 Found 1692 hits with Last Name = 'seiffert' and Initial = 'd'
Found 1692 hits with Last Name = 'seiffert' and Initial = 'd' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50260646
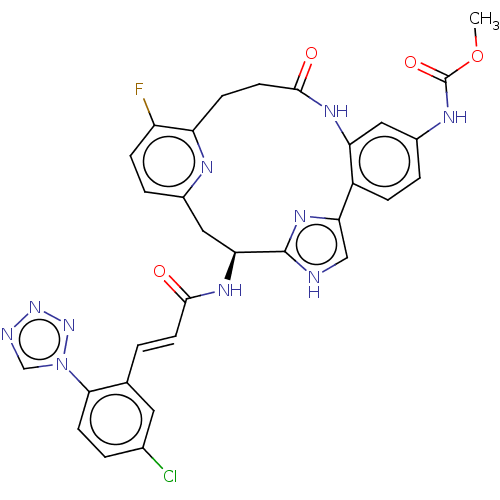
(CHEMBL4096251)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](Cc3ccc(F)c(CCC(=O)Nc2c1)n3)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r| Show InChI InChI=1S/C31H26ClFN10O4/c1-47-31(46)37-20-4-6-21-24(13-20)38-29(45)11-8-23-22(33)7-5-19(36-23)14-25(30-34-15-26(21)40-30)39-28(44)10-2-17-12-18(32)3-9-27(17)43-16-35-41-42-43/h2-7,9-10,12-13,15-16,25H,8,11,14H2,1H3,(H,34,40)(H,37,46)(H,38,45)(H,39,44)/b10-2+/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.0200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company, Research and Development, 350 Carter Road, Hopewell, NJ 08540 United States.
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using peptide substrate by spectrophotometry |
Bioorg Med Chem Lett 27: 4056-4060 (2017)
Article DOI: 10.1016/j.bmcl.2017.07.048
BindingDB Entry DOI: 10.7270/Q2TB19B3 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50230326
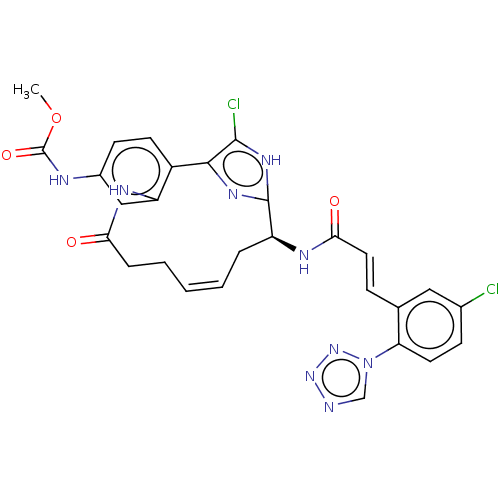
(CHEMBL4060950)Show SMILES COC(=O)Nc1ccc2-c3nc([nH]c3Cl)[C@H](C\C=C\CCC(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r,t:18| Show InChI InChI=1S/C28H25Cl2N9O4/c1-43-28(42)32-18-9-10-19-21(14-18)34-23(40)6-4-2-3-5-20(27-35-25(19)26(30)36-27)33-24(41)12-7-16-13-17(29)8-11-22(16)39-15-31-37-38-39/h2-3,7-15,20H,4-6H2,1H3,(H,32,42)(H,33,41)(H,34,40)(H,35,36)/b3-2+,12-7+/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method |
J Med Chem 60: 1060-1075 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01460
BindingDB Entry DOI: 10.7270/Q25D8V31 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50230326

