S/C18H19ClN4O3S/c1-11-12(3-2-4-13(11)19)9-23-14(10-24)20-17(25)15-16(23)21-18(27-15)22-5-7-26-8-6-22/h2-4,24H,5-10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50081221

(4-{5-[(S)-2-Butyl-4-(3-chloro-phenyl)-5-oxo-pipera...)Show SMILES CCCC[C@H]1CN(C(=O)CN1Cc1cncn1Cc1ccc(cc1)C#N)c1cccc(Cl)c1 Show InChI InChI=1S/C26H28ClN5O/c1-2-3-6-24-17-32(23-7-4-5-22(27)12-23)26(33)18-30(24)16-25-14-29-19-31(25)15-21-10-8-20(13-28)9-11-21/h4-5,7-12,14,19,24H,2-3,6,15-18H2,1H3/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into recombinant human K-Ras by Farnesyltransferase |
J Med Chem 42: 3779-84 (1999)
BindingDB Entry DOI: 10.7270/Q2F47NB1 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50079961
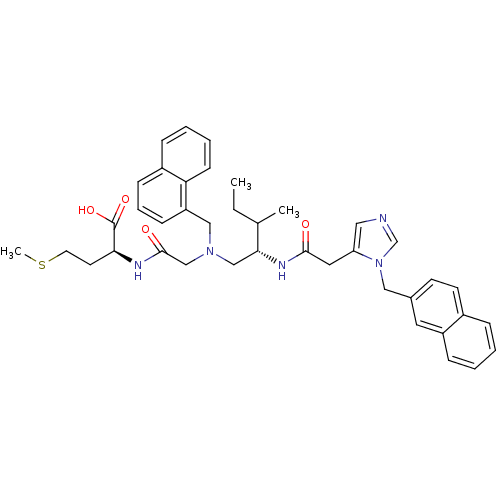
((S)-2-[2-({(S)-3-Methyl-2-[2-(3-naphthalen-2-ylmet...)Show SMILES CCC(C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC(=O)Cc1cncn1Cc1ccc2ccccc2c1 Show InChI InChI=1S/C40H47N5O4S/c1-4-28(2)37(43-38(46)21-34-22-41-27-45(34)23-29-16-17-30-10-5-6-12-32(30)20-29)25-44(26-39(47)42-36(40(48)49)18-19-50-3)24-33-14-9-13-31-11-7-8-15-35(31)33/h5-17,20,22,27-28,36-37H,4,18-19,21,23-26H2,1-3H3,(H,42,47)(H,43,46)(H,48,49)/t28?,36-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50079974
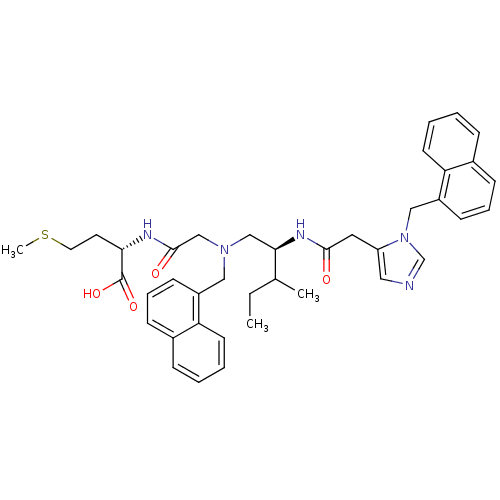
((S)-2-[2-({(S)-3-Methyl-2-[2-(3-naphthalen-1-ylmet...)Show SMILES CCC(C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC(=O)Cc1cncn1Cc1cccc2ccccc12 Show InChI InChI=1S/C40H47N5O4S/c1-4-28(2)37(43-38(46)21-33-22-41-27-45(33)24-32-16-10-14-30-12-6-8-18-35(30)32)25-44(26-39(47)42-36(40(48)49)19-20-50-3)23-31-15-9-13-29-11-5-7-17-34(29)31/h5-18,22,27-28,36-37H,4,19-21,23-26H2,1-3H3,(H,42,47)(H,43,46)(H,48,49)/t28?,36-,37+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50369443
