

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065147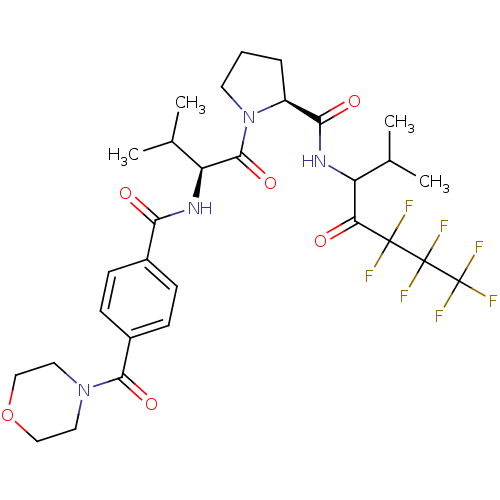 ((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50035495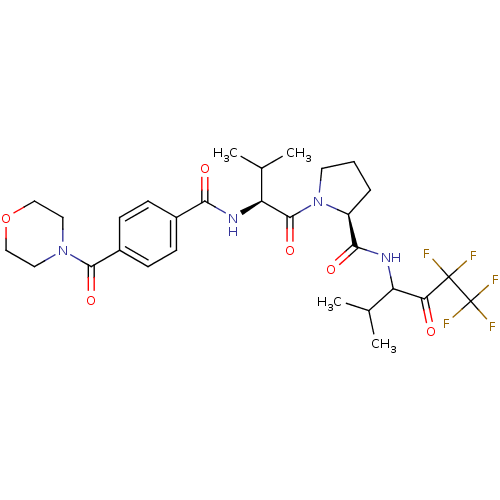 ((S)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065158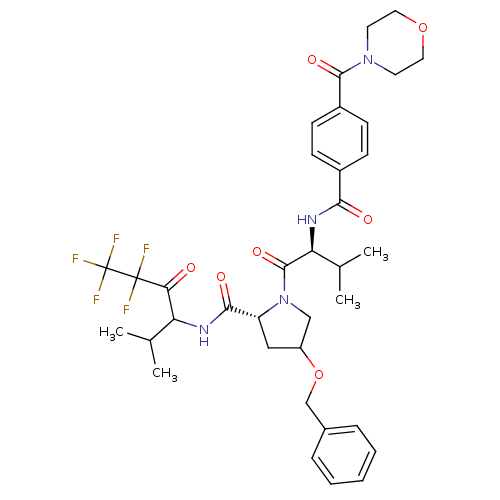 ((R)-4-Benzyloxy-1-{(S)-3-methyl-2-[4-(morpholine-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065157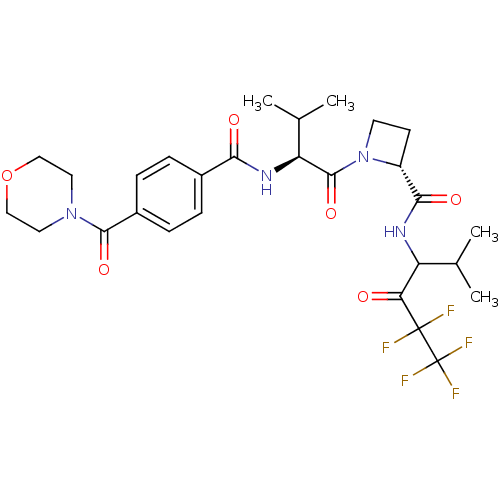 ((R)-1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 34 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065161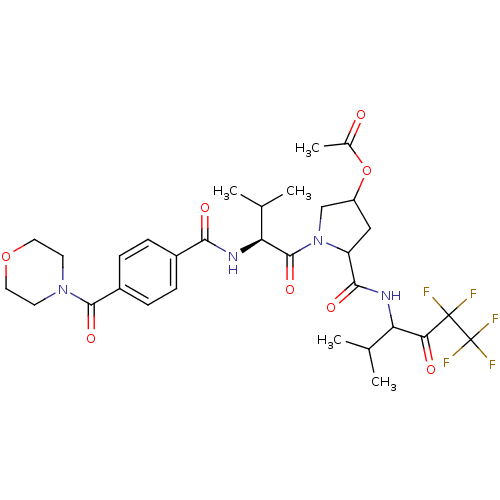 (Acetic acid 1-{(S)-3-methyl-2-[4-(morpholine-4-car...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065163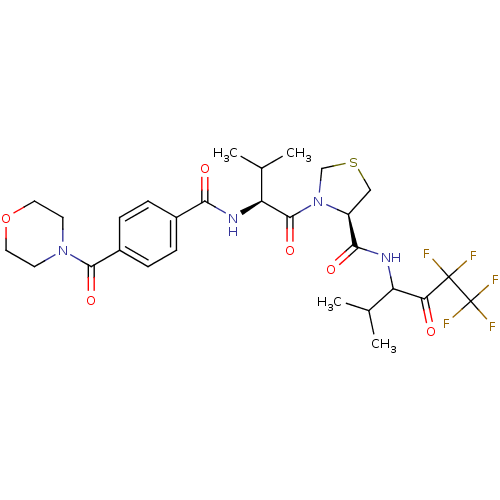 ((R)-3-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065155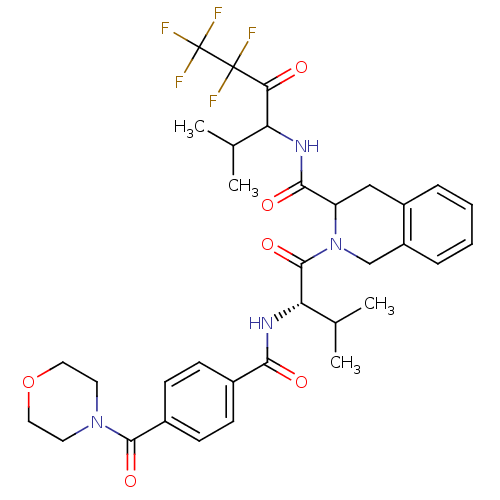 (2-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065154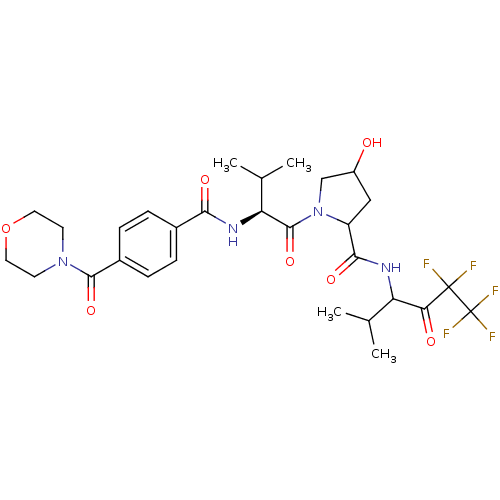 (4-Hydroxy-1-{(S)-3-methyl-2-[4-(morpholine-4-carbo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065146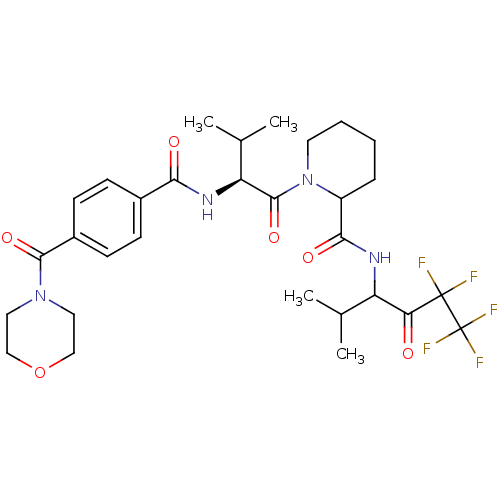 (1-{(S)-3-Methyl-2-[4-(morpholine-4-carbonyl)-benzo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065164 ((S)-1-{(R)-3,3-Dimethyl-2-[4-(morpholine-4-carbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065156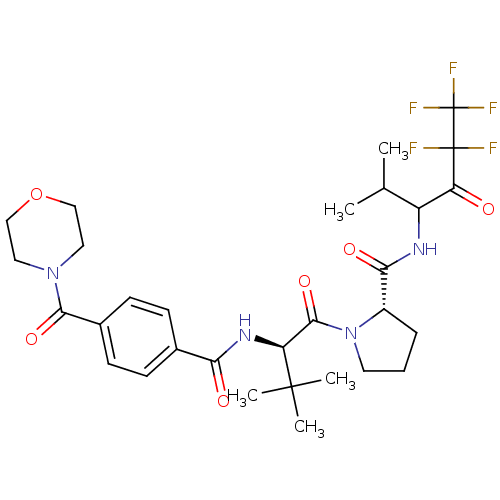 ((S)-1-{(R)-3,3-Dimethyl-2-[4-(morpholine-4-carbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 340 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065162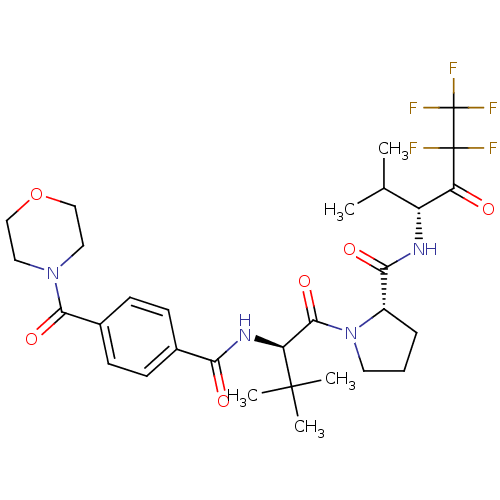 ((S)-1-{(R)-3,3-Dimethyl-2-[4-(morpholine-4-carbony...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065153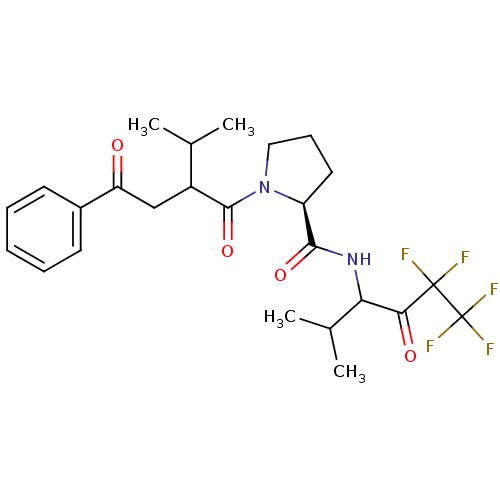 ((S)-1-[3-Methyl-2-(2-oxo-2-phenyl-ethyl)-butyryl]-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065151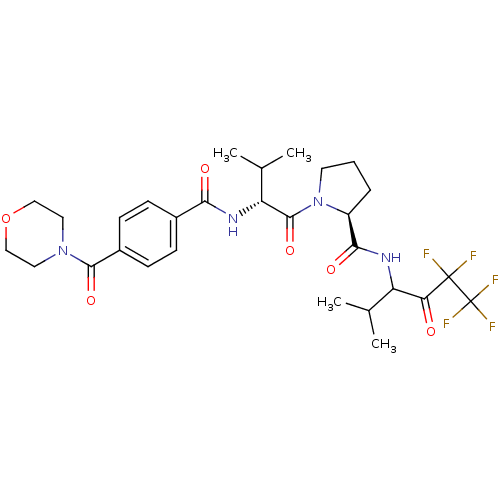 ((S)-1-{(R)-3-Methyl-2-[4-(morpholine-4-carbonyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065150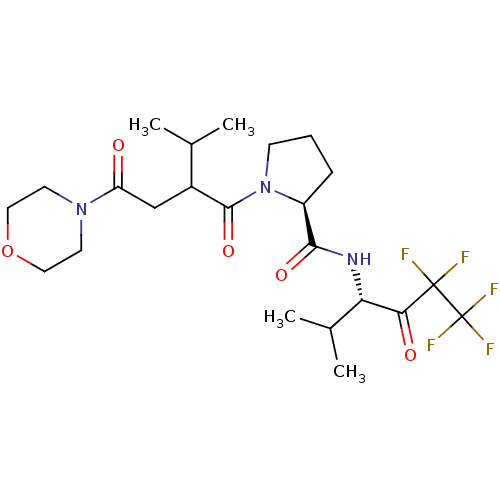 ((S)-1-[3-Methyl-2-(2-morpholin-4-yl-2-oxo-ethyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065148 (4-(Morpholine-4-carbonyl)-N-{(S)-2-oxo-1-[(S)-1-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065159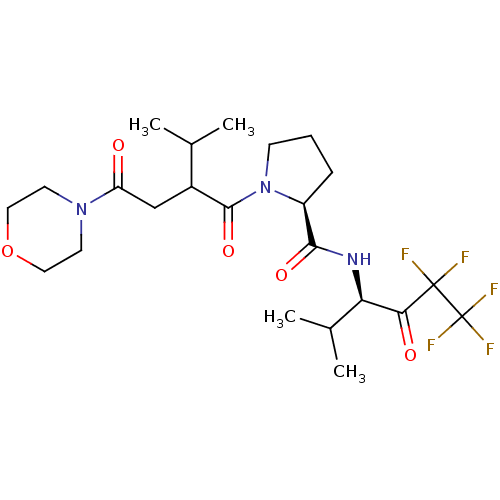 ((S)-1-[3-Methyl-2-(2-morpholin-4-yl-2-oxo-ethyl)-b...