 Found 101 hits with Last Name = 'scholz' and Initial = 't'
Found 101 hits with Last Name = 'scholz' and Initial = 't' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM26810
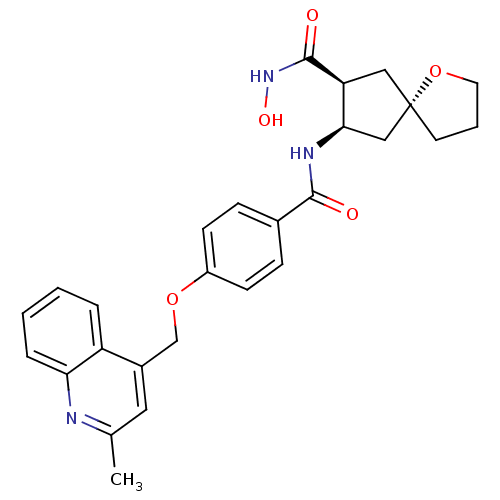
((5S,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@]3(CCCO3)C[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C27H29N3O5/c1-17-13-19(21-5-2-3-6-23(21)28-17)16-34-20-9-7-18(8-10-20)25(31)29-24-15-27(11-4-12-35-27)14-22(24)26(32)30-33/h2-3,5-10,13,22,24,33H,4,11-12,14-16H2,1H3,(H,29,31)(H,30,32)/t22-,24+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.25E+3 | -33.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM26809
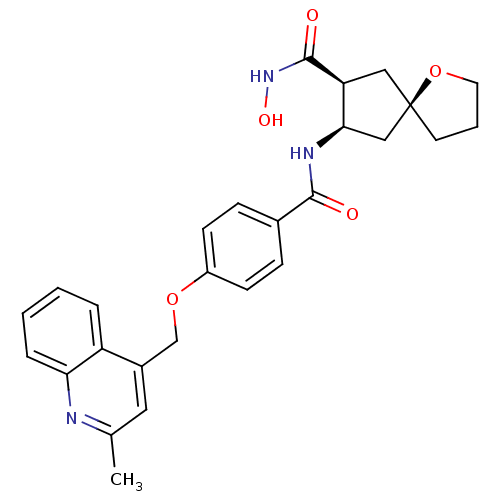
((5R,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@@]3(CCCO3)C[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C27H29N3O5/c1-17-13-19(21-5-2-3-6-23(21)28-17)16-34-20-9-7-18(8-10-20)25(31)29-24-15-27(11-4-12-35-27)14-22(24)26(32)30-33/h2-3,5-10,13,22,24,33H,4,11-12,14-16H2,1H3,(H,29,31)(H,30,32)/t22-,24+,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.36E+3 | -33.5 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM26809

((5R,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@@]3(CCCO3)C[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C27H29N3O5/c1-17-13-19(21-5-2-3-6-23(21)28-17)16-34-20-9-7-18(8-10-20)25(31)29-24-15-27(11-4-12-35-27)14-22(24)26(32)30-33/h2-3,5-10,13,22,24,33H,4,11-12,14-16H2,1H3,(H,29,31)(H,30,32)/t22-,24+,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.45E+3 | -33.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM26810

((5S,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@]3(CCCO3)C[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C27H29N3O5/c1-17-13-19(21-5-2-3-6-23(21)28-17)16-34-20-9-7-18(8-10-20)25(31)29-24-15-27(11-4-12-35-27)14-22(24)26(32)30-33/h2-3,5-10,13,22,24,33H,4,11-12,14-16H2,1H3,(H,29,31)(H,30,32)/t22-,24+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.92E+3 | -32.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
Matrix metalloproteinase-9
(Homo sapiens (Human)) | BDBM26808
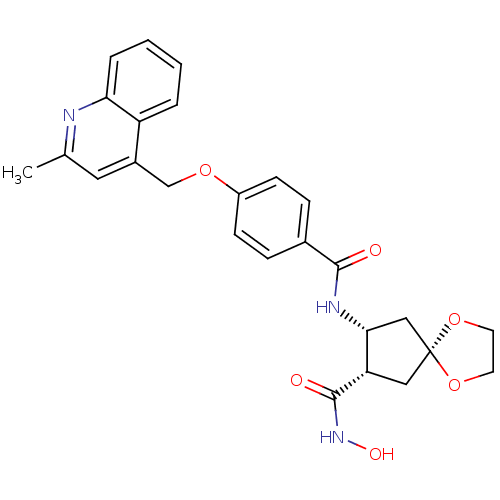
((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@@]3(C[C@@H]2C(=O)NO)OCCO3)c2ccccc2n1 |r| Show InChI InChI=1S/C26H27N3O6/c1-16-12-18(20-4-2-3-5-22(20)27-16)15-33-19-8-6-17(7-9-19)24(30)28-23-14-26(34-10-11-35-26)13-21(23)25(31)29-32/h2-9,12,21,23,32H,10-11,13-15H2,1H3,(H,28,30)(H,29,31)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >2.13E+3 | >-32.4 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
72 kDa type IV collagenase
(Homo sapiens (Human)) | BDBM26808

((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@@]3(C[C@@H]2C(=O)NO)OCCO3)c2ccccc2n1 |r| Show InChI InChI=1S/C26H27N3O6/c1-16-12-18(20-4-2-3-5-22(20)27-16)15-33-19-8-6-17(7-9-19)24(30)28-23-14-26(34-10-11-35-26)13-21(23)25(31)29-32/h2-9,12,21,23,32H,10-11,13-15H2,1H3,(H,28,30)(H,29,31)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.80E+3 | -31.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM26810

((5S,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@]3(CCCO3)C[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C27H29N3O5/c1-17-13-19(21-5-2-3-6-23(21)28-17)16-34-20-9-7-18(8-10-20)25(31)29-24-15-27(11-4-12-35-27)14-22(24)26(32)30-33/h2-3,5-10,13,22,24,33H,4,11-12,14-16H2,1H3,(H,29,31)(H,30,32)/t22-,24+,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.95E+3 | >-30.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM26808