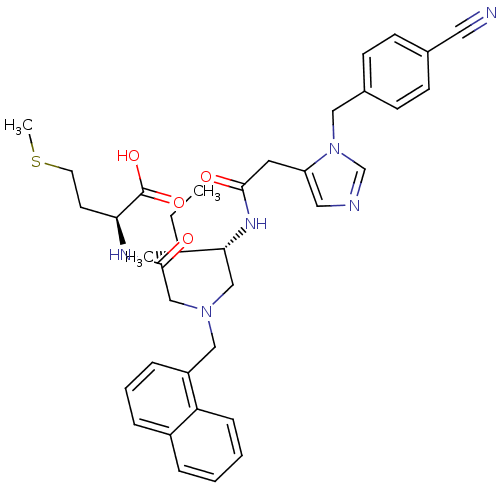
(CHEMBL252953)Show SMILES CC[C@@H](C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC(=O)Cc1cncn1Cc1ccc(cc1)C#N Show InChI InChI=1S/C37H44N6O4S/c1-4-26(2)34(41-35(44)18-31-20-39-25-43(31)21-28-14-12-27(19-38)13-15-28)23-42(24-36(45)40-33(37(46)47)16-17-48-3)22-30-10-7-9-29-8-5-6-11-32(29)30/h5-15,20,25-26,33-34H,4,16-18,21-24H2,1-3H3,(H,40,45)(H,41,44)(H,46,47)/t26-,33+,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50079956

((S)-2-[2-({(S)-2-[2-(3-Benzyl-3H-imidazol-4-yl)-ac...)Show SMILES CCC(C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC(=O)Cc1cncn1Cc1ccccc1 Show InChI InChI=1S/C36H45N5O4S/c1-4-26(2)33(39-34(42)19-30-20-37-25-41(30)21-27-11-6-5-7-12-27)23-40(24-35(43)38-32(36(44)45)17-18-46-3)22-29-15-10-14-28-13-8-9-16-31(28)29/h5-16,20,25-26,32-33H,4,17-19,21-24H2,1-3H3,(H,38,43)(H,39,42)(H,44,45)/t26?,32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50081212
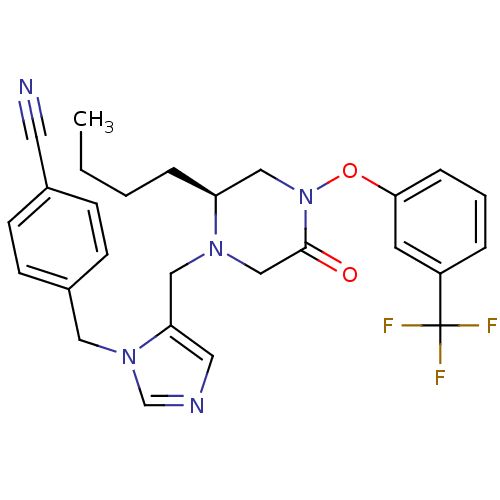
(4-{5-[(S)-2-Butyl-5-oxo-4-(3-trifluoromethyl-pheno...)Show SMILES CCCC[C@H]1CN(Oc2cccc(c2)C(F)(F)F)C(=O)CN1Cc1cncn1Cc1ccc(cc1)C#N Show InChI InChI=1S/C27H28F3N5O2/c1-2-3-6-23-17-35(37-25-7-4-5-22(12-25)27(28,29)30)26(36)18-33(23)16-24-14-32-19-34(24)15-21-10-8-20(13-31)9-11-21/h4-5,7-12,14,19,23H,2-3,6,15-18H2,1H3/t23-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | >0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into recombinant human K-Ras by Farnesyltransferase |
J Med Chem 42: 3779-84 (1999)
