

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511450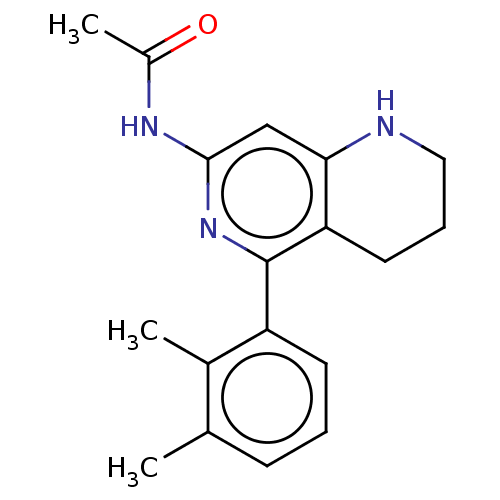 (CHEMBL4436749) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511450 (CHEMBL4436749) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Reversible inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50125472 (4-[2-carboxy-1-methylcarboxamido-(1S)-ethylcarboxa...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibitory concentration of the compound against HCV NS3 protease was determined | Bioorg Med Chem Lett 13: 1157-60 (2003) BindingDB Entry DOI: 10.7270/Q20R9NSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511450 (CHEMBL4436749) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.0430 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511453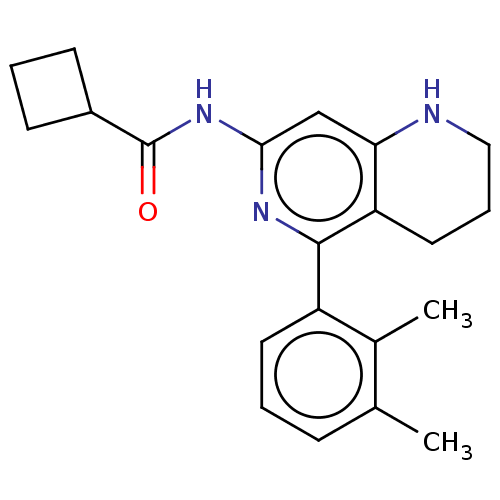 (CHEMBL4451394) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511431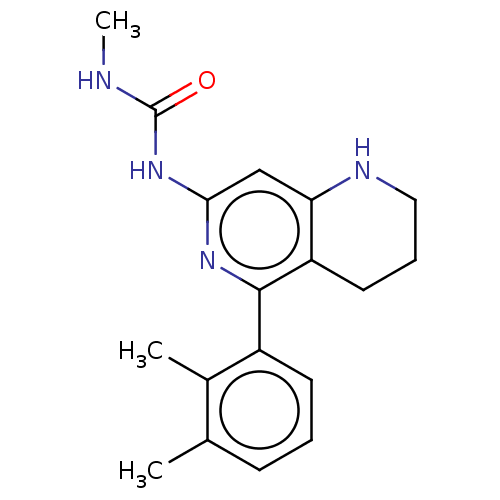 (CHEMBL4468099) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511430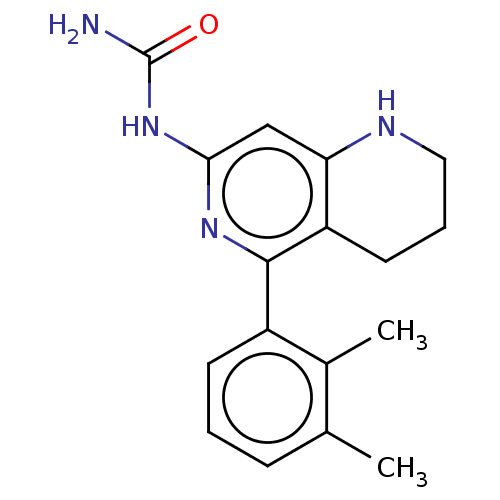 (CHEMBL4455353) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | <0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511452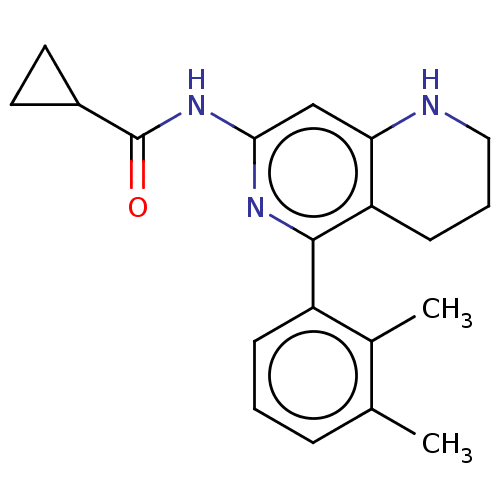 (CHEMBL4570266) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511454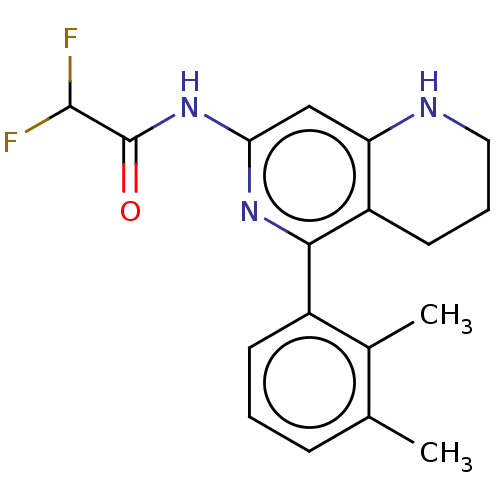 (CHEMBL4460228) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.110 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511432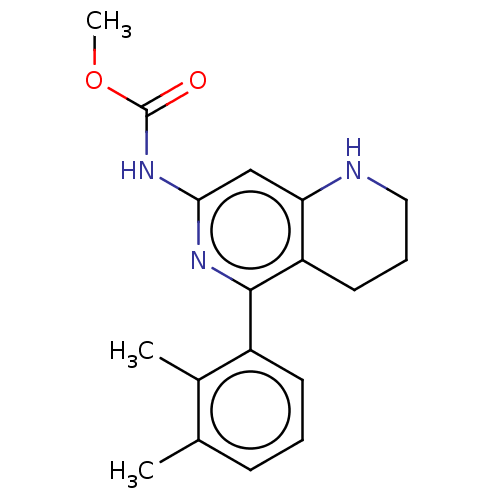 (CHEMBL4435945) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511466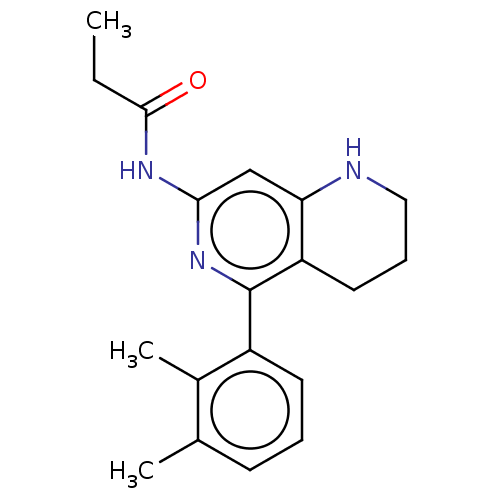 (CHEMBL4529335) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50152144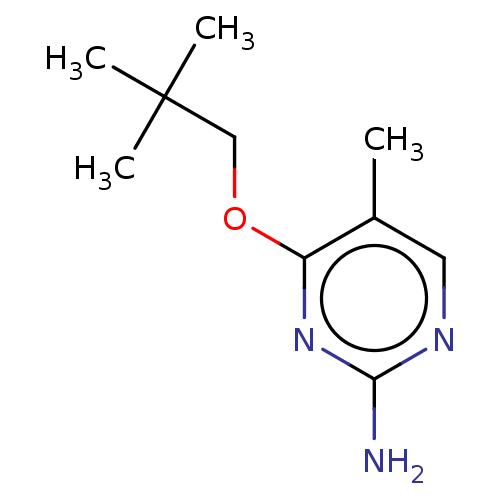 (CHEMBL3781661) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511448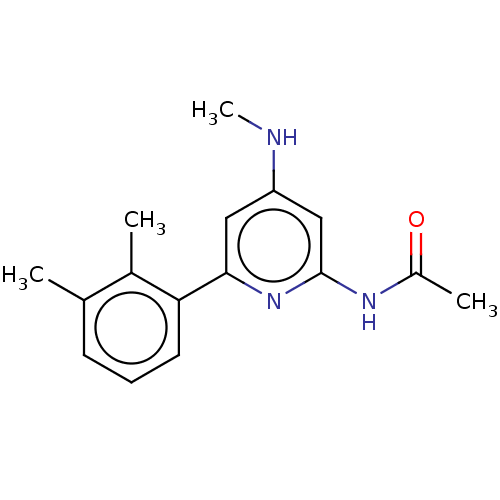 (CHEMBL4460446) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.330 