((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@@]3(C[C@@H]2C(=O)NO)OCCO3)c2ccccc2n1 |r| Show InChI InChI=1S/C26H27N3O6/c1-16-12-18(20-4-2-3-5-22(20)27-16)15-33-19-8-6-17(7-9-19)24(30)28-23-14-26(34-10-11-35-26)13-21(23)25(31)29-32/h2-9,12,21,23,32H,10-11,13-15H2,1H3,(H,28,30)(H,29,31)/t21-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.95E+3 | >-30.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
Interstitial collagenase
(Homo sapiens (Human)) | BDBM26809

((5R,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@@]3(CCCO3)C[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C27H29N3O5/c1-17-13-19(21-5-2-3-6-23(21)28-17)16-34-20-9-7-18(8-10-20)25(31)29-24-15-27(11-4-12-35-27)14-22(24)26(32)30-33/h2-3,5-10,13,22,24,33H,4,11-12,14-16H2,1H3,(H,29,31)(H,30,32)/t22-,24+,27+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >4.95E+3 | >-30.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50369371
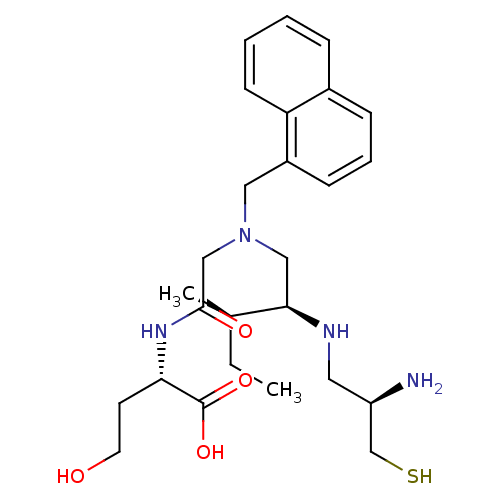
(CHEMBL1790750)Show SMILES CC[C@@H](C)[C@@H](CN(CC(=O)N[C@@H](CCO)C(O)=O)Cc1cccc2ccccc12)NC[C@@H](N)CS Show InChI InChI=1S/C26H40N4O4S/c1-3-18(2)24(28-13-21(27)17-35)15-30(16-25(32)29-23(11-12-31)26(33)34)14-20-9-6-8-19-7-4-5-10-22(19)20/h4-10,18,21,23-24,28,31,35H,3,11-17,27H2,1-2H3,(H,29,32)(H,33,34)/t18-,21-,23+,24-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.123 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant human Ha-Ras by Farnesyltransferase |
J Med Chem 41: 2651-6 (1998)
Article DOI: 10.1021/jm9800907
BindingDB Entry DOI: 10.7270/Q2WW7JCV |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM26810

((5S,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@]3(CCCO3)C[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C27H29N3O5/c1-17-13-19(21-5-2-3-6-23(21)28-17)16-34-20-9-7-18(8-10-20)25(31)29-24-15-27(11-4-12-35-27)14-22(24)26(32)30-33/h2-3,5-10,13,22,24,33H,4,11-12,14-16H2,1H3,(H,29,31)(H,30,32)/t22-,24+,27-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM26809

((5R,7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@@]3(CCCO3)C[C@@H]2C(=O)NO)c2ccccc2n1 |r| Show InChI InChI=1S/C27H29N3O5/c1-17-13-19(21-5-2-3-6-23(21)28-17)16-34-20-9-7-18(8-10-20)25(31)29-24-15-27(11-4-12-35-27)14-22(24)26(32)30-33/h2-3,5-10,13,22,24,33H,4,11-12,14-16H2,1H3,(H,29,31)(H,30,32)/t22-,24+,27+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
Disintegrin and metalloproteinase domain-containing protein 17
(Sus scrofa (pig)) | BDBM26808

((7S,8R)-7-N-hydroxy-8-N-{4-[(2-methylquinolin-4-yl...)Show SMILES Cc1cc(COc2ccc(cc2)C(=O)N[C@@H]2C[C@@]3(C[C@@H]2C(=O)NO)OCCO3)c2ccccc2n1 |r| Show InChI InChI=1S/C26H27N3O6/c1-16-12-18(20-4-2-3-5-22(20)27-16)15-33-19-8-6-17(7-9-19)24(30)28-23-14-26(34-10-11-35-26)13-21(23)25(31)29-32/h2-9,12,21,23,32H,10-11,13-15H2,1H3,(H,28,30)(H,29,31)/t21-,23+/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | 7.5 | 25 |
Bristol-Myers Squibb Company
| Assay Description
The compounds were tested for enzyme inhibition using fluorescence resonance energy transfer (FRET) assay. Fluorescence measurements were performed i... |
Bioorg Med Chem Lett 18: 1288-92 (2008)
Article DOI: 10.1016/j.bmcl.2008.01.030
BindingDB Entry DOI: 10.7270/Q2BV7DX2 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50366557
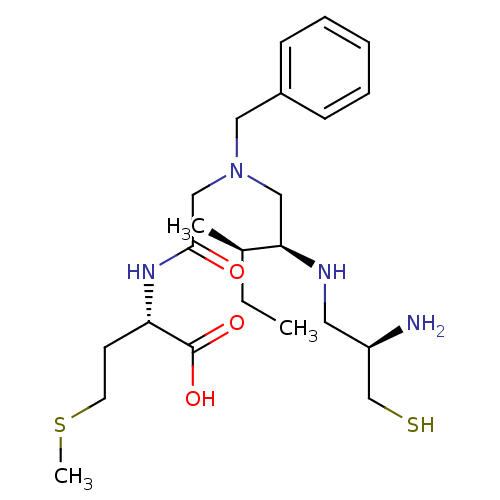
(CHEMBL1790748)Show SMILES CC[C@@H](C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1ccccc1)NC[C@@H](N)CS Show InChI InChI=1S/C23H40N4O3S2/c1-4-17(2)21(25-12-19(24)16-31)14-27(13-18-8-6-5-7-9-18)15-22(28)26-20(23(29)30)10-11-32-3/h5-9,17,19-21,25,31H,4,10-16,24H2,1-3H3,(H,26,28)(H,29,30)/t17-,19-,20+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant human Ha-Ras by Farnesyltransferase |
J Med Chem 41: 2651-6 (1998)
Article DOI: 10.1021/jm9800907
BindingDB Entry DOI: 10.7270/Q2WW7JCV |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50369364
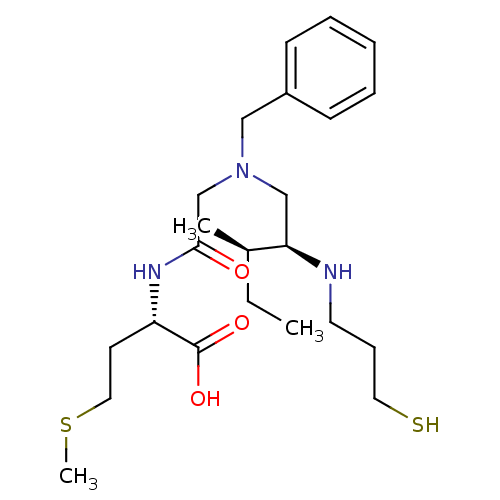
(CHEMBL1790760)Show SMILES CC[C@@H](C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1ccccc1)NCCCS Show InChI InChI=1S/C23H39N3O3S2/c1-4-18(2)21(24-12-8-13-30)16-26(15-19-9-6-5-7-10-19)17-22(27)25-20(23(28)29)11-14-31-3/h5-7,9-10,18,20-21,24,30H,4,8,11-17H2,1-3H3,(H,25,27)(H,28,29)/t18-,20+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant human Ha-Ras by Farnesyltransferase |
J Med Chem 41: 2651-6 (1998)
Article DOI: 10.1021/jm9800907
BindingDB Entry DOI: 10.7270/Q2WW7JCV |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50044312