BindingDB Entry DOI: 10.7270/Q2F47NB1 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50079966
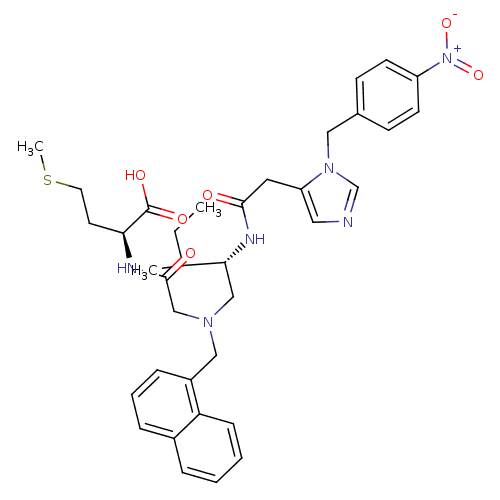
((S)-2-{2-[((S)-3-Methyl-2-{2-[3-(4-nitro-benzyl)-3...)Show SMILES CCC(C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC(=O)Cc1cncn1Cc1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C36H44N6O6S/c1-4-25(2)33(39-34(43)18-30-19-37-24-41(30)20-26-12-14-29(15-13-26)42(47)48)22-40(23-35(44)38-32(36(45)46)16-17-49-3)21-28-10-7-9-27-8-5-6-11-31(27)28/h5-15,19,24-25,32-33H,4,16-18,20-23H2,1-3H3,(H,38,44)(H,39,43)(H,45,46)/t25?,32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50081215
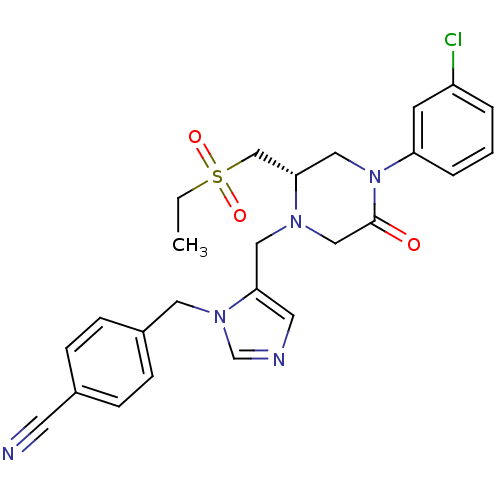
(4-{5-[(S)-4-(3-Chloro-phenyl)-2-ethanesulfonylmeth...)Show SMILES CCS(=O)(=O)C[C@@H]1CN(C(=O)CN1Cc1cncn1Cc1ccc(cc1)C#N)c1cccc(Cl)c1 Show InChI InChI=1S/C25H26ClN5O3S/c1-2-35(33,34)17-24-15-31(22-5-3-4-21(26)10-22)25(32)16-29(24)14-23-12-28-18-30(23)13-20-8-6-19(11-27)7-9-20/h3-10,12,18,24H,2,13-17H2,1H3/t24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into recombinant human K-Ras by Farnesyltransferase |
J Med Chem 42: 3779-84 (1999)
BindingDB Entry DOI: 10.7270/Q2F47NB1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420321

(CHEMBL2089112)Show SMILES Cc1c(Cn2c(CO)nc(=O)c3sc(nc23)N2CCOCC2)cccc1C(F)(F)F Show InChI InChI=1S/C19H19F3N4O3S/c1-11-12(3-2-4-13(11)19(20,21)22)9-26-14(10-27)23-17(28)15-16(26)24-18(30-15)25-5-7-29-8-6-25/h2-4,27H,5-10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420309

(CHEMBL2089120)Show SMILES Cc1c(Cl)cccc1Cn1c(N)nc(=O)c2sc(nc12)N1CCOCC1 Show InChI InChI=1S/C17H18ClN5O2S/c1-10-11(3-2-4-12(10)18)9-23-14-13(15(24)21-16(23)19)26-17(20-14)22-5-7-25-8-6-22/h2-4H,5-9H2,1H3,(H2,19,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420310