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511428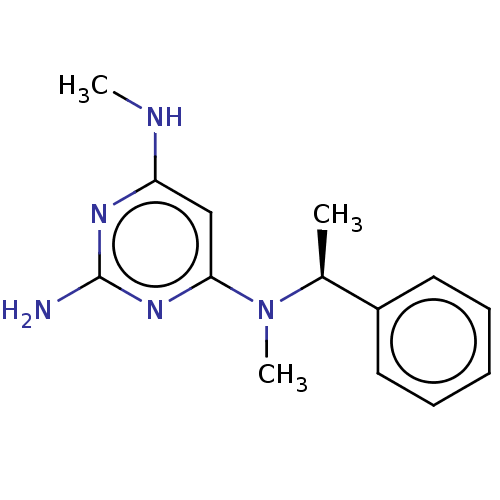 (CHEMBL4435983) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511463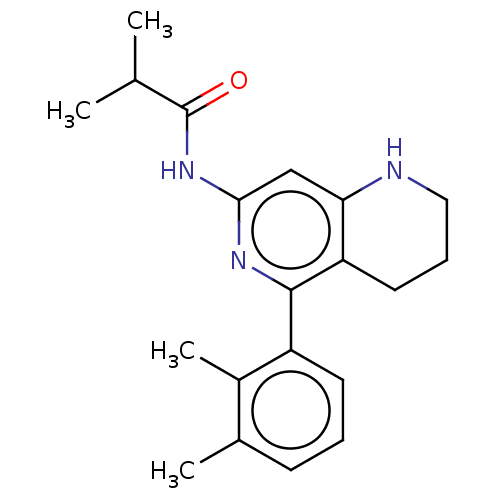 (CHEMBL4577002) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.610 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511457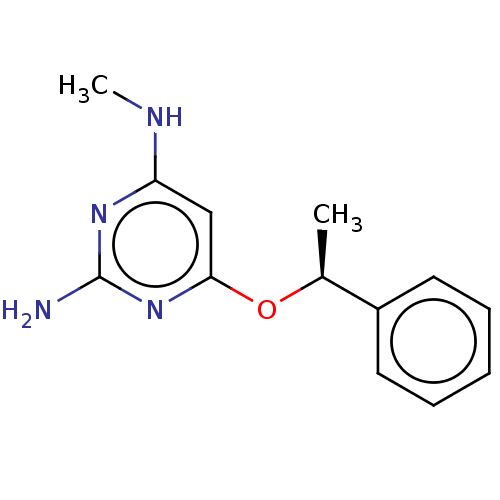 (CHEMBL4537820) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511439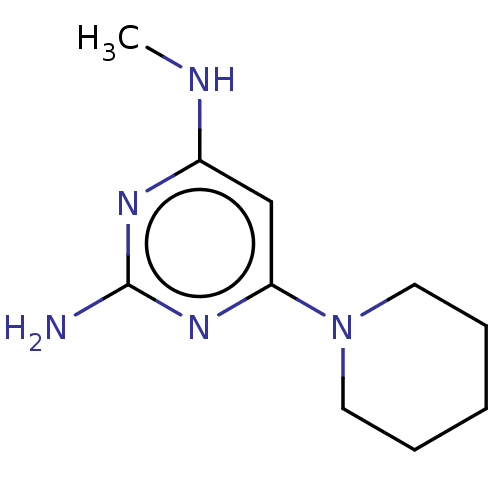 (CHEMBL4565830) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50152125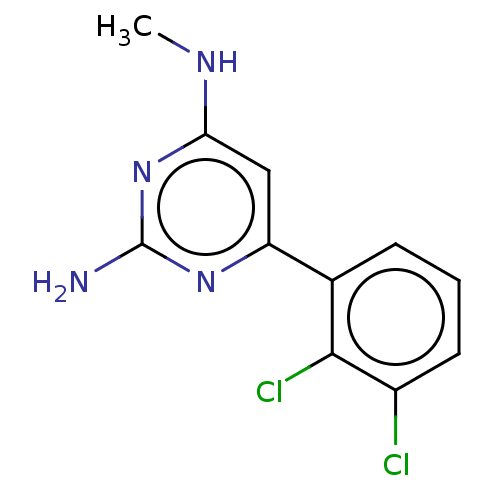 (CHEMBL3781316) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using dGTP as substrate measured after 30 mins by mala... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511458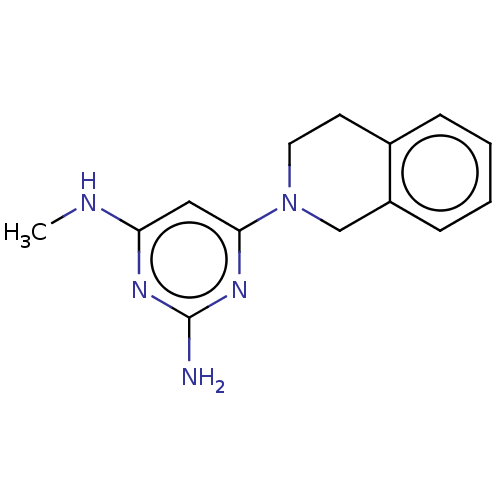 (CHEMBL4534555) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111938 (US8618107, 104) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 0.870 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111953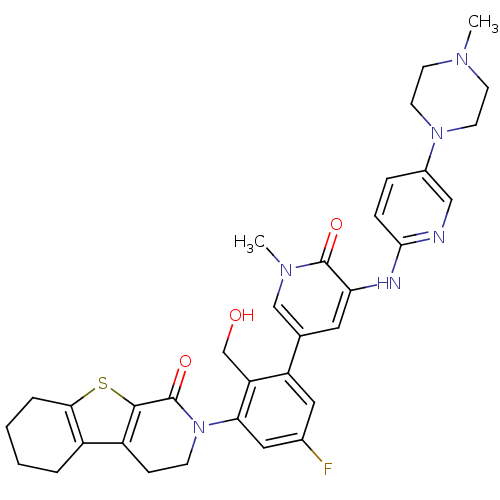 (US8618107, 212) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111940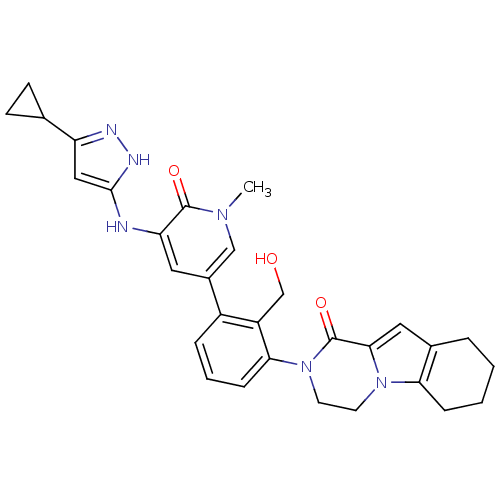 (US8618107, 106) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511441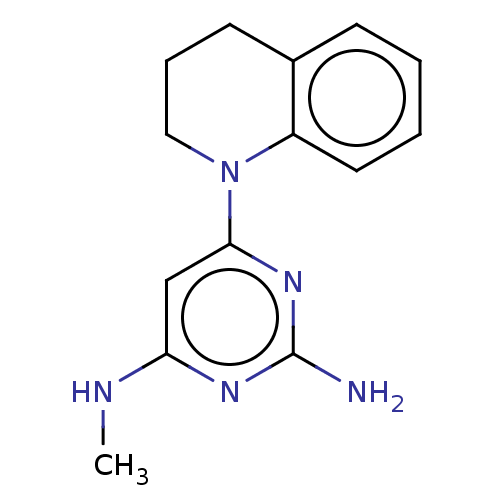 (CHEMBL4464305) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111975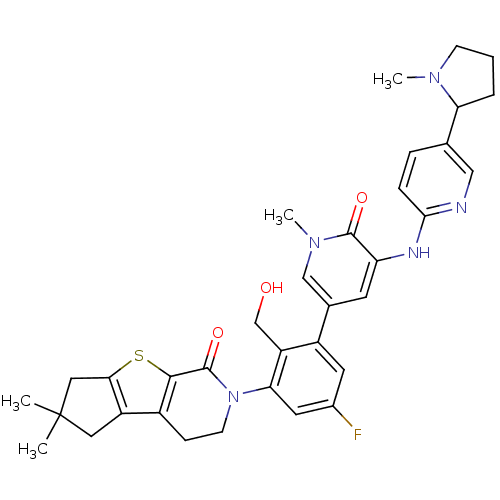 (US8618107, 287) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111978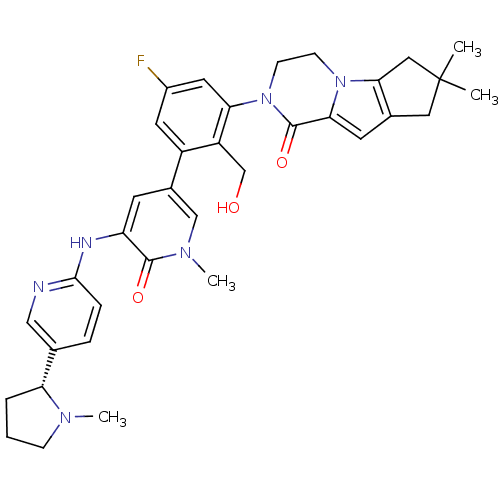 (US8618107, 291) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.74 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111951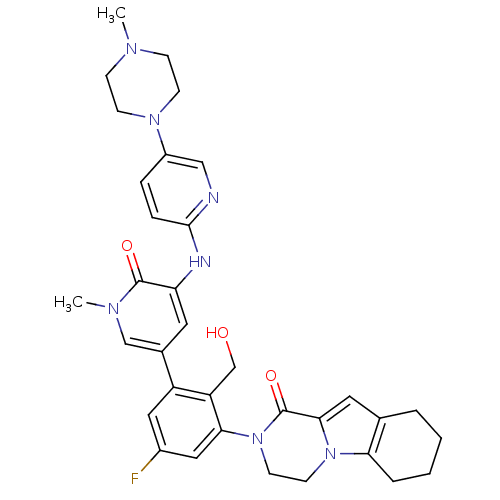 (US8618107, 197) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111956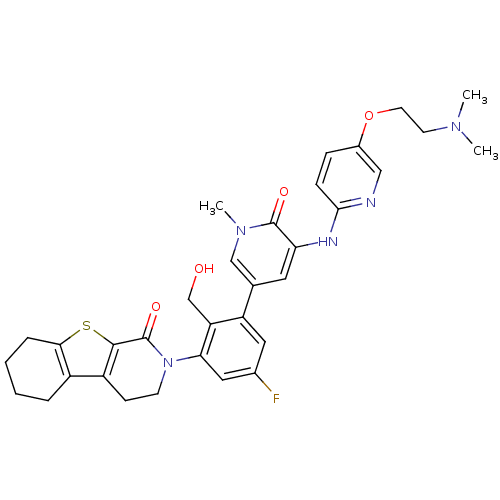 (US8618107, 268) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111935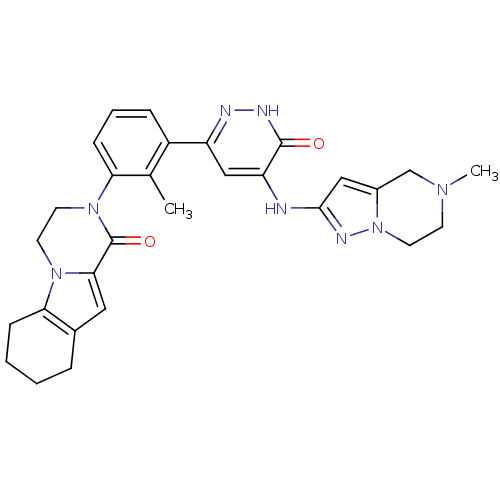 (US8618107, 101) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Genome polyprotein (Hepatitis C virus) | BDBM50125474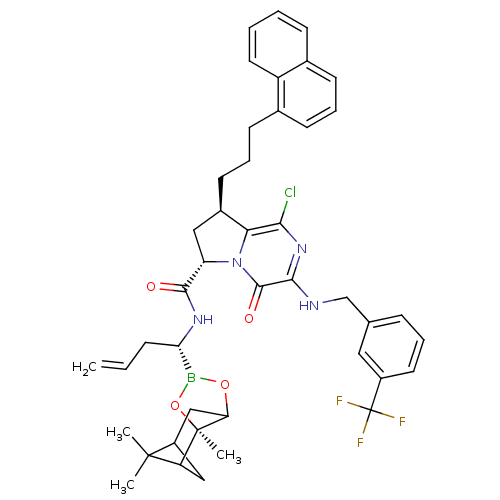 ((6S,8R)-1-Chloro-8-(3-naphthalen-1-yl-propyl)-4-ox...) | PDB MMDB UniProtKB/TrEMBL B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Curated by ChEMBL | Assay Description Inhibitory concentration against HCV NS3 protease. | Bioorg Med Chem Lett 13: 1157-60 (2003) BindingDB Entry DOI: 10.7270/Q20R9NSS | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111949 (US8618107, 131) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111976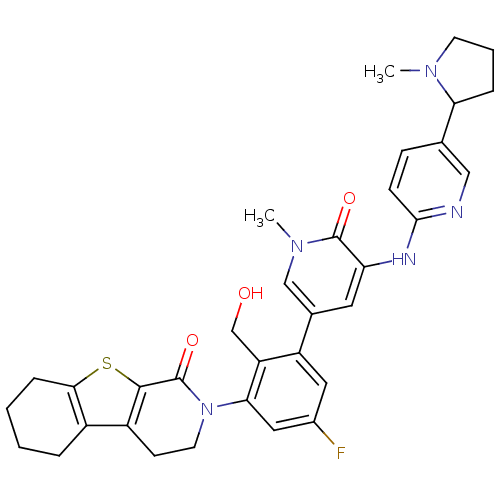 (US8618107, 288) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111964 (US8618107, 276) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111982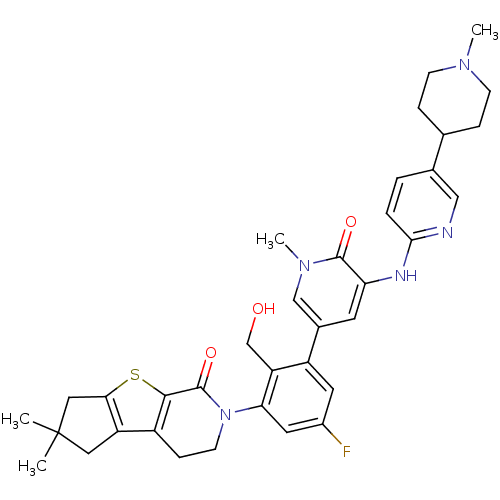 (US8618107, 295) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50255583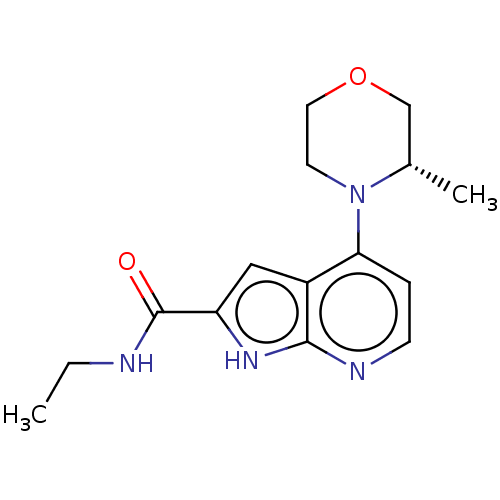 (CHEMBL4096813) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111962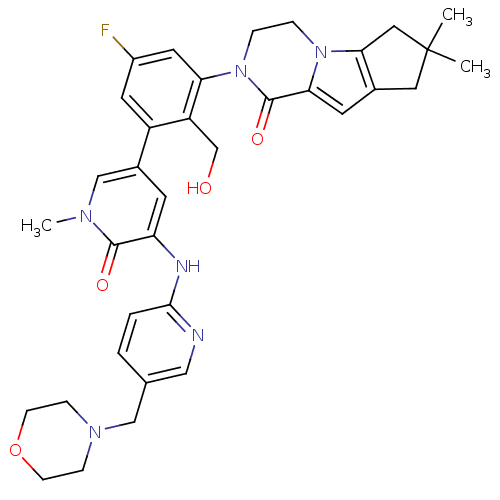 (US8618107, 274) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.34 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111966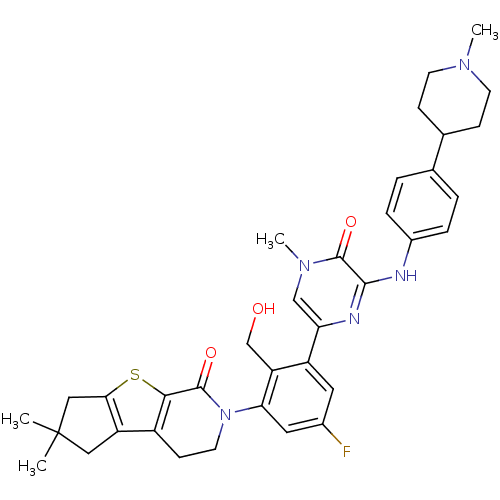 (US8618107, 278) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111954 (US8618107, 265) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111972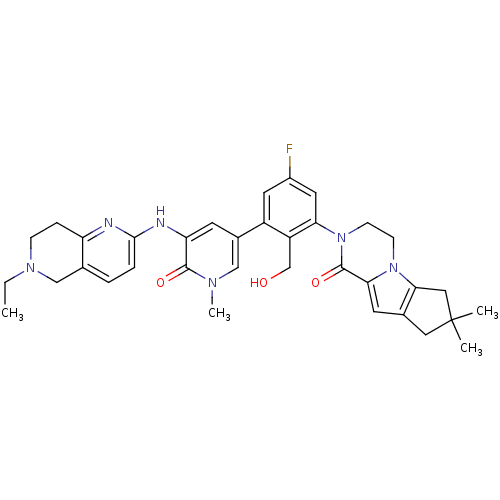 (US8618107, 284) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111983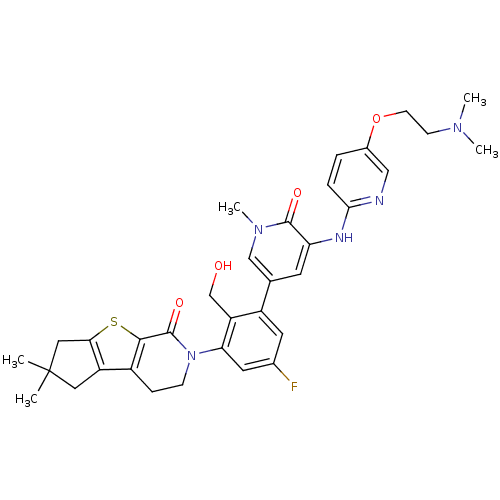 (US8618107, 296) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111948 (US8618107, 123) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111994 (US8618107, 308) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.30 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111965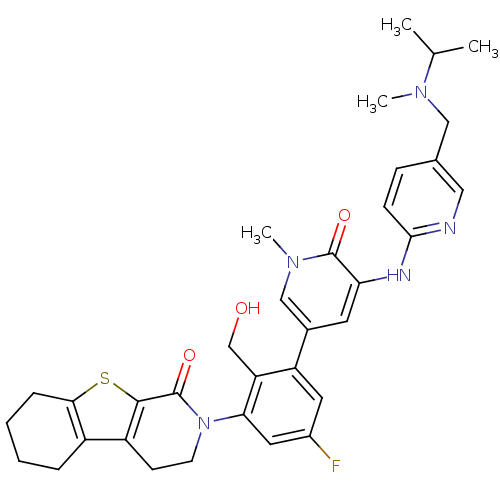 (US8618107, 277) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111974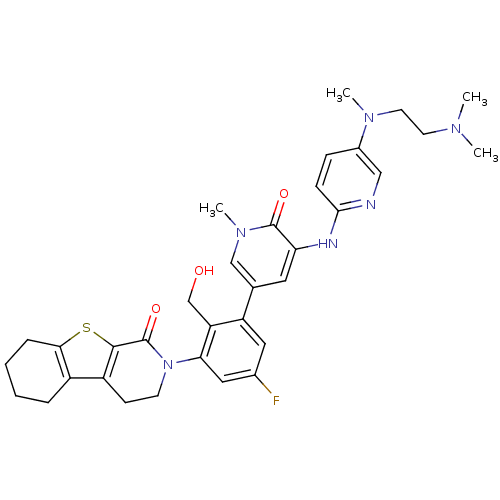 (US8618107, 286) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111987 (US8618107, 300) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111973 (US8618107, 285) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxidized purine nucleoside triphosphate hydrolase (Homo sapiens (Human)) | BDBM50511464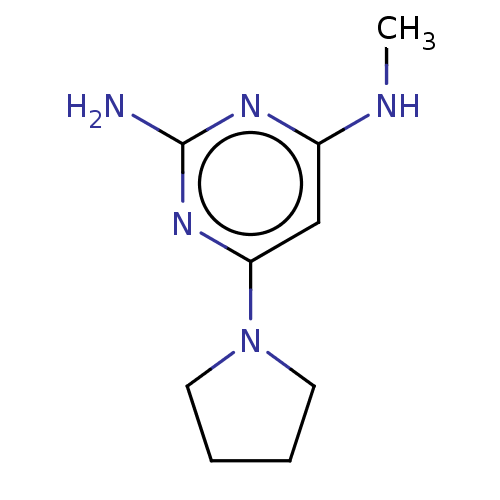 (CHEMBL4437933) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Gilead Sciences, Inc. Curated by ChEMBL | Assay Description Inhibition of human recombinant human His-tagged MTH1 expressed in Escherichia coli BL21 (DE3) using 8-oxo-dGTP as substrate incubated for 15 mins fo... | ACS Med Chem Lett 11: 358-364 (2020) Article DOI: 10.1021/acsmedchemlett.9b00420 BindingDB Entry DOI: 10.7270/Q2PV6PPZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111955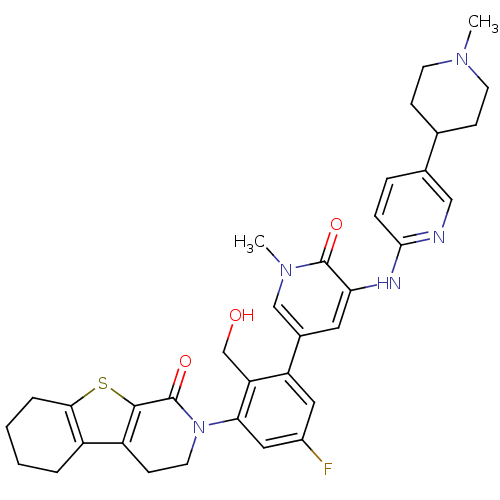 (US8618107, 267) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.80 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111952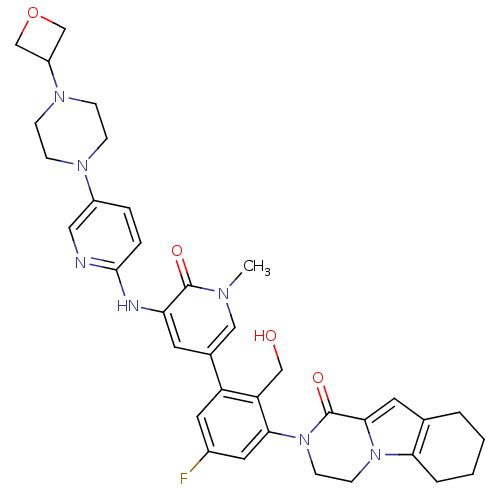 (US8618107, 210) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111958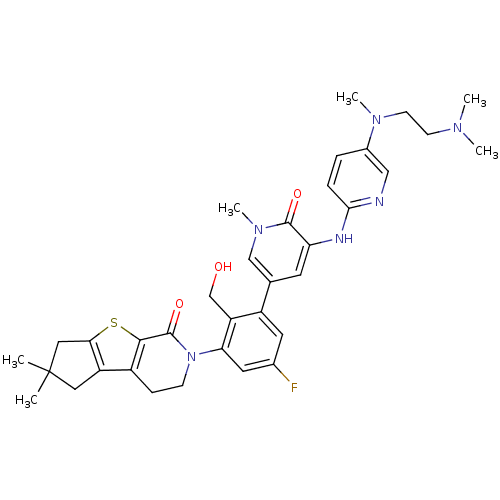 (US8618107, 270) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosine-protein kinase BTK (Homo sapiens (Human)) | BDBM111970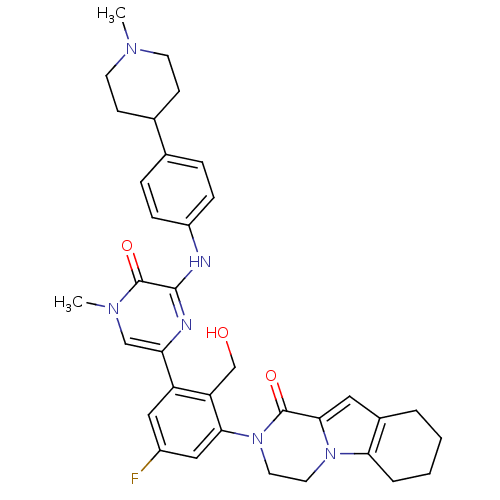 (US8618107, 282) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.5 | n/a |
Gilead Connecticut, Inc.; Genentech, Inc. US Patent | Assay Description A generalized procedure for a standard biochemical Btk Kinase Assay that can be used to test Formula I compounds. Alternatively, the Lanthascreen ass... | US Patent US8618107 (2013) BindingDB Entry DOI: 10.7270/Q2T72G3C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 311 total ) | Next | Last >> |