(CHEMBL302780 | Fluoro-(octane-1-sulfonyl)-methanes...)Show InChI InChI=1S/C9H20FNO4S2/c1-2-3-4-5-6-7-8-16(12,13)9(10)17(11,14)15/h9H,2-8H2,1H3,(H2,11,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human erythrocyte carbonic anhydrase II |
J Med Chem 36: 2134-41 (1993)
BindingDB Entry DOI: 10.7270/Q2GX49N1 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50369370
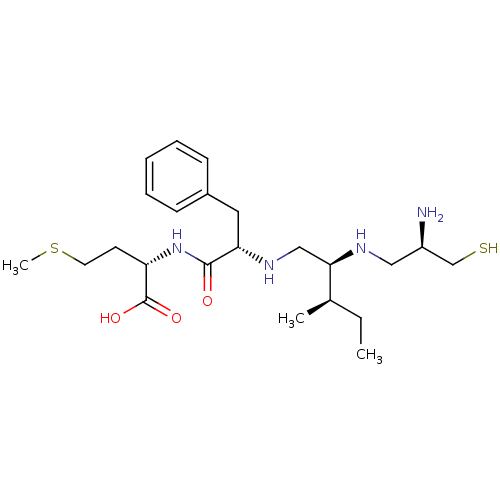
(CHEMBL1790745)Show SMILES CC[C@@H](C)[C@@H](CN[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCSC)C(O)=O)NC[C@@H](N)CS Show InChI InChI=1S/C23H40N4O3S2/c1-4-16(2)21(25-13-18(24)15-31)14-26-20(12-17-8-6-5-7-9-17)22(28)27-19(23(29)30)10-11-32-3/h5-9,16,18-21,25-26,31H,4,10-15,24H2,1-3H3,(H,27,28)(H,29,30)/t16-,18-,19+,20+,21-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant human Ha-Ras by Farnesyltransferase |
J Med Chem 41: 2651-6 (1998)
Article DOI: 10.1021/jm9800907
BindingDB Entry DOI: 10.7270/Q2WW7JCV |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50031179

((S)-2-((R)-2-Amino-3-mercapto-propylamino)-3-methy...)Show SMILES CCC(C)[C@H](NC[C@@H](N)CS)C(=O)Nc1cccc(C)c1 Show InChI InChI=1S/C16H27N3OS/c1-4-12(3)15(18-9-13(17)10-21)16(20)19-14-7-5-6-11(2)8-14/h5-8,12-13,15,18,21H,4,9-10,17H2,1-3H3,(H,19,20)/t12?,13-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of bovine Geranylgeranyl transferase type I |
J Med Chem 38: 3967-71 (1995)
BindingDB Entry DOI: 10.7270/Q2XS5TD4 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50031169
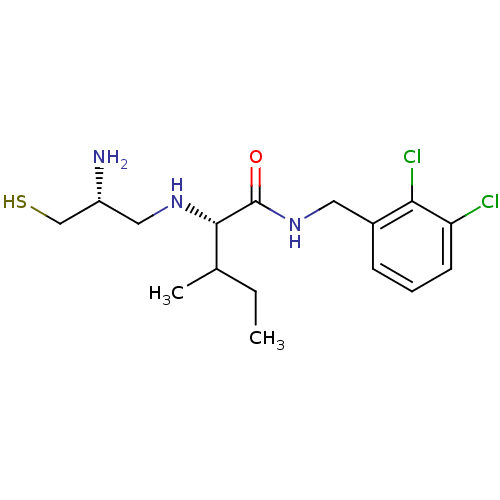
((S)-2-((R)-2-Amino-3-mercapto-propylamino)-3-methy...)Show SMILES CCC(C)[C@H](NC[C@@H](N)CS)C(=O)NCc1cccc(Cl)c1Cl Show InChI InChI=1S/C16H25Cl2N3OS/c1-3-10(2)15(20-8-12(19)9-23)16(22)21-7-11-5-4-6-13(17)14(11)18/h4-6,10,12,15,20,23H,3,7-9,19H2,1-2H3,(H,21,22)/t10?,12-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of bovine Geranylgeranyl transferase type I |
J Med Chem 38: 3967-71 (1995)
BindingDB Entry DOI: 10.7270/Q2XS5TD4 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50031167

((S)-2-((R)-2-Amino-3-mercapto-propylamino)-3-methy...)Show SMILES CCC(C)[C@H](NC[C@@H](N)CS)C(=O)Nc1cccc(C)c1C Show InChI InChI=1S/C17H29N3OS/c1-5-11(2)16(19-9-14(18)10-22)17(21)20-15-8-6-7-12(3)13(15)4/h6-8,11,14,16,19,22H,5,9-10,18H2,1-4H3,(H,20,21)/t11?,14-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of bovine Geranylgeranyl transferase type I |
J Med Chem 38: 3967-71 (1995)
BindingDB Entry DOI: 10.7270/Q2XS5TD4 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50031170