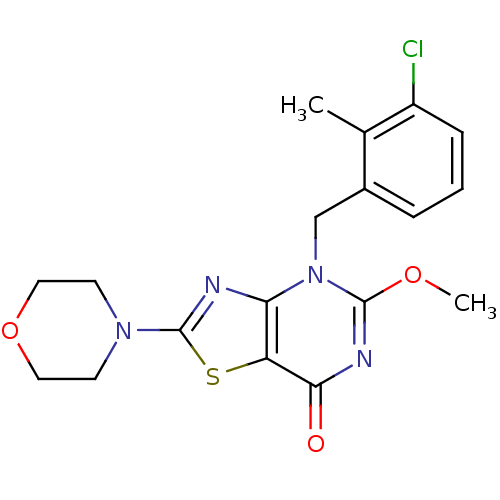
(CHEMBL2089116)Show SMILES COc1nc(=O)c2sc(nc2n1Cc1cccc(Cl)c1C)N1CCOCC1 Show InChI InChI=1S/C18H19ClN4O3S/c1-11-12(4-3-5-13(11)19)10-23-15-14(16(24)21-17(23)25-2)27-18(20-15)22-6-8-26-9-7-22/h3-5H,6-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420317
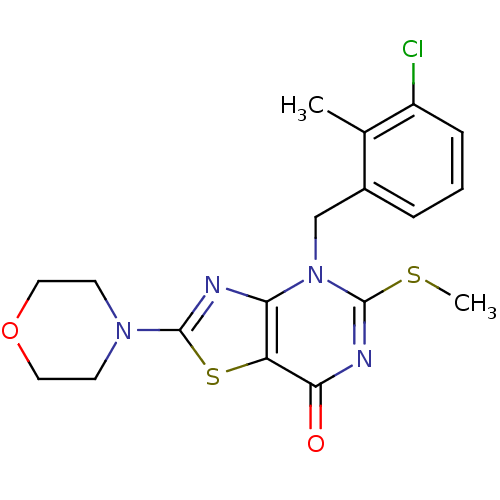
(CHEMBL2089118)Show SMILES CSc1nc(=O)c2sc(nc2n1Cc1cccc(Cl)c1C)N1CCOCC1 Show InChI InChI=1S/C18H19ClN4O2S2/c1-11-12(4-3-5-13(11)19)10-23-15-14(16(24)21-18(23)26-2)27-17(20-15)22-6-8-25-9-7-22/h3-5H,6-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50489439
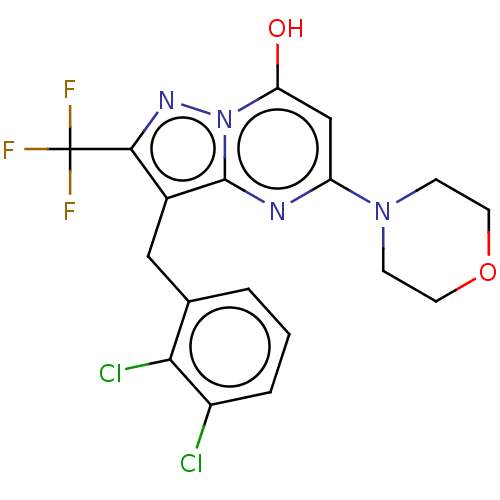
(CHEMBL2322340)Show SMILES Oc1cc(nc2c(Cc3cccc(Cl)c3Cl)c(nn12)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C18H15Cl2F3N4O2/c19-12-3-1-2-10(15(12)20)8-11-16(18(21,22)23)25-27-14(28)9-13(24-17(11)27)26-4-6-29-7-5-26/h1-3,9,28H,4-8H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50489445
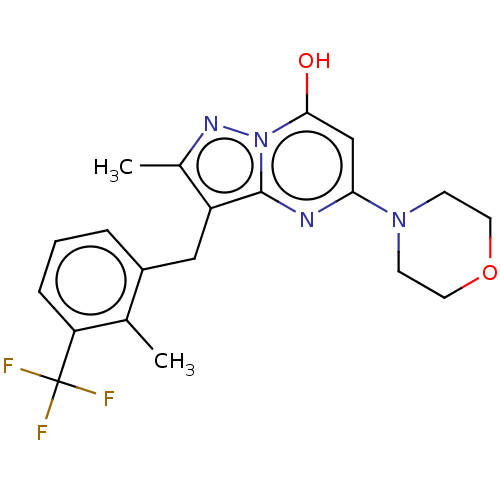
(CHEMBL2322331)Show SMILES Cc1nn2c(O)cc(nc2c1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C20H21F3N4O2/c1-12-14(4-3-5-16(12)20(21,22)23)10-15-13(2)25-27-18(28)11-17(24-19(15)27)26-6-8-29-9-7-26/h3-5,11,28H,6-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.398 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50381156

(CHEMBL2018220 | D3RKN_94)Show SMILES CSc1nn2c(nc(cc2=O)N2CCOCC2)n1Cc1cccc(c1C)C(F)(F)F Show InChI InChI=1S/C19H20F3N5O2S/c1-12-13(4-3-5-14(12)19(20,21)22)11-26-17-23-15(25-6-8-29-9-7-25)10-16(28)27(17)24-18(26)30-2/h3-5,10H,6-9,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta by continuous read time resolved fluorescence resonance energy transfer displacement assay |
