

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM470468 (1-[(3R)-1-acryloylpiperidin-3-yl]-5-amino-3-[4-(4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377836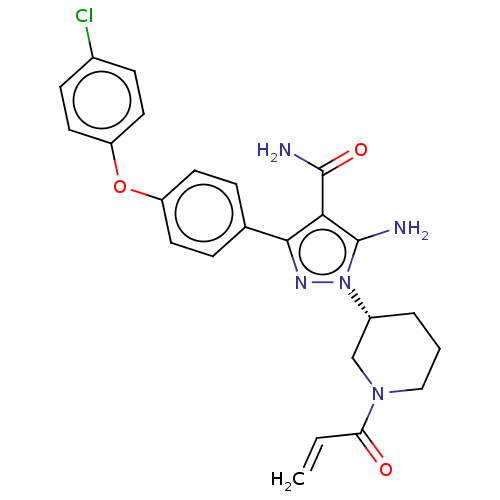 (1-[(3R)-1-acryloyl piperidin-3-yl]-5-amino-3-[4-(4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303543 (US10138229, Example 54 | US10266513, Example 127 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM303543 (US10138229, Example 54 | US10266513, Example 127 |...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 0.180 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130880 (CHEMBL407196 | NT(1-13) | neurotensin | pGlu-Leu-T...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377842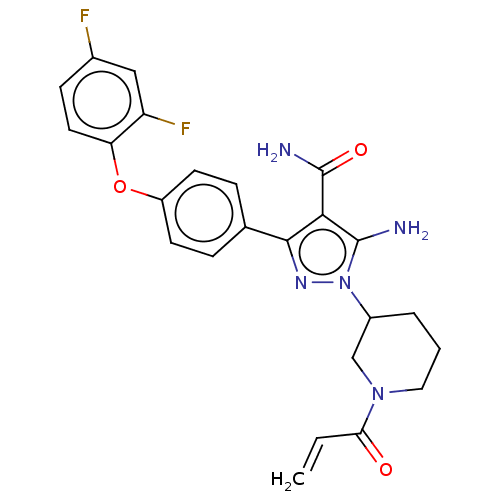 (1-[(3S)-1-acryloyl piperidin-3-yl]-5-amino-3-[4-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377842 (1-[(3S)-1-acryloyl piperidin-3-yl]-5-amino-3-[4-(2...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130895 (CHEMBL408127 | DTPA-Cha(tr-4-Mam)-Pro-Arg-Gly(PipA...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130898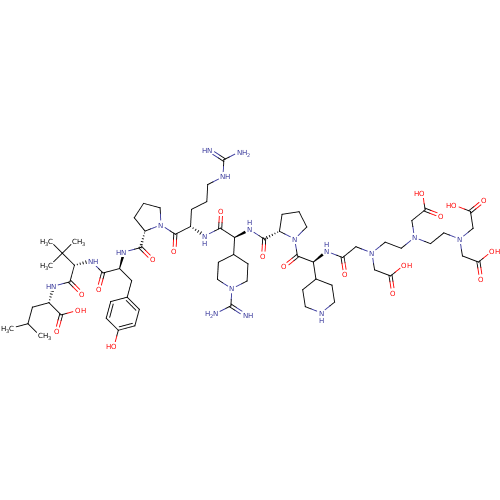 (CHEMBL408381 | DTPA-Gly(Pip)-Pro-Arg-Gly(PipAm)-Ar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377707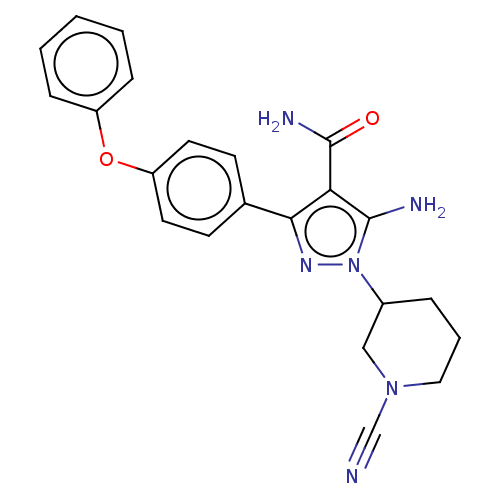 (5-amino-1-(1-cyanopiperidin-3-yl)-3-(4-phenoxyphen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377707 (5-amino-1-(1-cyanopiperidin-3-yl)-3-(4-phenoxyphen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377839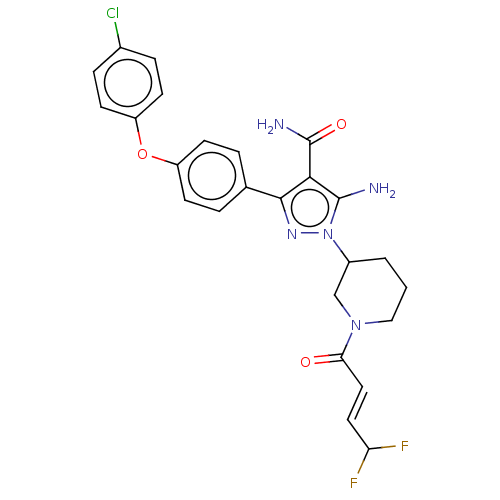 (5-amino-3-[4-(4-chlorophenoxy)phenyl]-1-{(3R)-1-[(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377839 (5-amino-3-[4-(4-chlorophenoxy)phenyl]-1-{(3R)-1-[(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377831 (5-amino-1-(1-cyanopiperidin-3-yl)-3-[4-(phenylthio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130899 (CHEMBL408638 | DTPA-DLys-Pro-Arg-Gly(PipAm)-Arg-Pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130885 (CHEMBL412699 | DTPA-DLys-Pro-Arg-Phe(4-Gu)-Pro-Tyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130882 (CHEMBL406127 | DTPA-DLys-Pro-Arg-Gly(PipAm)-Arg-Pr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130876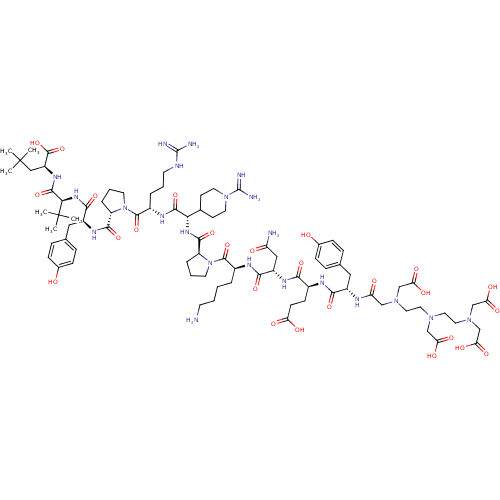 (CHEMBL404942 | DTPA-DTyr-Glu-Asn-Lys-Pro-Gly(PipAm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130873 (CHEMBL409477 | DTPA-Arg-Arg-Pro-Tur-Ile-Leu-OH) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377722 (5-amino-1-(1-cyanopiperidin-3-yl)-3-[4-(3,4-dimeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377831 (5-amino-1-(1-cyanopiperidin-3-yl)-3-[4-(phenylthio...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM470355 ((R)-5-amino-1-(1-cyanopiperidin-3-yl)-3-[4-(3,4-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377850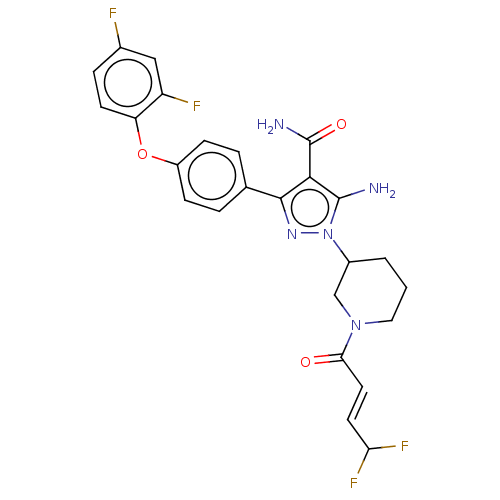 (5-amino-1-{(3R)-1-[(2E)-4,4-difluorobut-2-enoyl]pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377850 (5-amino-1-{(3R)-1-[(2E)-4,4-difluorobut-2-enoyl]pi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.410 