

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050632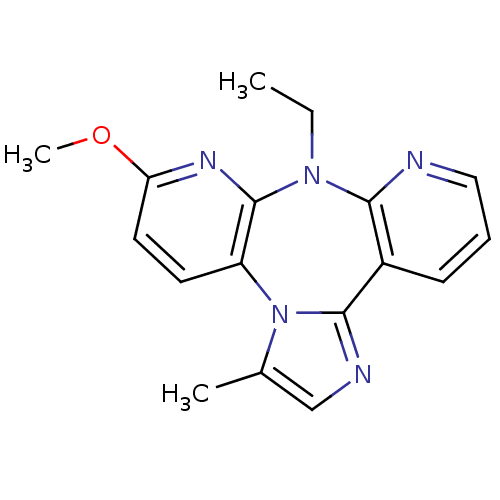 (8-ETHYL-6-METHOXY-3-METHYL-8H-1,3A,7,8,9-PENTAAZA-...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-FCRDC Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 39: 1645-50 (1996) Article DOI: 10.1021/jm9508088 BindingDB Entry DOI: 10.7270/Q2VD6XJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2753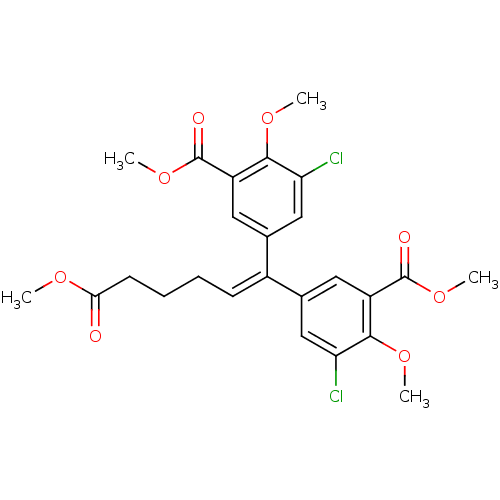 (Alkenyldiarylmethanes (ADAM) 22 | CHEMBL294840 | M...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2739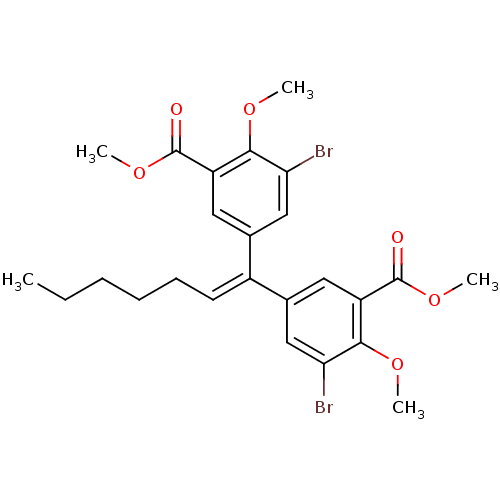 (Alkenyldiarylmethane (ADAM) 1 | Alkenyldiarylmetha...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 380 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050628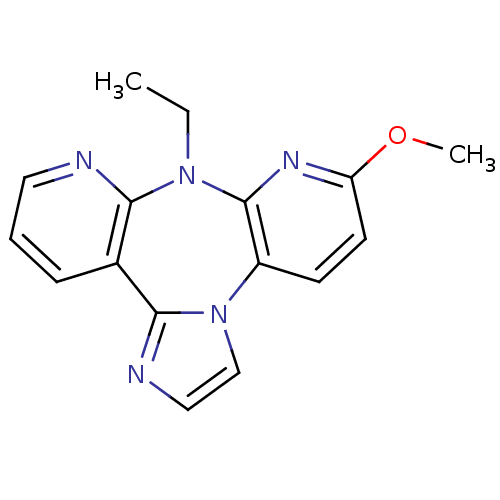 (8-Ethyl-6-methoxy-8H-1,3a,7,8,9-pentaaza-dibenzo[e...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-FCRDC Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 39: 1645-50 (1996) Article DOI: 10.1021/jm9508088 BindingDB Entry DOI: 10.7270/Q2VD6XJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050640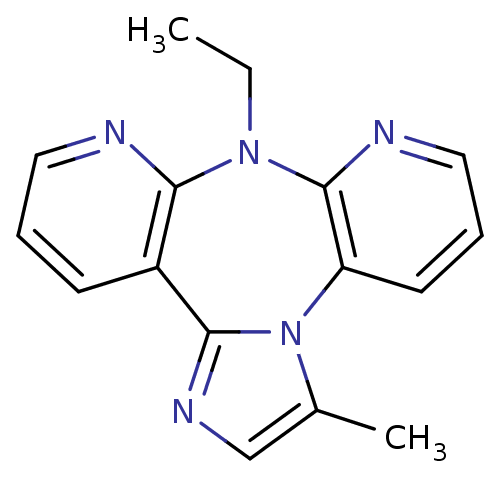 (8-Ethyl-3-methyl-8H-1,3a,7,8,9-pentaaza-dibenzo[e,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-FCRDC Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 39: 1645-50 (1996) Article DOI: 10.1021/jm9508088 BindingDB Entry DOI: 10.7270/Q2VD6XJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050633 (8-Ethyl-4-methyl-8H-1,3a,7,8,9-pentaaza-dibenzo[e,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-FCRDC Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 39: 1645-50 (1996) Article DOI: 10.1021/jm9508088 BindingDB Entry DOI: 10.7270/Q2VD6XJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2751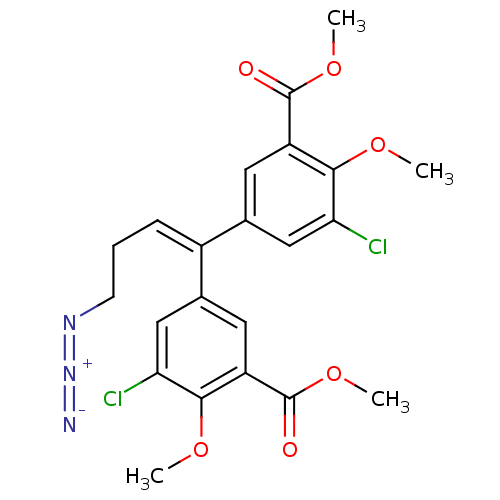 (4-Azido-3,3-dichloro-4,4-dimethoxy-5,5-bis(methoxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050636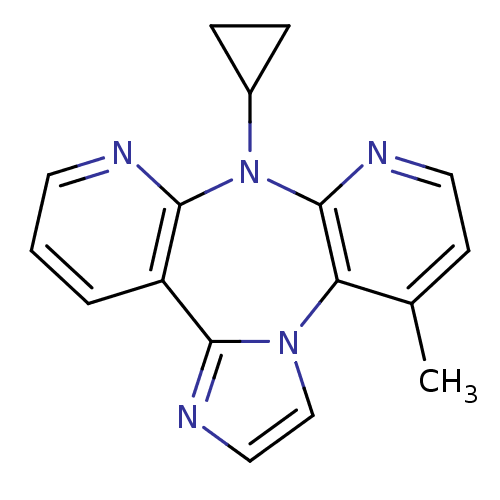 (8-Cyclopropyl-4-methyl-8H-1,3a,7,8,9-pentaaza-dibe...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-FCRDC Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 39: 1645-50 (1996) Article DOI: 10.1021/jm9508088 BindingDB Entry DOI: 10.7270/Q2VD6XJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050634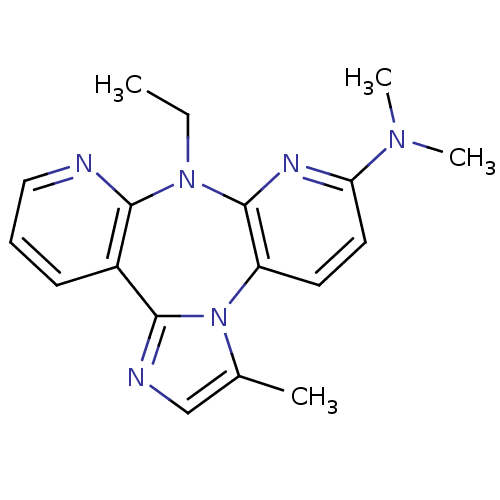 ((8-Ethyl-3-methyl-8H-1,3a,7,8,9-pentaaza-dibenzo[e...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-FCRDC Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 39: 1645-50 (1996) Article DOI: 10.1021/jm9508088 BindingDB Entry DOI: 10.7270/Q2VD6XJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050635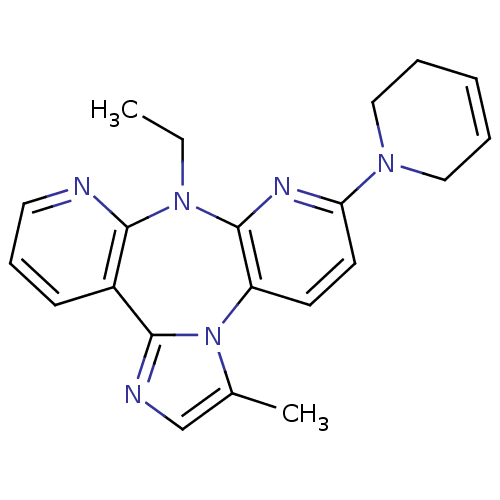 (6-(3,6-Dihydro-2H-pyridin-1-yl)-8-ethyl-3-methyl-8...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-FCRDC Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 39: 1645-50 (1996) Article DOI: 10.1021/jm9508088 BindingDB Entry DOI: 10.7270/Q2VD6XJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2760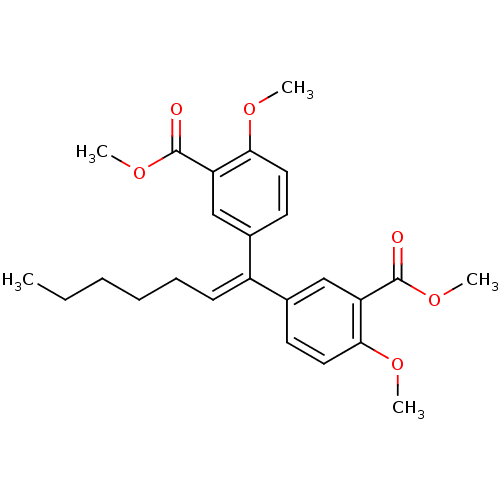 (4,4-Dimethoxy-3,3-bis(methoxycarbonyl)-1,1-dipheny...