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065160 (4-(Morpholine-4-carbonyl)-N-{(R)-2-oxo-1-[(3,3,4,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065149 (4-(Morpholine-4-carbonyl)-N-{(R)-2-oxo-1-[(3,3,4,4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Neutrophil elastase (Homo sapiens (Human)) | BDBM50065152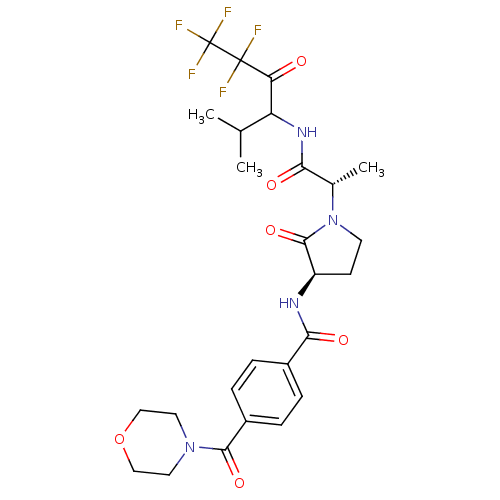 (4-(Morpholine-4-carbonyl)-N-{(R)-2-oxo-1-[(S)-1-(3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.10E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hoechst Marion Roussel Inc. Curated by ChEMBL | Assay Description Inhibition of human neutrophil elastase in hamster lungs following 25 mg/kg pre-treatment before instillation of elastase. | J Med Chem 41: 2461-80 (1998) Article DOI: 10.1021/jm970812e BindingDB Entry DOI: 10.7270/Q23X85S8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089758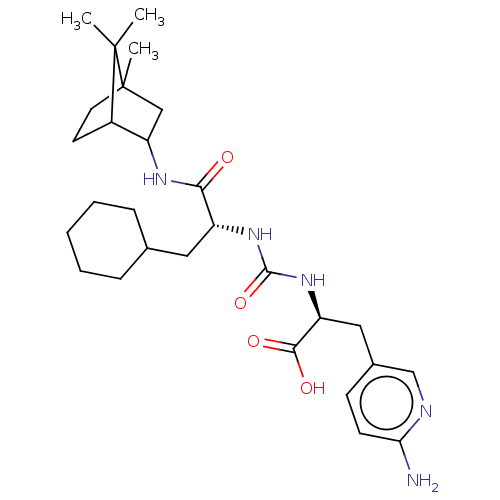 (CHEMBL3577442) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B (Homo sapiens (Human)) | BDBM50089691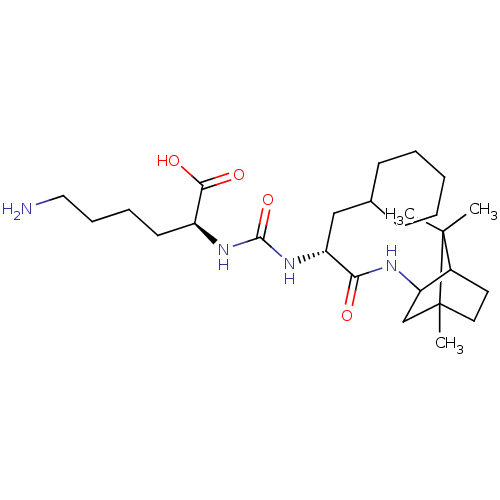 (CHEMBL3577425) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of CBP (unknown origin) | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089687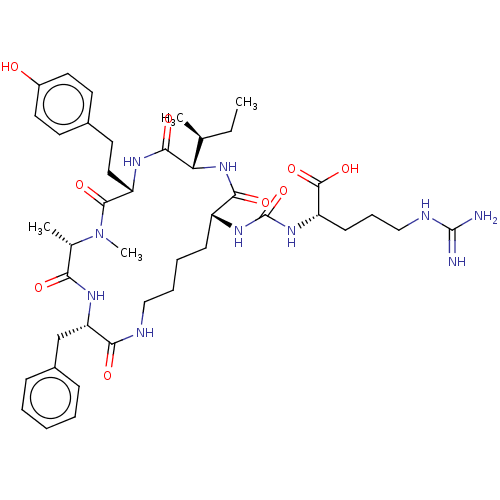 (Anabaenopeptin F) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089688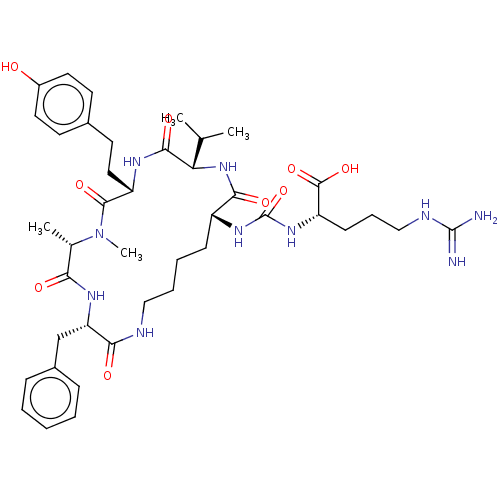 (ANABAENOPEPTIN B) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089686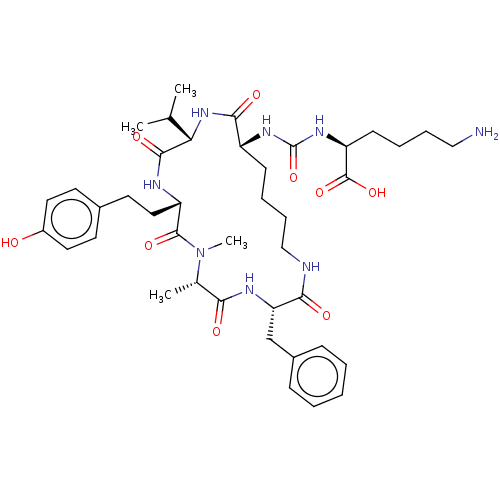 (CHEMBL3577334) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50197573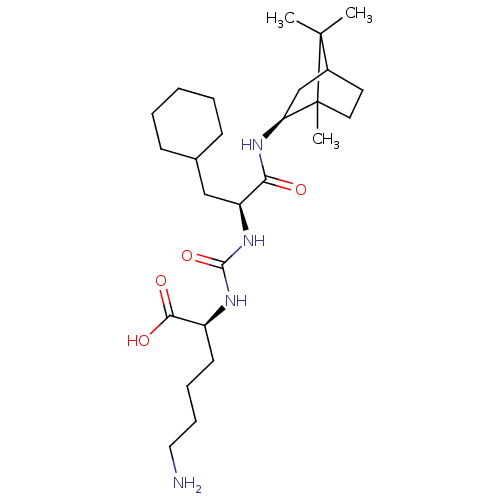 (CHEMBL3912764) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Inhibition of activated human plasma TAFI incubated for 15 mins by chromogenic assay | J Med Chem 59: 9567-9573 (2016) Article DOI: 10.1021/acs.jmedchem.6b01276 BindingDB Entry DOI: 10.7270/Q2MG7RG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089691 (CHEMBL3577425) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089756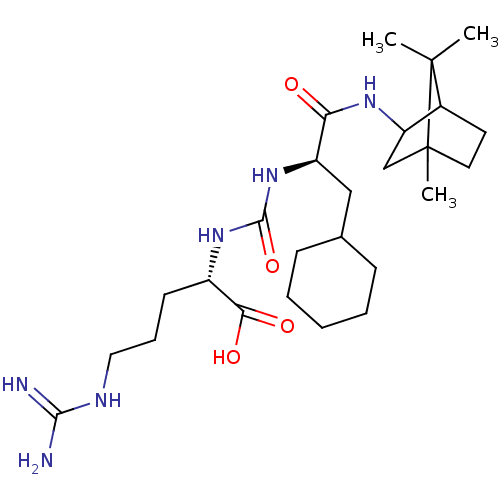 (CHEMBL3577440) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089751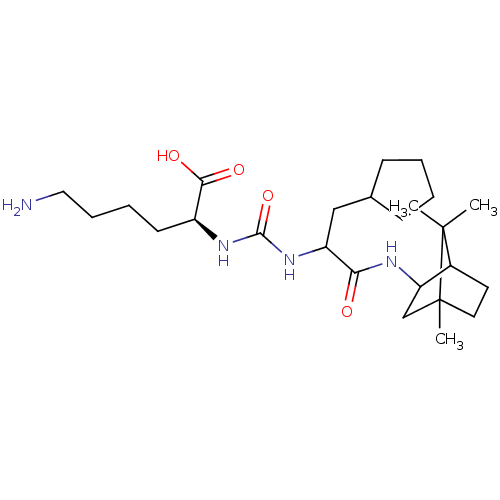 (CHEMBL3577435) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089755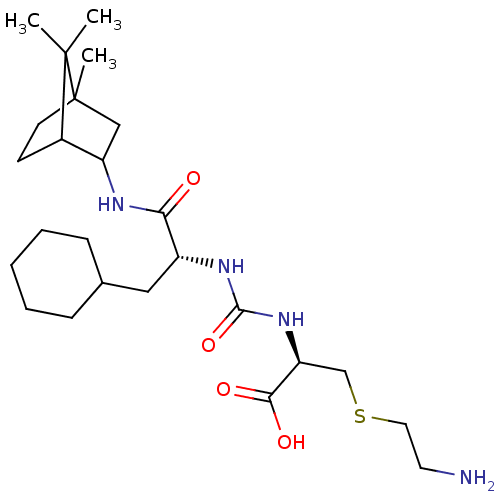 (CHEMBL3577439) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089691 (CHEMBL3577425) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins in presence