Bioorg Med Chem Lett 22: 3198-202 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.039
BindingDB Entry DOI: 10.7270/Q2TD9ZBP |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50079978
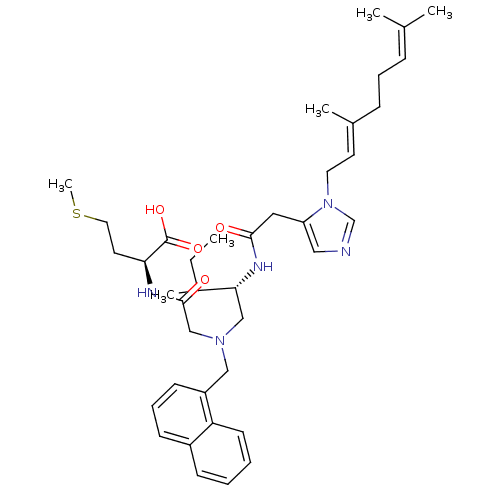
((S)-2-{2-[((S)-2-{2-[3-((E)-3,7-Dimethyl-octa-2,6-...)Show SMILES [#6]-[#6]-[#6](-[#6])-[#6@@H](-[#6]-[#7](-[#6]-[#6](=O)-[#7]-[#6@@H](-[#6]-[#6]-[#16]-[#6])-[#6](-[#8])=O)-[#6]-c1cccc2ccccc12)-[#7]-[#6](=O)-[#6]-c1cncn1-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6] Show InChI InChI=1S/C39H55N5O4S/c1-7-30(5)36(42-37(45)22-33-23-40-27-44(33)20-18-29(4)13-10-12-28(2)3)25-43(26-38(46)41-35(39(47)48)19-21-49-6)24-32-16-11-15-31-14-8-9-17-34(31)32/h8-9,11-12,14-18,23,27,30,35-36H,7,10,13,19-22,24-26H2,1-6H3,(H,41,46)(H,42,45)(H,47,48)/b29-18+/t30?,35-,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
Dimer of Protein farnesyltransferase subunit beta
(Homo sapiens (Human)) | BDBM50081217
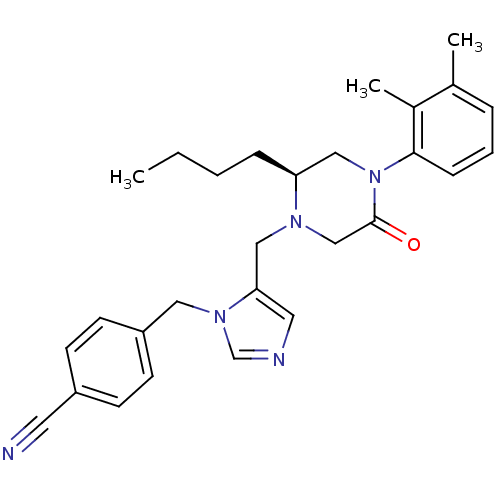
(4-{5-[(S)-2-Butyl-4-(2,3-dimethyl-phenyl)-5-oxo-pi...)Show SMILES CCCC[C@H]1CN(C(=O)CN1Cc1cncn1Cc1ccc(cc1)C#N)c1cccc(C)c1C Show InChI InChI=1S/C28H33N5O/c1-4-5-8-25-18-33(27-9-6-7-21(2)22(27)3)28(34)19-31(25)17-26-15-30-20-32(26)16-24-12-10-23(14-29)11-13-24/h6-7,9-13,15,20,25H,4-5,8,16-19H2,1-3H3/t25-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into recombinant human K-Ras by Farnesyltransferase |
J Med Chem 42: 3779-84 (1999)
BindingDB Entry DOI: 10.7270/Q2F47NB1 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50079971