((S)-2-((R)-2-Amino-3-mercapto-propylamino)-3-methy...)Show SMILES CCC(C)[C@H](NC[C@@H](N)CS)C(=O)NCc1ccc(Cl)cc1Cl Show InChI InChI=1S/C16H25Cl2N3OS/c1-3-10(2)15(20-8-13(19)9-23)16(22)21-7-11-4-5-12(17)6-14(11)18/h4-6,10,13,15,20,23H,3,7-9,19H2,1-2H3,(H,21,22)/t10?,13-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of bovine Geranylgeranyl transferase type I |
J Med Chem 38: 3967-71 (1995)
BindingDB Entry DOI: 10.7270/Q2XS5TD4 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50369371

(CHEMBL1790750)Show SMILES CC[C@@H](C)[C@@H](CN(CC(=O)N[C@@H](CCO)C(O)=O)Cc1cccc2ccccc12)NC[C@@H](N)CS Show InChI InChI=1S/C26H40N4O4S/c1-3-18(2)24(28-13-21(27)17-35)15-30(16-25(32)29-23(11-12-31)26(33)34)14-20-9-6-8-19-7-4-5-10-22(19)20/h4-10,18,21,23-24,28,31,35H,3,11-17,27H2,1-2H3,(H,29,32)(H,33,34)/t18-,21-,23+,24-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of bovine Geranylgeranyl transferase type I |
J Med Chem 41: 2651-6 (1998)
Article DOI: 10.1021/jm9800907
BindingDB Entry DOI: 10.7270/Q2WW7JCV |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM10880

(AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...)Show InChI InChI=1S/C4H6N4O3S2/c1-2(9)6-3-7-8-4(12-3)13(5,10)11/h1H3,(H2,5,10,11)(H,6,7,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human erythrocyte carbonic anhydrase II |
J Med Chem 36: 2134-41 (1993)
BindingDB Entry DOI: 10.7270/Q2GX49N1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50044313
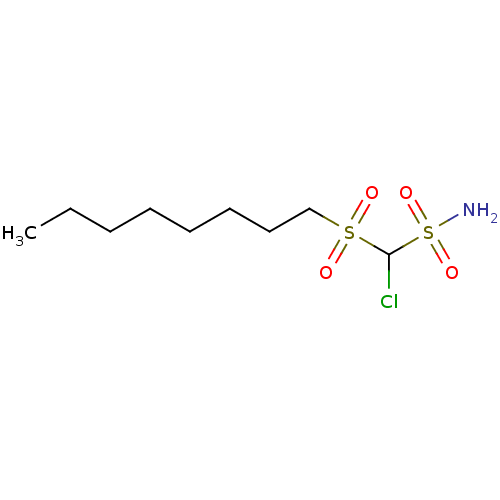
(CHEMBL304131 | Chloro-(octane-1-sulfonyl)-methanes...)Show InChI InChI=1S/C9H20ClNO4S2/c1-2-3-4-5-6-7-8-16(12,13)9(10)17(11,14)15/h9H,2-8H2,1H3,(H2,11,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human erythrocyte carbonic anhydrase II |
J Med Chem 36: 2134-41 (1993)
BindingDB Entry DOI: 10.7270/Q2GX49N1 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50044311
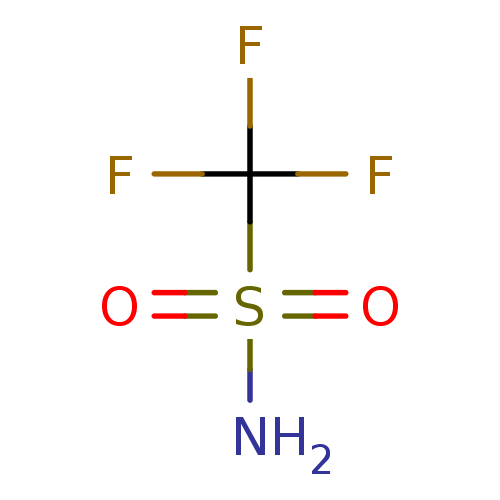
(CHEMBL67511 | TRIFLUOROMETHANE SULFONAMIDE | Trifl...)Show InChI InChI=1S/CH2F3NO2S/c2-1(3,4)8(5,6)7/h(H2,5,6,7) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| MMDB
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human erythrocyte carbonic anhydrase II |
J Med Chem 36: 2134-41 (1993)
BindingDB Entry DOI: 10.7270/Q2GX49N1 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50407296
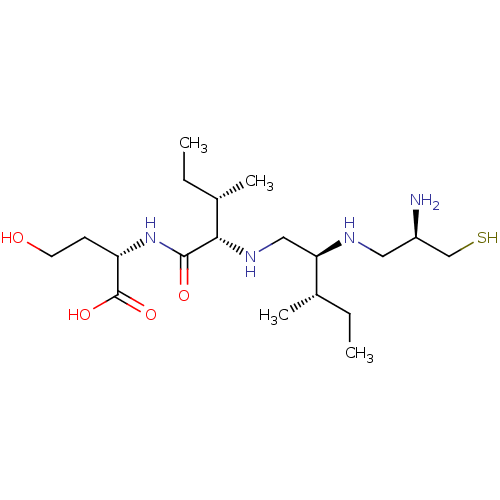
(CHEMBL2052018 | L-731735)Show SMILES CC[C@H](C)[C@@H](CN[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCO)C(O)=O)NC[C@@H](N)CS Show InChI InChI=1S/C19H40N4O4S/c1-5-12(3)16(21-9-14(20)11-28)10-22-17(13(4)6-2)18(25)23-15(7-8-24)19(26)27/h12-17,21-22,24,28H,5-11,20H2,1-4H3,(H,23,25)(H,26,27)/t12-,13-,14+,15-,16+,17-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant human Ha-Ras by Farnesyltransferase |
J Med Chem 41: 2651-6 (1998)
Article DOI: 10.1021/jm9800907
BindingDB Entry DOI: 10.7270/Q2WW7JCV |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
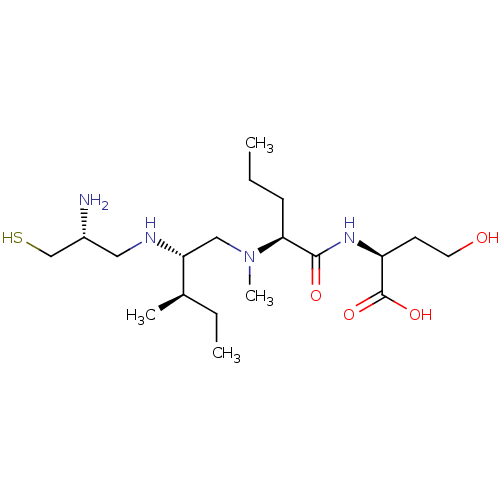
(Homo sapiens (Human)) | BDBM50369368
(CHEMBL1790744)Show SMILES CCC[C@H](N(C)C[C@@H](NC[C@@H](N)CS)[C@H](C)CC)C(=O)N[C@@H](CCO)C(O)=O Show InChI InChI=1S/C19H40N4O4S/c1-5-7-17(18(25)22-15(8-9-24)19(26)27)23(4)11-16(13(3)6-2)21-10-14(20)12-28/h13-17,21,24,28H,5-12,20H2,1-4H3,(H,22,25)(H,26,27)/t13-,14-,15+,16-,17+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant human Ha-Ras by Farnesyltransferase |
J Med Chem 41: 2651-6 (1998)
Article DOI: 10.1021/jm9800907
BindingDB Entry DOI: 10.7270/Q2WW7JCV |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50031168
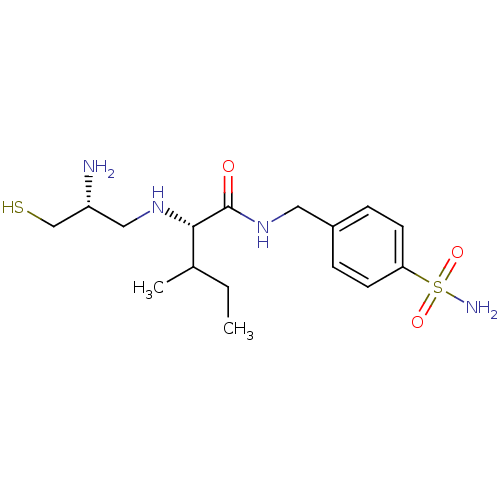
((S)-2-((R)-2-Amino-3-mercapto-propylamino)-3-methy...)Show SMILES CCC(C)[C@H](NC[C@@H](N)CS)C(=O)NCc1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C16H28N4O3S2/c1-3-11(2)15(19-9-13(17)10-24)16(21)20-8-12-4-6-14(7-5-12)25(18,22)23/h4-7,11,13,15,19,24H,3,8-10,17H2,1-2H3,(H,20,21)(H2,18,22,23)/t11?,13-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of bovine Geranylgeranyl transferase type I |
J Med Chem 38: 3967-71 (1995)
BindingDB Entry DOI: 10.7270/Q2XS5TD4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50044309