of 1% human serum albumin by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089749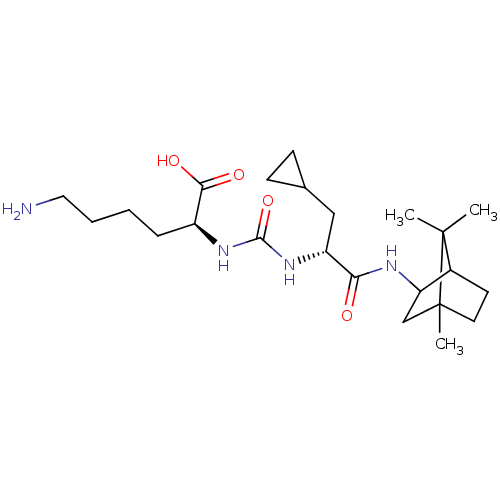 (CHEMBL3577433) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089750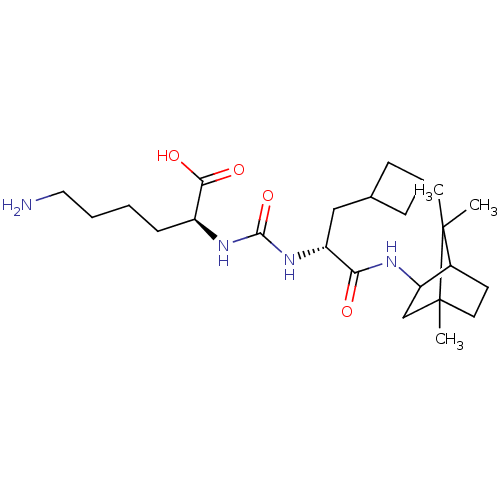 (CHEMBL3577434) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50197580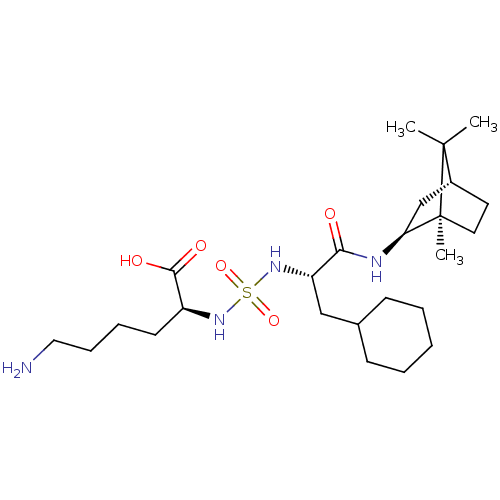 (CHEMBL3933831) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Inhibition of activated human plasma TAFI incubated for 15 mins by chromogenic assay | J Med Chem 59: 9567-9573 (2016) Article DOI: 10.1021/acs.jmedchem.6b01276 BindingDB Entry DOI: 10.7270/Q2MG7RG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089742 (CHEMBL3577426) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089748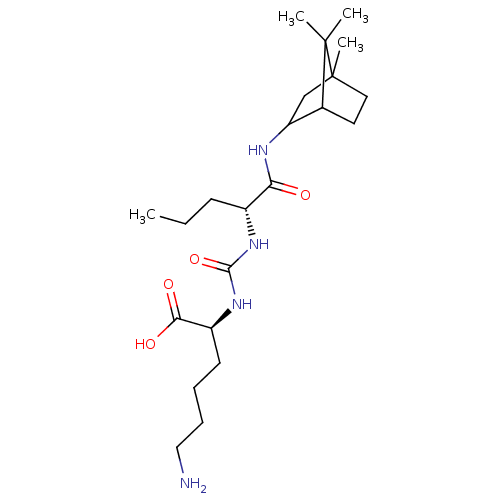 (CHEMBL3577432) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089752 (CHEMBL3577436) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089707 (CHEMBL3577336) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50197528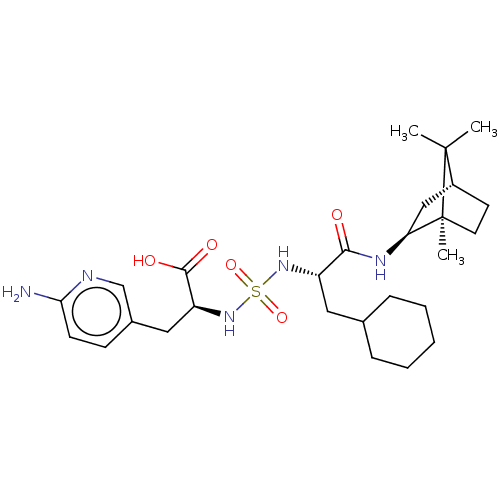 (CHEMBL3917216) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Inhibition of activated human plasma TAFI incubated for 15 mins in