((S)-2-{2-[((S)-2-{2-[3-(4-Fluoro-benzyl)-3H-imidaz...)Show SMILES CCC(C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC(=O)Cc1cncn1Cc1ccc(F)cc1 Show InChI InChI=1S/C36H44FN5O4S/c1-4-25(2)33(40-34(43)18-30-19-38-24-42(30)20-26-12-14-29(37)15-13-26)22-41(23-35(44)39-32(36(45)46)16-17-47-3)21-28-10-7-9-27-8-5-6-11-31(27)28/h5-15,19,24-25,32-33H,4,16-18,20-23H2,1-3H3,(H,39,44)(H,40,43)(H,45,46)/t25?,32-,33+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420313

(CHEMBL2089108)Show SMILES CCc1nc(=O)c2sc(nc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C20H21F3N4O2S/c1-3-15-24-18(28)16-17(25-19(30-16)26-7-9-29-10-8-26)27(15)11-13-5-4-6-14(12(13)2)20(21,22)23/h4-6H,3,7-11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420314
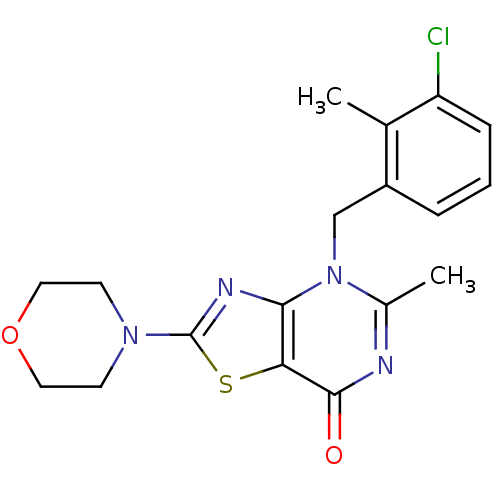
(CHEMBL2089107)Show InChI InChI=1S/C18H19ClN4O2S/c1-11-13(4-3-5-14(11)19)10-23-12(2)20-17(24)15-16(23)21-18(26-15)22-6-8-25-9-7-22/h3-5H,6-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420312
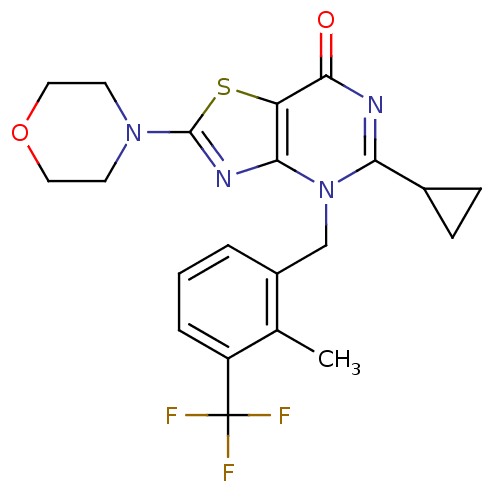
(CHEMBL2089109)Show SMILES Cc1c(Cn2c(nc(=O)c3sc(nc23)N2CCOCC2)C2CC2)cccc1C(F)(F)F Show InChI InChI=1S/C21H21F3N4O2S/c1-12-14(3-2-4-15(12)21(22,23)24)11-28-17(13-5-6-13)25-19(29)16-18(28)26-20(31-16)27-7-9-30-10-8-27/h2-4,13H,5-11H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Kinesin-like protein KIF11
(Homo sapiens (Human)) | BDBM50181139
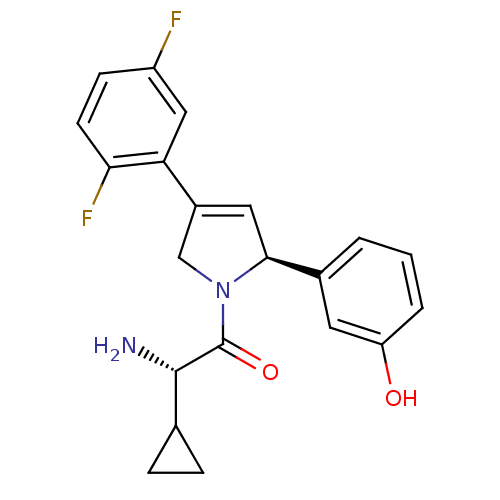
((S)-2-amino-2-cyclopropyl-1-((S)-4-(2,5-difluoroph...)Show SMILES N[C@@H](C1CC1)C(=O)N1CC(=C[C@H]1c1cccc(O)c1)c1cc(F)ccc1F |c:10| Show InChI InChI=1S/C21H20F2N2O2/c22-15-6-7-18(23)17(10-15)14-9-19(13-2-1-3-16(26)8-13)25(11-14)21(27)20(24)12-4-5-12/h1-3,6-10,12,19-20,26H,4-5,11,24H2/t19-,20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of KSP by ATPase assay |