(CHEMBL4060950)Show SMILES COC(=O)Nc1ccc2-c3nc([nH]c3Cl)[C@H](C\C=C\CCC(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r,t:18| Show InChI InChI=1S/C28H25Cl2N9O4/c1-43-28(42)32-18-9-10-19-21(14-18)34-23(40)6-4-2-3-5-20(27-35-25(19)26(30)36-27)33-24(41)12-7-16-13-17(29)8-11-22(16)39-15-31-37-38-39/h2-3,7-15,20H,4-6H2,1H3,(H,32,42)(H,33,41)(H,34,40)(H,35,36)/b3-2+,12-7+/t20-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 27: 3833-3839 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.058
BindingDB Entry DOI: 10.7270/Q2GT5QPS |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50096792
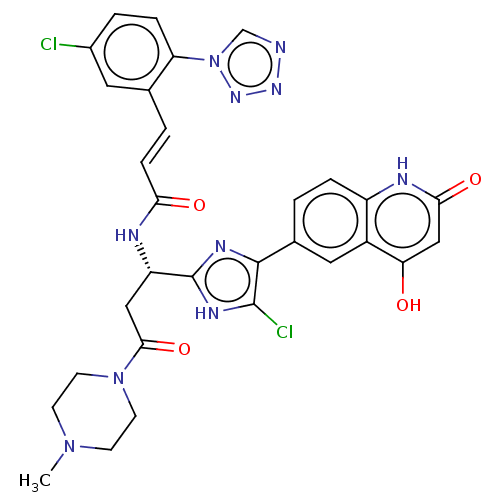
(CHEMBL3580759)Show SMILES CN1CCN(CC1)C(=O)C[C@H](NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1)c1nc(c(Cl)[nH]1)-c1ccc2[nH]c(=O)cc(O)c2c1 |r| Show InChI InChI=1S/C30H26Cl2N10O4/c1-40-8-10-41(11-9-40)27(46)14-22(35-25(44)7-3-17-12-19(31)4-6-23(17)42-16-33-38-39-42)30-36-28(29(32)37-30)18-2-5-21-20(13-18)24(43)15-26(45)34-21/h2-7,12-13,15-16,22,43H,8-11,14H2,1H3,(H,35,44)/b7-3+,28-18-/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometry |
ACS Med Chem Lett 6: 590-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00066
BindingDB Entry DOI: 10.7270/Q2B27X24 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50192770
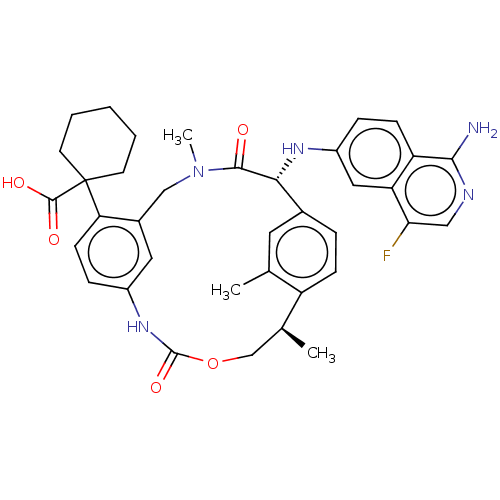
(CHEMBL3956096)Show SMILES C[C@H]1COC(=O)Nc2ccc(c(CN(C)C(=O)[C@H](Nc3ccc4c(N)ncc(F)c4c3)c3ccc1c(C)c3)c2)C1(CCCCC1)C(O)=O |r| Show InChI InChI=1S/C37H40FN5O5/c1-21-15-23-7-10-27(21)22(2)20-48-36(47)42-25-9-12-30(37(35(45)46)13-5-4-6-14-37)24(16-25)19-43(3)34(44)32(23)41-26-8-11-28-29(17-26)31(38)18-40-33(28)39/h7-12,15-18,22,32,41H,4-6,13-14,19-20H2,1-3H3,(H2,39,40)(H,42,47)(H,45,46)/t22-,32+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
| PDB
Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TF-factor 7a using factor 10 as substrate at 37 degC |
Bioorg Med Chem Lett 26: 5051-5057 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.088
BindingDB Entry DOI: 10.7270/Q24X59Q6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50269207