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2754 (3,3-Dichloro-4,4-dimethoxy-5,5-bis(methoxycarbonyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050639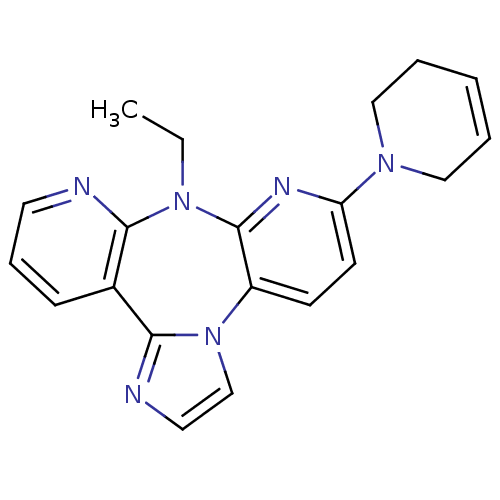 (6-(3,6-Dihydro-2H-pyridin-1-yl)-8-ethyl-8H-1,3a,7,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-FCRDC Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 39: 1645-50 (1996) Article DOI: 10.1021/jm9508088 BindingDB Entry DOI: 10.7270/Q2VD6XJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050637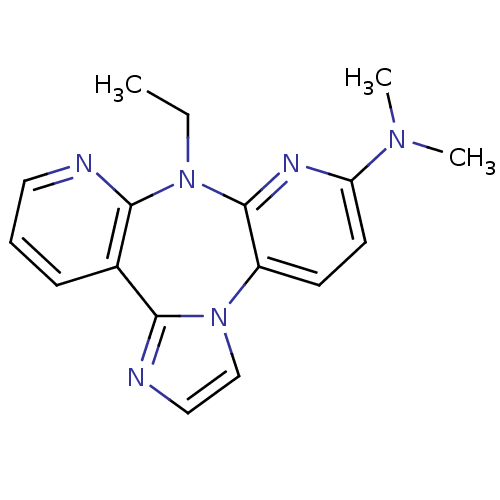 ((8-Ethyl-8H-1,3a,7,8,9-pentaaza-dibenzo[e,h]azulen...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-FCRDC Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 39: 1645-50 (1996) Article DOI: 10.1021/jm9508088 BindingDB Entry DOI: 10.7270/Q2VD6XJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2745 (3,3-Dibromo-4,4-dimethoxy-5,5-bis(methoxycarbonyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050631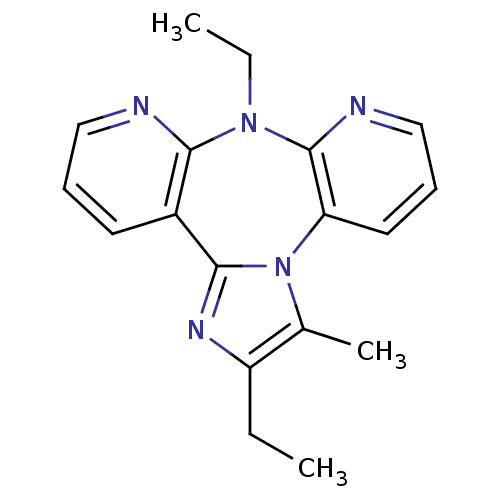 (2,8-Diethyl-3-methyl-8H-1,3a,7,8,9-pentaaza-dibenz...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.63E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-FCRDC Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 39: 1645-50 (1996) Article DOI: 10.1021/jm9508088 BindingDB Entry DOI: 10.7270/Q2VD6XJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1434 (11-cyclopropyl-5,11-dihydro-4-methyl-6H-dipyrido[3...