Bioorg Med Chem Lett 16: 1780-3 (2006)
Article DOI: 10.1016/j.bmcl.2005.12.094
BindingDB Entry DOI: 10.7270/Q2PZ58D2 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50079968
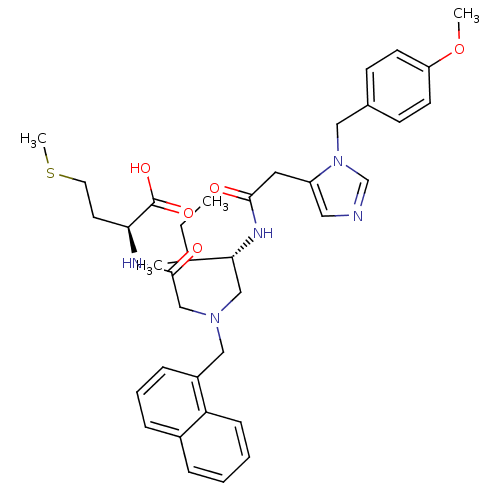
((S)-2-{2-[((S)-2-{2-[3-(4-Methoxy-benzyl)-3H-imida...)Show SMILES CCC(C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1cccc2ccccc12)NC(=O)Cc1cncn1Cc1ccc(OC)cc1 Show InChI InChI=1S/C37H47N5O5S/c1-5-26(2)34(40-35(43)19-30-20-38-25-42(30)21-27-13-15-31(47-3)16-14-27)23-41(24-36(44)39-33(37(45)46)17-18-48-4)22-29-11-8-10-28-9-6-7-12-32(28)29/h6-16,20,25-26,33-34H,5,17-19,21-24H2,1-4H3,(H,39,44)(H,40,43)(H,45,46)/t26?,33-,34+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant [Leu68]-RAS1CVIM by Farnesyltransferase |
J Med Chem 42: 3356-68 (1999)
Article DOI: 10.1021/jm990080l
BindingDB Entry DOI: 10.7270/Q27P9020 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316183

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(OC[C@H](N)Cc3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-18-13-19(33-14-16(25)12-15-8-6-5-7-9-15)27-17(10-11-24(2,3)32)20(18)28-23(31)21-22(26)30-34-29-21/h5-9,13,16,32H,4,12,14,25H2,1-3H3,(H2,26,30)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420315
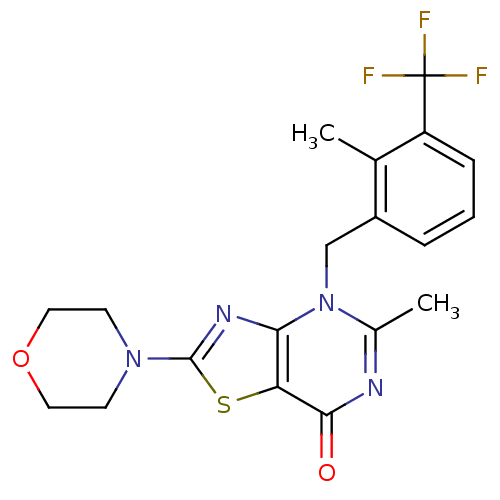
(CHEMBL2089106)Show SMILES Cc1nc(=O)c2sc(nc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C19H19F3N4O2S/c1-11-13(4-3-5-14(11)19(20,21)22)10-26-12(2)23-17(27)15-16(26)24-18(29-15)25-6-8-28-9-7-25/h3-5H,6-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of PI3K beta by continuous TR-FRET assay |