presence of 1% human serum albumin by chromogenic assay | J Med Chem 59: 9567-9573 (2016) Article DOI: 10.1021/acs.jmedchem.6b01276 BindingDB Entry DOI: 10.7270/Q2MG7RG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089702 (CHEMBL3577335) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50197541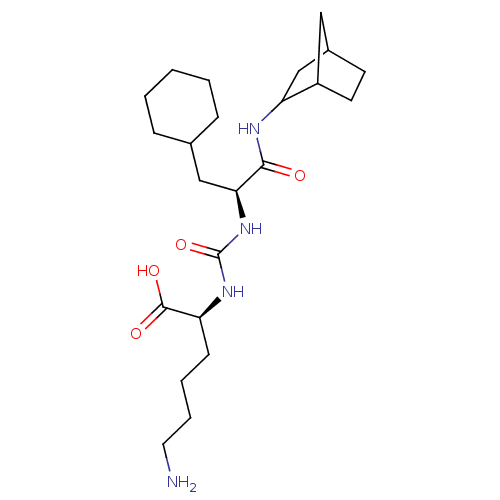 (CHEMBL3949673) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Inhibition of activated human plasma TAFI incubated for 15 mins in presence of 1% human serum albumin by chromogenic assay | J Med Chem 59: 9567-9573 (2016) Article DOI: 10.1021/acs.jmedchem.6b01276 BindingDB Entry DOI: 10.7270/Q2MG7RG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089754 (CHEMBL3577438) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 38 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089741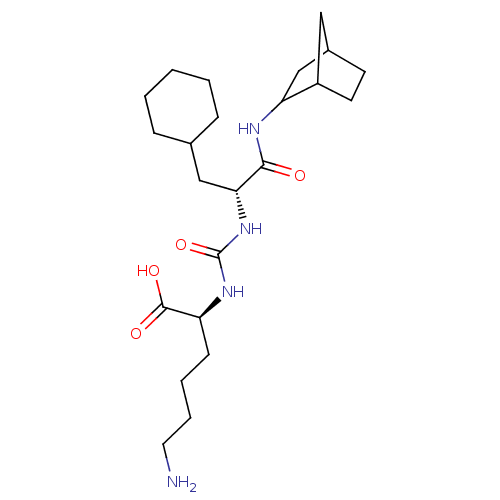 (CHEMBL3577424) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50197542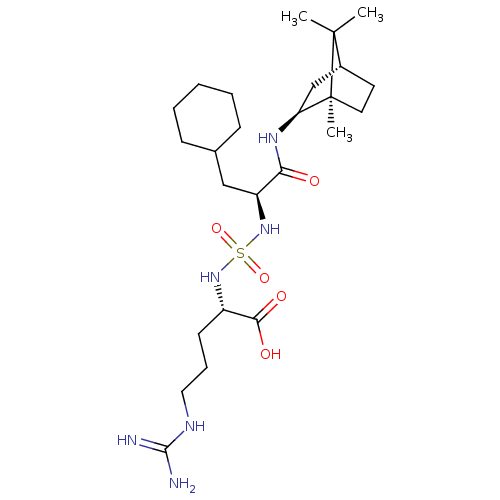 (CHEMBL3967145) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 49 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Inhibition of activated human plasma TAFI incubated for 15 mins in presence of 