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377734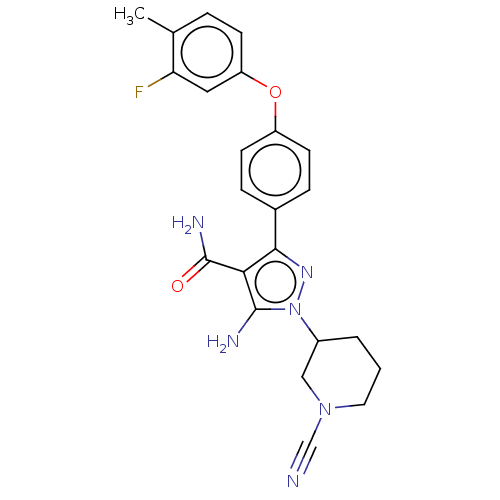 (5-amino-1-(1-cyanopiperidin-3-yl)-3-[4-(3-fluoro-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377734 (5-amino-1-(1-cyanopiperidin-3-yl)-3-[4-(3-fluoro-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.430 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377754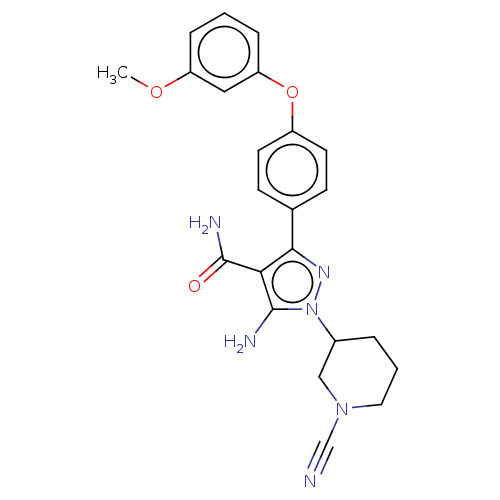 (5-amino-1-(1-cyanopiperidin-3-yl)- 3-[4-(3-methoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377754 (5-amino-1-(1-cyanopiperidin-3-yl)- 3-[4-(3-methoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.440 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377827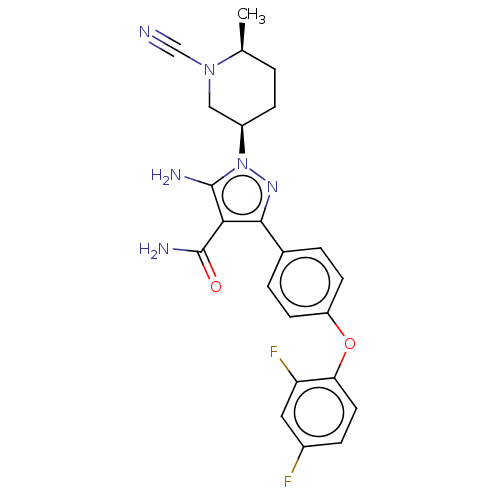 (5-amino-1-[(3R*,6S*)-1-cyano-6-methylpiperidin-3-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377827 (5-amino-1-[(3R*,6S*)-1-cyano-6-methylpiperidin-3-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM470491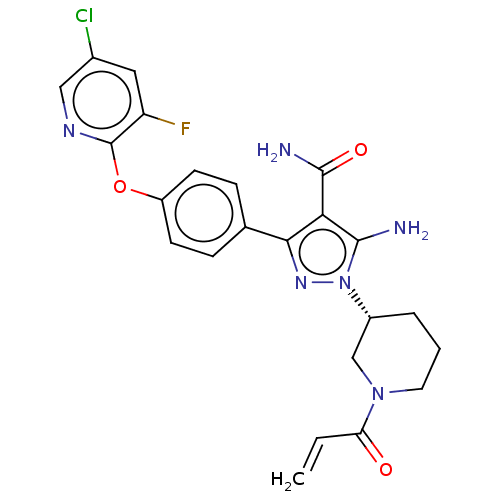 (1-[(3R)-1-acryloylpiperidin-3-yl]-5-amino-3-{4-[(5...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377859 (1-[(3R)-1-acryloyl piperidin-3-yl]-5-amino-3-{4-[(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.480 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130886 (CHEMBL437288 | alpha-FITC-Gly(Pip)-Pro-Gly(PipAm)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50370236 (CHEMBL1790850) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377778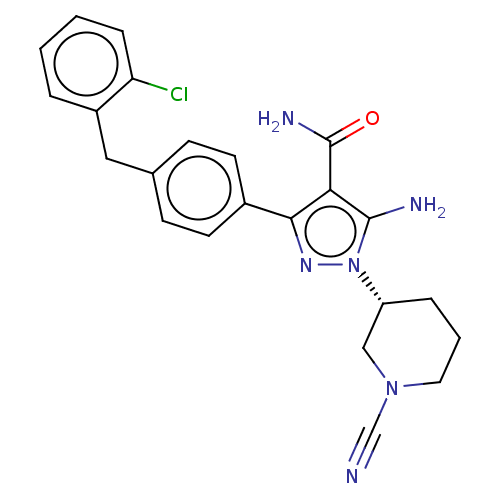 ((R)-5-amino-3-(4-(2-chlorobenzyl)phenyl)-1-(1-cyan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377778 ((R)-5-amino-3-(4-(2-chlorobenzyl)phenyl)-1-(1-cyan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130883 (CHEMBL406456 | epsilon-FITC-DLys-Pro-Gly(PipAm)-Ar...