ACS Med Chem Lett 3: 524-529 (2012)
Article DOI: 10.1021/ml300045b
BindingDB Entry DOI: 10.7270/Q2J967PT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50381161
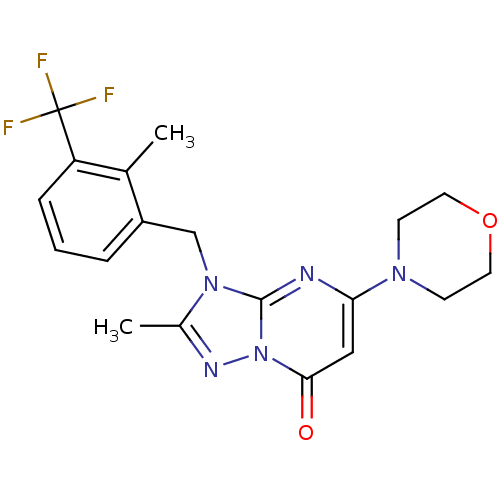
(CHEMBL2018219)Show SMILES Cc1nn2c(nc(cc2=O)N2CCOCC2)n1Cc1cccc(c1C)C(F)(F)F Show InChI InChI=1S/C19H20F3N5O2/c1-12-14(4-3-5-15(12)19(20,21)22)11-26-13(2)24-27-17(28)10-16(23-18(26)27)25-6-8-29-9-7-25/h3-5,10H,6-9,11H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta by continuous read time resolved fluorescence resonance energy transfer displacement assay |
Bioorg Med Chem Lett 22: 3198-202 (2012)
Article DOI: 10.1016/j.bmcl.2012.03.039
BindingDB Entry DOI: 10.7270/Q2TD9ZBP |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50420315

(CHEMBL2089106)Show SMILES Cc1nc(=O)c2sc(nc2n1Cc1cccc(c1C)C(F)(F)F)N1CCOCC1 Show InChI InChI=1S/C19H19F3N4O2S/c1-11-13(4-3-5-14(11)19(20,21)22)10-26-12(2)23-17(27)15-16(26)24-18(29-15)25-6-8-28-9-7-25/h3-5H,6-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50489454
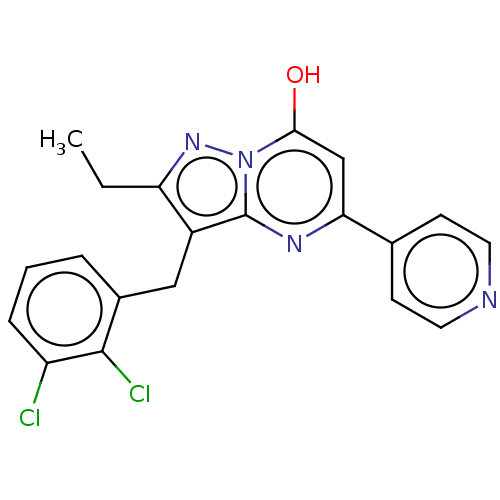
(CHEMBL2322338)Show SMILES CCc1nn2c(O)cc(nc2c1Cc1cccc(Cl)c1Cl)-c1ccncc1 Show InChI InChI=1S/C20H16Cl2N4O/c1-2-16-14(10-13-4-3-5-15(21)19(13)22)20-24-17(11-18(27)26(20)25-16)12-6-8-23-9-7-12/h3-9,11,27H,2,10H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.631 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) |
ACS Med Chem Lett 4: 230-4 (2013)
Article DOI: 10.1021/ml300330m
BindingDB Entry DOI: 10.7270/Q2HD7ZJT |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data