1% human serum albumin by chromogenic assay | J Med Chem 59: 9567-9573 (2016) Article DOI: 10.1021/acs.jmedchem.6b01276 BindingDB Entry DOI: 10.7270/Q2MG7RG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50197526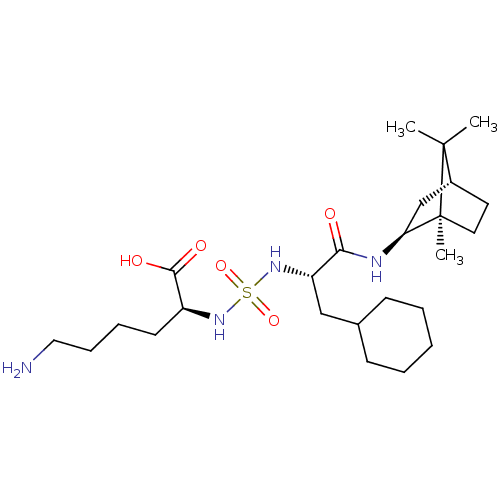 (CHEMBL3982647) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Inhibition of activated human plasma TAFI incubated for 15 mins in presence of 1% human serum albumin by chromogenic assay | J Med Chem 59: 9567-9573 (2016) Article DOI: 10.1021/acs.jmedchem.6b01276 BindingDB Entry DOI: 10.7270/Q2MG7RG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089743 (CHEMBL3577427) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50197549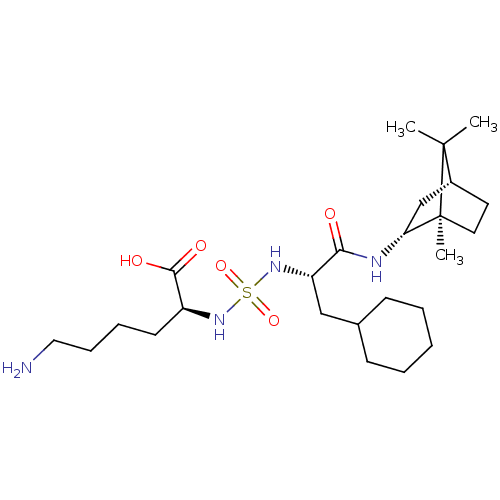 (CHEMBL3922450) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Inhibition of activated human plasma TAFI incubated for 15 mins by chromogenic assay | J Med Chem 59: 9567-9573 (2016) Article DOI: 10.1021/acs.jmedchem.6b01276 BindingDB Entry DOI: 10.7270/Q2MG7RG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50197565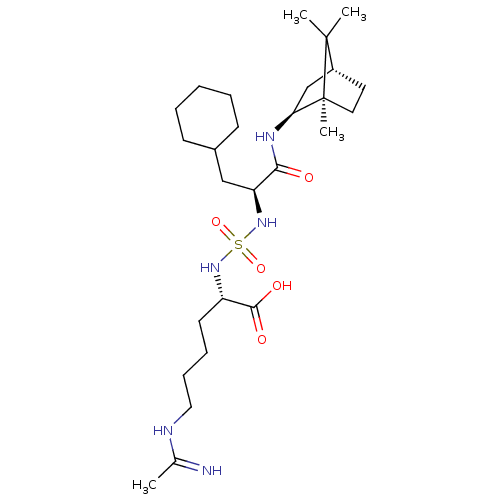 (CHEMBL3937445) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Inhibition of activated human