((Octane-1-sulfonyl)-methanesulfonamide | CHEMBL421...)Show InChI InChI=1S/C9H21NO4S2/c1-2-3-4-5-6-7-8-15(11,12)9-16(10,13)14/h2-9H2,1H3,(H2,10,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human erythrocyte carbonic anhydrase II |
J Med Chem 36: 2134-41 (1993)
BindingDB Entry DOI: 10.7270/Q2GX49N1 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50031169

((S)-2-((R)-2-Amino-3-mercapto-propylamino)-3-methy...)Show SMILES CCC(C)[C@H](NC[C@@H](N)CS)C(=O)NCc1cccc(Cl)c1Cl Show InChI InChI=1S/C16H25Cl2N3OS/c1-3-10(2)15(20-8-12(19)9-23)16(22)21-7-11-5-4-6-13(17)14(11)18/h4-6,10,12,15,20,23H,3,7-9,19H2,1-2H3,(H,21,22)/t10?,12-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro concentration required to reduce Farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ha-Ras protein by 50% |
J Med Chem 38: 3967-71 (1995)
BindingDB Entry DOI: 10.7270/Q2XS5TD4 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50369372

(CHEMBL1790756)Show SMILES CC[C@@H](C)[C@@H](CN(C)[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCSC)C(O)=O)NC[C@@H](N)CS Show InChI InChI=1S/C21H44N4O3S2/c1-7-14(3)18(23-11-16(22)13-29)12-25(5)19(15(4)8-2)20(26)24-17(21(27)28)9-10-30-6/h14-19,23,29H,7-13,22H2,1-6H3,(H,24,26)(H,27,28)/t14-,15+,16-,17+,18-,19+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant human Ha-Ras by Farnesyltransferase |
J Med Chem 41: 2651-6 (1998)
Article DOI: 10.1021/jm9800907
BindingDB Entry DOI: 10.7270/Q2WW7JCV |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50369366
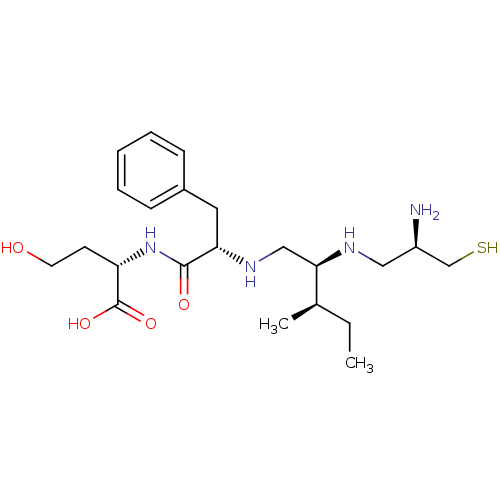
(CHEMBL1790752)Show SMILES CC[C@@H](C)[C@@H](CN[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCO)C(O)=O)NC[C@@H](N)CS Show InChI InChI=1S/C22H38N4O4S/c1-3-15(2)20(24-12-17(23)14-31)13-25-19(11-16-7-5-4-6-8-16)21(28)26-18(9-10-27)22(29)30/h4-8,15,17-20,24-25,27,31H,3,9-14,23H2,1-2H3,(H,26,28)(H,29,30)/t15-,17-,18+,19+,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain Farnesyltransferase at 1 nM |
J Med Chem 41: 2651-6 (1998)
Article DOI: 10.1021/jm9800907
BindingDB Entry DOI: 10.7270/Q2WW7JCV |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50369375
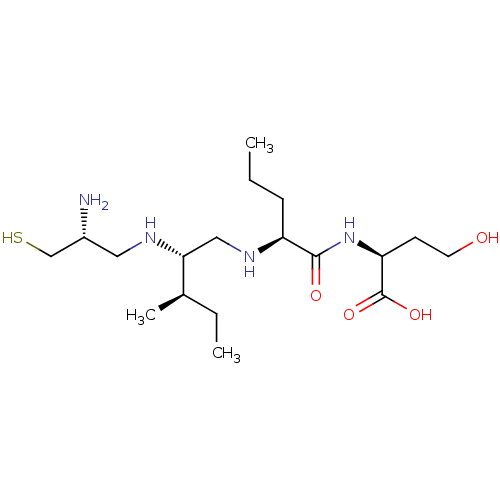
(CHEMBL1790753)Show SMILES CCC[C@H](NC[C@@H](NC[C@@H](N)CS)[C@H](C)CC)C(=O)N[C@@H](CCO)C(O)=O Show InChI InChI=1S/C18H38N4O4S/c1-4-6-14(17(24)22-15(7-8-23)18(25)26)21-10-16(12(3)5-2)20-9-13(19)11-27/h12-16,20-21,23,27H,4-11,19H2,1-3H3,(H,22,24)(H,25,26)/t12-,13-,14+,15+,16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain Farnesyltransferase at 10 pM |
J Med Chem 41: 2651-6 (1998)
Article DOI: 10.1021/jm9800907
BindingDB Entry DOI: 10.7270/Q2WW7JCV |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50031178