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377775 ((R)-5-amino-1-(1-cyanopiperidin-3-yl)-3-(4-(3-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neurotensin receptor type 1 (Homo sapiens (Human)) | BDBM50130875 (CHEMBL386387 | DTPA-DTyr-Glu-Asn-Lys-Pro-Gly(PipAm...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington University School of Medicine Curated by ChEMBL | Assay Description Binding affinity towards neurotensin receptor in membranes prepared from HT-29 cell line, relative to [111In]-labeled neurotensin peptide | J Med Chem 46: 3403-11 (2003) Article DOI: 10.1021/jm030081k BindingDB Entry DOI: 10.7270/Q2222VHR | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377775 ((R)-5-amino-1-(1-cyanopiperidin-3-yl)-3-(4-(3-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377719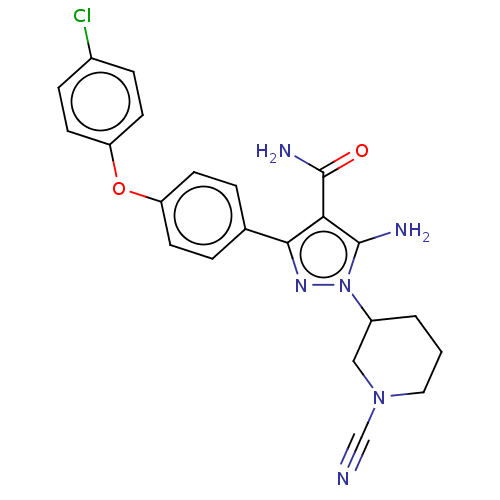 (5-amino-3-[4-(4-chlorophenoxy)phenyl]-1-(1-cyanopi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM50512857 (CHEMBL4456283 | US10815213, Example 15) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.640 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377861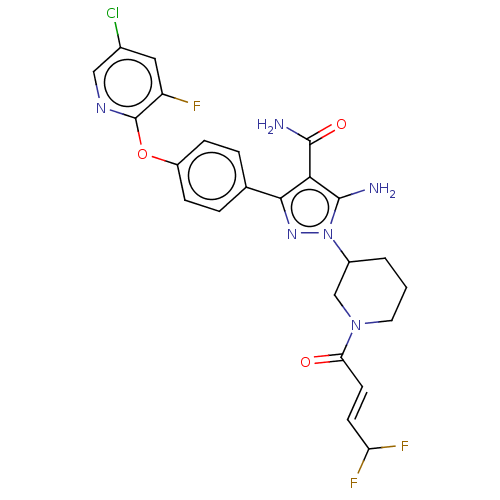 (5-amino-3-{4-[(5-chloro-3-fluoropyridin-2-yl)oxy]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM470493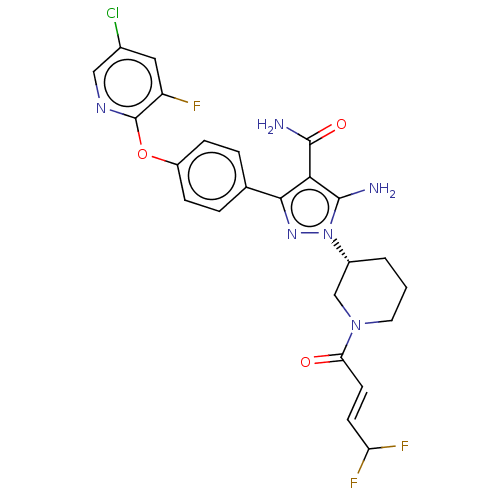 (5-amino-3-{4-[(5-chloro-3-fluoropyridin-2-yl)oxy]p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377830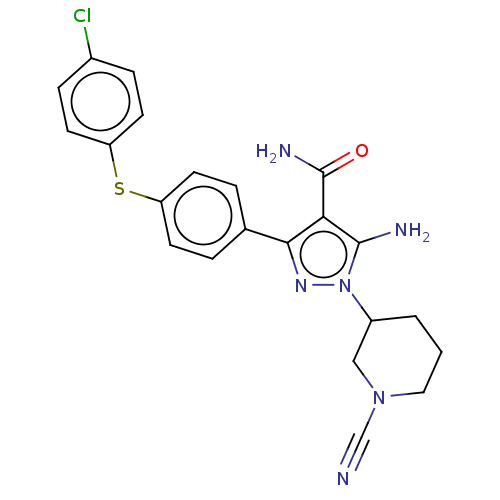 (5-amino-3-{4-[(4-chlorophenyl)thio]phenyl}-1-(1-cy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377830 (5-amino-3-{4-[(4-chlorophenyl)thio]phenyl}-1-(1-cy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.740 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377749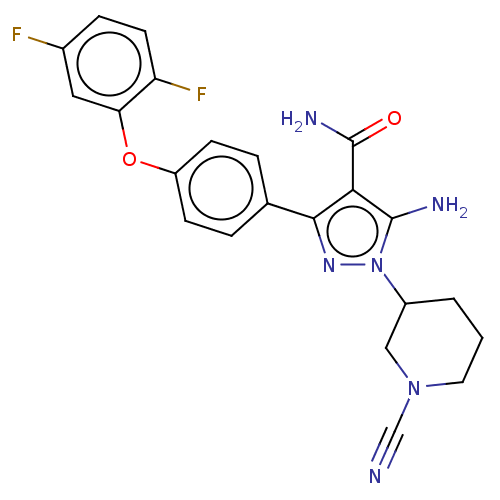 (5-amino-1-(1-cyanopiperidin-3-yl)-3-[4-(2, 5-diflu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377749 (5-amino-1-(1-cyanopiperidin-3-yl)-3-[4-(2, 5-diflu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.770 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM377741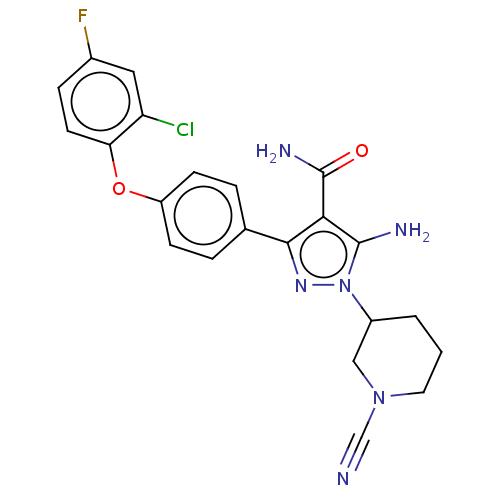 (5-amino-3-[4-(2-chloro-4-fluorophenoxy)phenyl]-1-(...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Institut Claudius Regaud | Assay Description TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide peptid... | J Med Chem 48: 287-91 (2005) BindingDB Entry DOI: 10.7270/Q2P271FD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM470375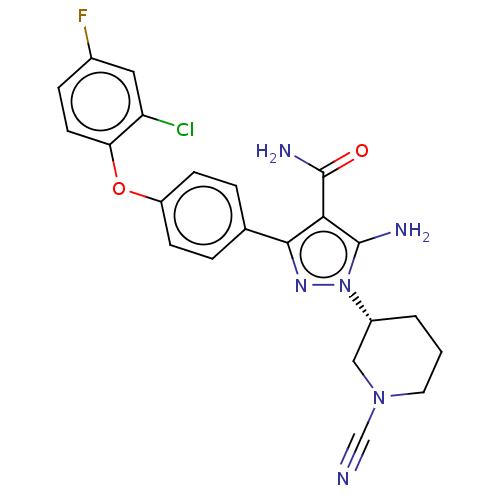 ((R)-5-amino-3-[4-(2-chloro-4-fluorophenoxy)phenyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description BTK: TR-FRET LanthaScreen assays were performed by incubating a dilution series of inhibitor concentrations with 50 μM ATP, 100 nM FAM-Srctide p... | US Patent US10815213 (2020) BindingDB Entry DOI: 10.7270/Q2W95D87 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 408 total ) | Next | Last >> |