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank MMDB PDB Article PubMed | n/a | n/a | 6.44E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-FCRDC Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 39: 1645-50 (1996) Article DOI: 10.1021/jm9508088 BindingDB Entry DOI: 10.7270/Q2VD6XJ4 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM1517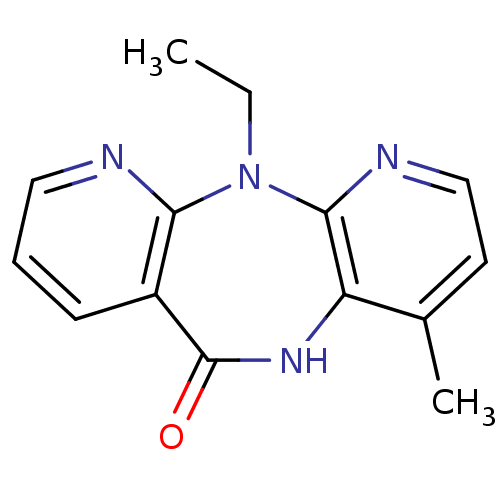 (2-ethyl-7-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-FCRDC Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 39: 1645-50 (1996) Article DOI: 10.1021/jm9508088 BindingDB Entry DOI: 10.7270/Q2VD6XJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2761 (3,3-Diiodo-4,4-dimethoxy-5,5-bis(methoxycarbonyl)-...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050638 (8-Ethyl-2-methyl-8H-1,3a,7,8,9-pentaaza-dibenzo[e,...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-FCRDC Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 39: 1645-50 (1996) Article DOI: 10.1021/jm9508088 BindingDB Entry DOI: 10.7270/Q2VD6XJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050630 (8-Cyclopropyl-3-methyl-8H-1,2,3a,7,8,9-hexaaza-dib...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-FCRDC Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 39: 1645-50 (1996) Article DOI: 10.1021/jm9508088 BindingDB Entry DOI: 10.7270/Q2VD6XJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Reverse transcriptase/RNaseH (Human immunodeficiency virus 1) | BDBM50050629 (8-Ethyl-3,4-dimethyl-7H,8H-1,3a,7,8,9-pentaaza-dib...) | PDB MMDB UniProtKB/TrEMBL B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