((S)-2-((R)-2-Amino-3-mercapto-propylamino)-3-methy...)Show SMILES CCC(C)[C@H](NC[C@@H](N)CS)C(=O)NCc1ccc(C)cc1C Show InChI InChI=1S/C18H31N3OS/c1-5-13(3)17(20-10-16(19)11-23)18(22)21-9-15-7-6-12(2)8-14(15)4/h6-8,13,16-17,20,23H,5,9-11,19H2,1-4H3,(H,21,22)/t13?,16-,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of bovine Geranylgeranyl transferase type I |
J Med Chem 38: 3967-71 (1995)
BindingDB Entry DOI: 10.7270/Q2XS5TD4 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50369367
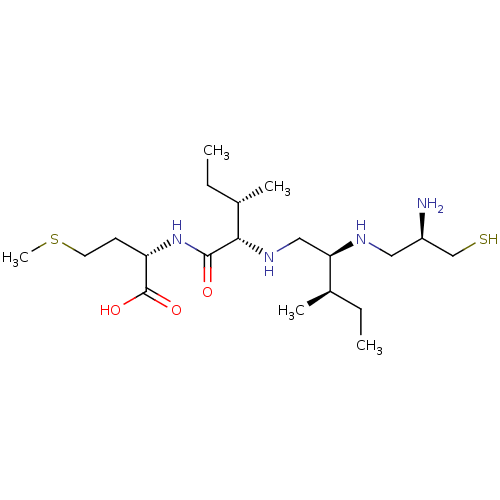
(CHEMBL1790757)Show SMILES CC[C@@H](C)[C@@H](CN[C@@H]([C@@H](C)CC)C(=O)N[C@@H](CCSC)C(O)=O)NC[C@@H](N)CS Show InChI InChI=1S/C20H42N4O3S2/c1-6-13(3)17(22-10-15(21)12-28)11-23-18(14(4)7-2)19(25)24-16(20(26)27)8-9-29-5/h13-18,22-23,28H,6-12,21H2,1-5H3,(H,24,25)(H,26,27)/t13-,14+,15-,16+,17-,18+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant human Ha-Ras by Farnesyltransferase |
J Med Chem 41: 2651-6 (1998)
Article DOI: 10.1021/jm9800907
BindingDB Entry DOI: 10.7270/Q2WW7JCV |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50031167

((S)-2-((R)-2-Amino-3-mercapto-propylamino)-3-methy...)Show SMILES CCC(C)[C@H](NC[C@@H](N)CS)C(=O)Nc1cccc(C)c1C Show InChI InChI=1S/C17H29N3OS/c1-5-11(2)16(19-9-14(18)10-22)17(21)20-15-8-6-7-12(3)13(15)4/h6-8,11,14,16,19,22H,5,9-10,18H2,1-4H3,(H,20,21)/t11?,14-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro concentration required to reduce Farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ha-Ras protein by 50% |
J Med Chem 38: 3967-71 (1995)
BindingDB Entry DOI: 10.7270/Q2XS5TD4 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Homo sapiens (Human)) | BDBM50369363
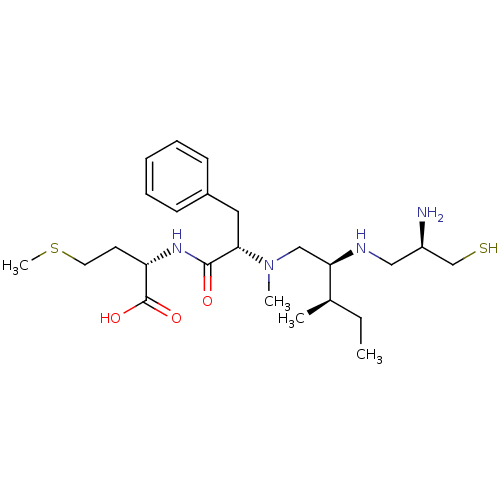
(CHEMBL1790747)Show SMILES CC[C@@H](C)[C@@H](CN(C)[C@@H](Cc1ccccc1)C(=O)N[C@@H](CCSC)C(O)=O)NC[C@@H](N)CS Show InChI InChI=1S/C24H42N4O3S2/c1-5-17(2)21(26-14-19(25)16-32)15-28(3)22(13-18-9-7-6-8-10-18)23(29)27-20(24(30)31)11-12-33-4/h6-10,17,19-22,26,32H,5,11-16,25H2,1-4H3,(H,27,29)(H,30,31)/t17-,19-,20+,21-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]FPP incorporation into recombinant human Ha-Ras by Farnesyltransferase |
J Med Chem 41: 2651-6 (1998)
Article DOI: 10.1021/jm9800907
BindingDB Entry DOI: 10.7270/Q2WW7JCV |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50031179