plasma TAFI incubated for 15 mins by chromogenic assay | J Med Chem 59: 9567-9573 (2016) Article DOI: 10.1021/acs.jmedchem.6b01276 BindingDB Entry DOI: 10.7270/Q2MG7RG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50197576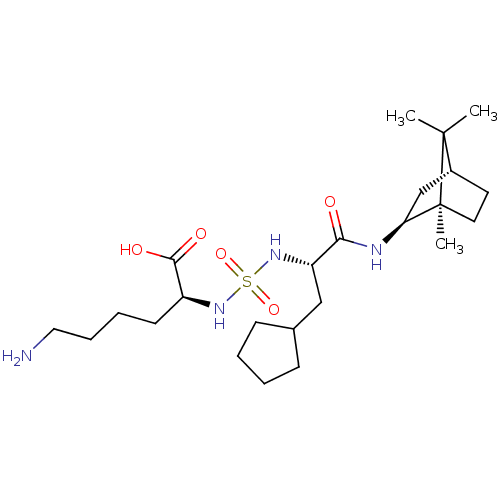 (CHEMBL3961311) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 81 | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi R&D Curated by ChEMBL | Assay Description Inhibition of activated human plasma TAFI incubated for 15 mins by chromogenic assay | J Med Chem 59: 9567-9573 (2016) Article DOI: 10.1021/acs.jmedchem.6b01276 BindingDB Entry DOI: 10.7270/Q2MG7RG2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carboxypeptidase B2 (Homo sapiens (Human)) | BDBM50089757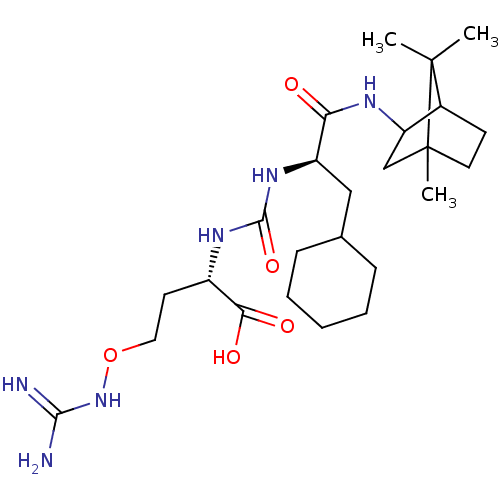 (CHEMBL3577441) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute for Infection Research Curated by ChEMBL | Assay Description Inhibition of human activated TAFI incubated for 15 mins by microtiter plate reader based assay | J Med Chem 58: 4839-44 (2015) Article DOI: 10.1021/jm501840b BindingDB Entry DOI: 10.7270/Q24B3314 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 142 total ) | Next | Last >> |