National Cancer Institute-FCRDC Curated by ChEMBL | Assay Description Inhibitory activity against HIV-1 reverse transcriptase | J Med Chem 39: 1645-50 (1996) Article DOI: 10.1021/jm9508088 BindingDB Entry DOI: 10.7270/Q2VD6XJ4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2747 (Alkenyldiarylmethane (ADAM) 15 | Alkenyldiarylmeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+4 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2741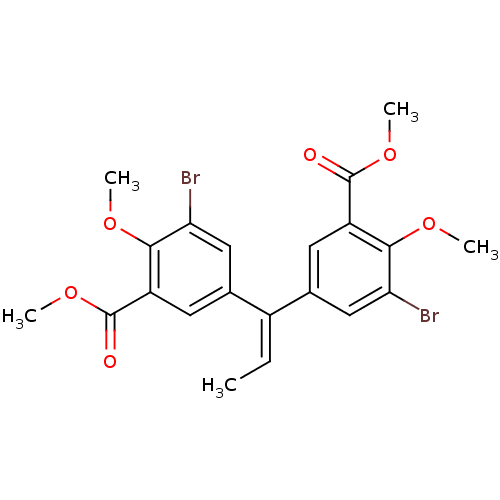 (3,3-Dibromo-4,4-dimethoxy-5,5-bis(methoxycarbonyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+4 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2743 (3,3-Dibromo-4-hydroxy-4,4-dimethoxy-5,5-bis-(metho...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2752 (4-Amino-3,3-dichloro-4,4-dimethoxy-5,5-bis(methoxy...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.30E+4 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2744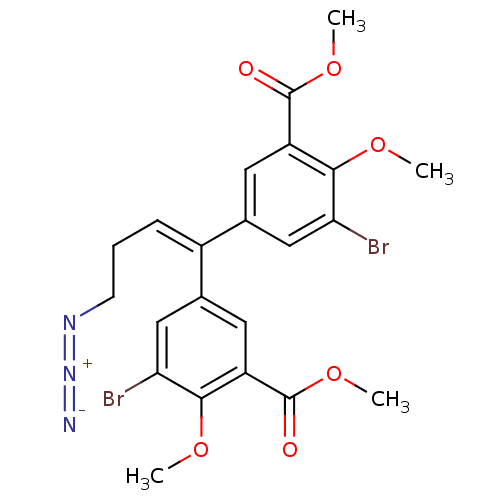 (4-Azido-3,3-dibromo-4,4-dimethoxy-5,5-bis(methoxyc...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.40E+4 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2750 (3,3-Dichloro-4-methanesulfonyloxy-4,4-dimethoxy-5,...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.60E+4 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2759 (1-Bromo-4,4-bis[8,8-dichloro-2,2,2,2-tetramethyl-4...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2758 (8-chloro-6-[1-(8-chloro-2,2-dimethyl-4-oxo-2,4-dih...