((S)-2-((R)-2-Amino-3-mercapto-propylamino)-3-methy...)Show SMILES CCC(C)[C@H](NC[C@@H](N)CS)C(=O)Nc1cccc(C)c1 Show InChI InChI=1S/C16H27N3OS/c1-4-12(3)15(18-9-13(17)10-21)16(20)19-14-7-5-6-11(2)8-14/h5-8,12-13,15,18,21H,4,9-10,17H2,1-3H3,(H,19,20)/t12?,13-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 46 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro concentration required to reduce Farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ha-Ras protein by 50% |
J Med Chem 38: 3967-71 (1995)
BindingDB Entry DOI: 10.7270/Q2XS5TD4 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50031175

((S)-2-((R)-2-Amino-3-mercapto-propylamino)-3-methy...)Show SMILES CCC(C)[C@H](NC[C@@H](N)CS)C(=O)Nc1ccc(C)cc1C Show InChI InChI=1S/C17H29N3OS/c1-5-12(3)16(19-9-14(18)10-22)17(21)20-15-7-6-11(2)8-13(15)4/h6-8,12,14,16,19,22H,5,9-10,18H2,1-4H3,(H,20,21)/t12?,14-,16+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro concentration required to reduce Farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ha-Ras protein by 50% |
J Med Chem 38: 3967-71 (1995)
BindingDB Entry DOI: 10.7270/Q2XS5TD4 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50031176

((S)-2-((R)-2-Amino-3-mercapto-propylamino)-3-methy...)Show InChI InChI=1S/C15H25N3OS/c1-3-11(2)14(17-9-12(16)10-20)15(19)18-13-7-5-4-6-8-13/h4-8,11-12,14,17,20H,3,9-10,16H2,1-2H3,(H,18,19)/t11?,12-,14+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro concentration required to reduce Farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ha-Ras protein by 50% |
J Med Chem 38: 3967-71 (1995)
BindingDB Entry DOI: 10.7270/Q2XS5TD4 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50369365

(CHEMBL1790754)Show SMILES CC[C@@H](C)[C@@H](CN(C)CC(=O)N[C@@H](CCO)C(O)=O)NC[C@@H](N)CS Show InChI InChI=1S/C16H34N4O4S/c1-4-11(2)14(18-7-12(17)10-25)8-20(3)9-15(22)19-13(5-6-21)16(23)24/h11-14,18,21,25H,4-10,17H2,1-3H3,(H,19,22)(H,23,24)/t11-,12-,13+,14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of bovine brain Farnesyltransferase at 10 pM |
J Med Chem 41: 2651-6 (1998)
Article DOI: 10.1021/jm9800907
BindingDB Entry DOI: 10.7270/Q2WW7JCV |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50031162

((S)-2-((R)-2-Amino-3-mercapto-propylamino)-3-methy...)Show InChI InChI=1S/C16H27N3OS/c1-3-12(2)15(18-10-14(17)11-21)16(20)19-9-13-7-5-4-6-8-13/h4-8,12,14-15,18,21H,3,9-11,17H2,1-2H3,(H,19,20)/t12?,14-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of bovine Geranylgeranyl transferase type I |
J Med Chem 38: 3967-71 (1995)
BindingDB Entry DOI: 10.7270/Q2XS5TD4 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50031170

((S)-2-((R)-2-Amino-3-mercapto-propylamino)-3-methy...)Show SMILES CCC(C)[C@H](NC[C@@H](N)CS)C(=O)NCc1ccc(Cl)cc1Cl Show InChI InChI=1S/C16H25Cl2N3OS/c1-3-10(2)15(20-8-13(19)9-23)16(22)21-7-11-4-5-12(17)6-14(11)18/h4-6,10,13,15,20,23H,3,7-9,19H2,1-2H3,(H,21,22)/t10?,13-,15+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro concentration required to reduce Farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ha-Ras protein by 50% |
J Med Chem 38: 3967-71 (1995)
BindingDB Entry DOI: 10.7270/Q2XS5TD4 |
More data for this
Ligand-Target Pair | |
Geranylgeranyl transferase type-1 subunit beta/Protein farnesyltransferase/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50031163
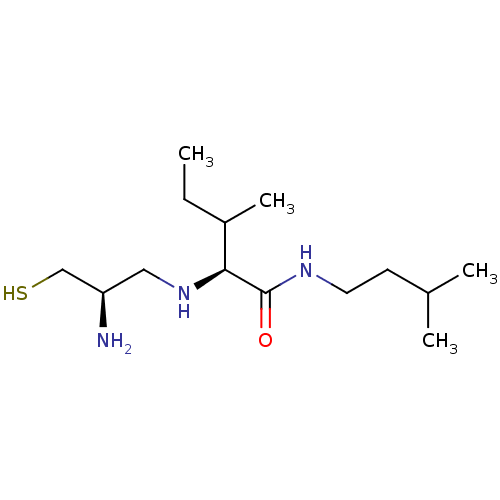
((S)-2-((R)-2-Amino-3-mercapto-propylamino)-3-methy...)Show InChI InChI=1S/C14H31N3OS/c1-5-11(4)13(17-8-12(15)9-19)14(18)16-7-6-10(2)3/h10-13,17,19H,5-9,15H2,1-4H3,(H,16,18)/t11?,12-,13+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of bovine Geranylgeranyl transferase type I |
J Med Chem 38: 3967-71 (1995)
BindingDB Entry DOI: 10.7270/Q2XS5TD4 |
More data for this
Ligand-Target Pair | |
UDP-N-acetylglucosamine 1-carboxyvinyltransferase
(Escherichia coli K-12 (Enterobacteria)) | BDBM50024894
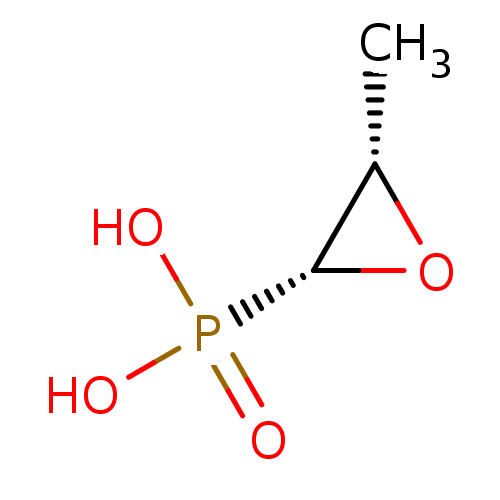
((1R, 2S)-1,2-epoxypropyl-phosphonic acid | (2R,3S)...)Show InChI InChI=1S/C3H7O4P/c1-2-3(7-2)8(4,5)6/h2-3H,1H3,(H2,4,5,6)/t2-,3+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Heidelberg University
Curated by ChEMBL
| Assay Description
Inhibition of Escherichia coli MurA expressed in Escherichia coli BL21(lamdaDE3) using UNAG and PEP as substrate incubated for 10 mins prior to PEP a... |
Bioorg Med Chem 21: 795-804 (2013)
Article DOI: 10.1016/j.bmc.2012.11.018
BindingDB Entry DOI: 10.7270/Q2HM59SD |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Mus musculus) | BDBM50065394