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2757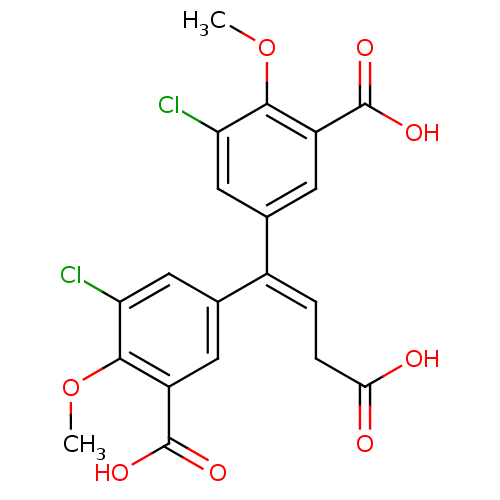 (3,3-Dichloro-4,4-dimethoxy-5,5-bis(carboxy)-4,4-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2756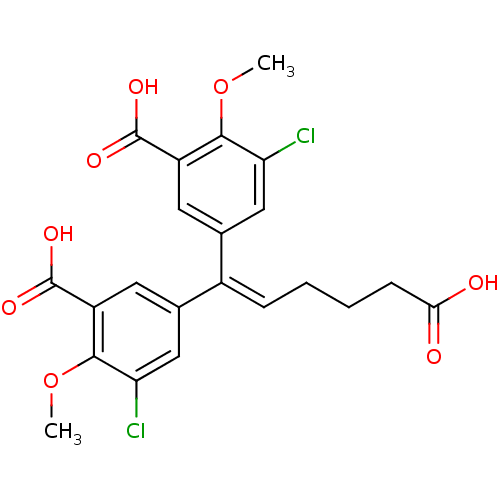 (3,3-Dichloro-4,4-dimethoxy-5,5-bis(carboxy)-6,6-di...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2755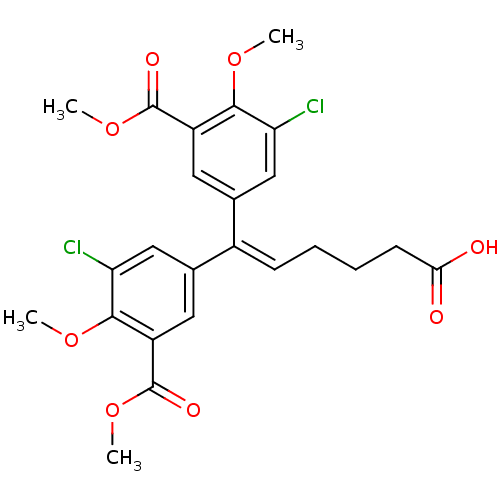 (3,3-Dichloro-4,4-dimethoxy-5,5-bis(methoxycarbonyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2749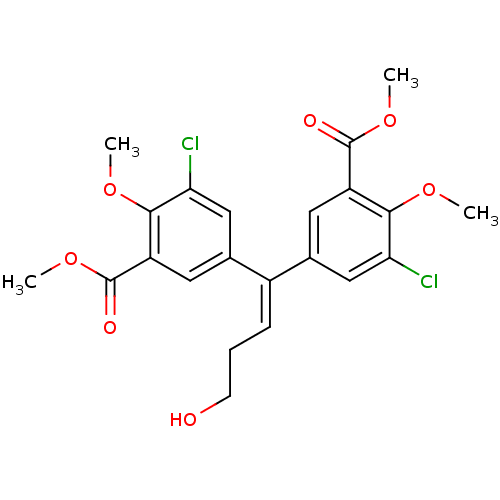 (3,3-Dichloro-4-hydroxy-4,4-dimethoxy-5,5-bis-(meth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2748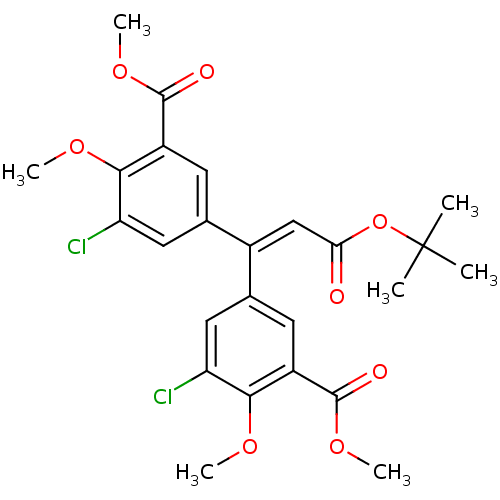 (Alkenyldiarylmethane (ADAM) 16 | Alkenyldiarylmeth...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2746 (3,3-Dichloro-4,4-dimethoxy-5,5-bis(methoxycarbonyl...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2742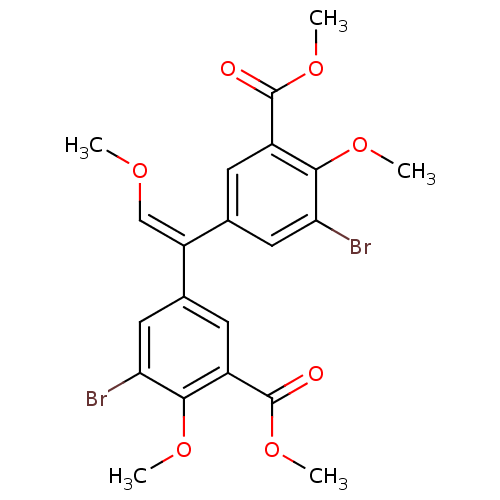 (3,3-Dibromo-4,4-dimethoxy-5,5-bis(methoxycarbonyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Gag-Pol polyprotein [588-1027]/[588-1147] (Human immunodeficiency virus type 1) | BDBM2740 (3,3-Dibromo-4,4-dimethoxy-5,5-bis(methoxycarbonyl)...) | PDB MMDB UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.9 | n/a |
Purdue University | Assay Description The IC50 of reverse transcriptase is the concentration that inhibits 50% of recombinant HIV-1 RT RNA-directed DNA polymerase activity in vitro. | J Med Chem 41: 2076-89 (1998) Article DOI: 10.1021/jm9800595 BindingDB Entry DOI: 10.7270/Q2251GC2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||