((S)-2-(2-{[(S)-2-((R)-2-Amino-3-mercapto-propylami...)Show SMILES CCC(C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(=O)OC)Cc1ccccc1)NC[C@@H](N)CS Show InChI InChI=1S/C24H42N4O3S2/c1-5-18(2)22(26-13-20(25)17-32)15-28(14-19-9-7-6-8-10-19)16-23(29)27-21(11-12-33-4)24(30)31-3/h6-10,18,20-22,26,32H,5,11-17,25H2,1-4H3,(H,27,29)/t18?,20-,21+,22-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of post-translational processing of v-Ras protein in NIH3T3 cells |
J Med Chem 41: 2651-6 (1998)
Article DOI: 10.1021/jm9800907
BindingDB Entry DOI: 10.7270/Q2WW7JCV |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50044317
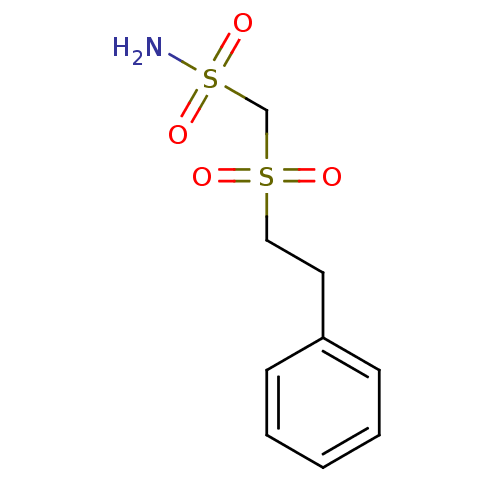
((2-Phenyl-ethanesulfonyl)-methanesulfonamide | CHE...)Show InChI InChI=1S/C9H13NO4S2/c10-16(13,14)8-15(11,12)7-6-9-4-2-1-3-5-9/h1-5H,6-8H2,(H2,10,13,14) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human erythrocyte carbonic anhydrase II |
J Med Chem 36: 2134-41 (1993)
BindingDB Entry DOI: 10.7270/Q2GX49N1 |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
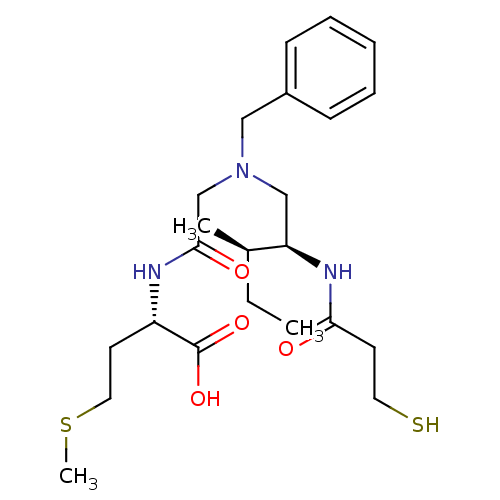
(Homo sapiens (Human)) | BDBM50369369
(CHEMBL1790759)Show SMILES CC[C@@H](C)[C@@H](CN(CC(=O)N[C@@H](CCSC)C(O)=O)Cc1ccccc1)NC(=O)CCS Show InChI InChI=1S/C23H37N3O4S2/c1-4-17(2)20(25-21(27)10-12-31)15-26(14-18-8-6-5-7-9-18)16-22(28)24-19(23(29)30)11-13-32-3/h5-9,17,19-20,31H,4,10-16H2,1-3H3,(H,24,28)(H,25,27)(H,29,30)/t17-,19+,20-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of [3H]-FPP incorporation into recombinant human Ha-Ras by Farnesyltransferase |
J Med Chem 41: 2651-6 (1998)
Article DOI: 10.1021/jm9800907
BindingDB Entry DOI: 10.7270/Q2WW7JCV |
More data for this
Ligand-Target Pair | |
Protein farnesyltransferase subunit beta/geranylgeranyltransferase type-1 subunit alpha
(Bos taurus (bovine)) | BDBM50031178

((S)-2-((R)-2-Amino-3-mercapto-propylamino)-3-methy...)Show SMILES CCC(C)[C@H](NC[C@@H](N)CS)C(=O)NCc1ccc(C)cc1C Show InChI InChI=1S/C18H31N3OS/c1-5-13(3)17(20-10-16(19)11-23)18(22)21-9-15-7-6-12(2)8-14(15)4/h6-8,13,16-17,20,23H,5,9-11,19H2,1-4H3,(H,21,22)/t13?,16-,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro concentration required to reduce Farnesyltransferase catalyzed incorporation of [3H]-FPP into recombinant Ha-Ras protein by 50% |
J Med Chem 38: 3967-71 (1995)
BindingDB Entry DOI: 10.7270/Q2XS5TD4 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50044320

((3-Phenyl-propane-1-sulfonyl)-methanesulfonamide |...)Show InChI InChI=1S/C10H15NO4S2/c11-17(14,15)9-16(12,13)8-4-7-10-5-2-1-3-6-10/h1-3,5-6H,4,7-9H2,(H2,11,14,15) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 190 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of purified human erythrocyte carbonic anhydrase II |
J Med Chem 36: 2134-41 (1993)
BindingDB Entry DOI: 10.7270/Q2GX49N1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data