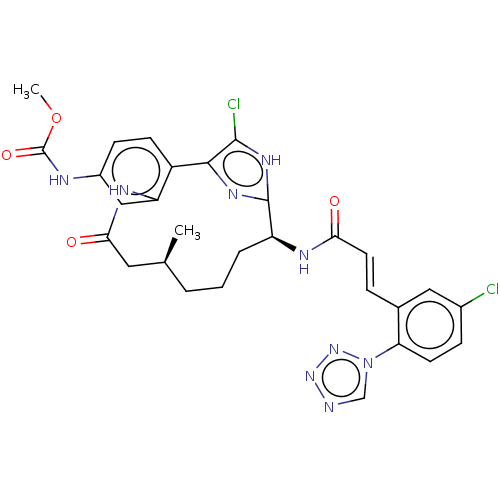
(CHEMBL4097522)Show SMILES COC(=O)Nc1ccc2-c3nc([nH]c3Cl)[C@H](CCC[C@H](C)CC(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r| Show InChI InChI=1S/C29H29Cl2N9O4/c1-16-4-3-5-21(34-24(41)11-6-17-13-18(30)7-10-23(17)40-15-32-38-39-40)28-36-26(27(31)37-28)20-9-8-19(33-29(43)44-2)14-22(20)35-25(42)12-16/h6-11,13-16,21H,3-5,12H2,1-2H3,(H,33,43)(H,34,41)(H,35,42)(H,36,37)/b11-6+/t16-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 27: 3833-3839 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.058
BindingDB Entry DOI: 10.7270/Q2GT5QPS |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50269199
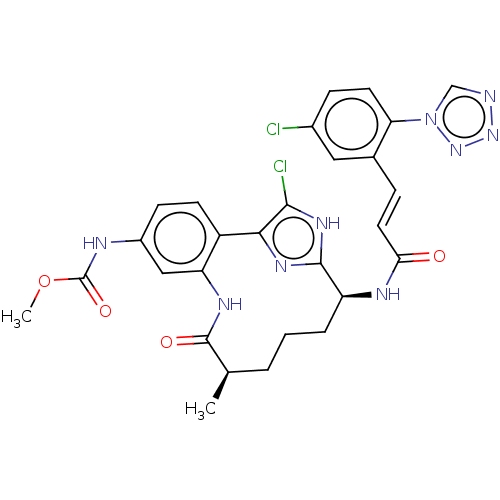
(CHEMBL4083769)Show SMILES COC(=O)Nc1ccc2-c3nc([nH]c3Cl)[C@H](CCC[C@@H](C)C(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r| Show InChI InChI=1S/C28H27Cl2N9O4/c1-15-4-3-5-20(33-23(40)11-6-16-12-17(29)7-10-22(16)39-14-31-37-38-39)26-35-24(25(30)36-26)19-9-8-18(32-28(42)43-2)13-21(19)34-27(15)41/h6-15,20H,3-5H2,1-2H3,(H,32,42)(H,33,40)(H,34,41)(H,35,36)/b11-6+/t15-,20+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 27: 3833-3839 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.058
BindingDB Entry DOI: 10.7270/Q2GT5QPS |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50250493
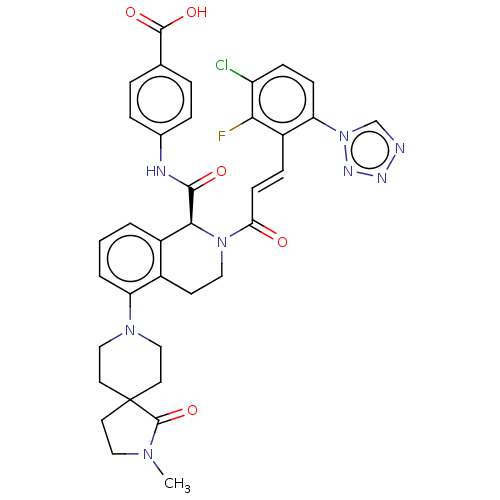
(CHEMBL4068445)Show SMILES CN1CCC2(CCN(CC2)c2cccc3[C@H](N(CCc23)C(=O)\C=C\c2c(F)c(Cl)ccc2-n2cnnn2)C(=O)Nc2ccc(cc2)C(O)=O)C1=O |r| Show InChI InChI=1S/C36H34ClFN8O5/c1-43-18-14-36(35(43)51)15-19-44(20-16-36)28-4-2-3-25-24(28)13-17-45(32(25)33(48)40-23-7-5-22(6-8-23)34(49)50)30(47)12-9-26-29(46-21-39-41-42-46)11-10-27(37)31(26)38/h2-12,21,32H,13-20H2,1H3,(H,40,48)(H,49,50)/b12-9+/t32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method |
J Med Chem 60: 9703-9723 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01171
BindingDB Entry DOI: 10.7270/Q2K64MHN |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50250492
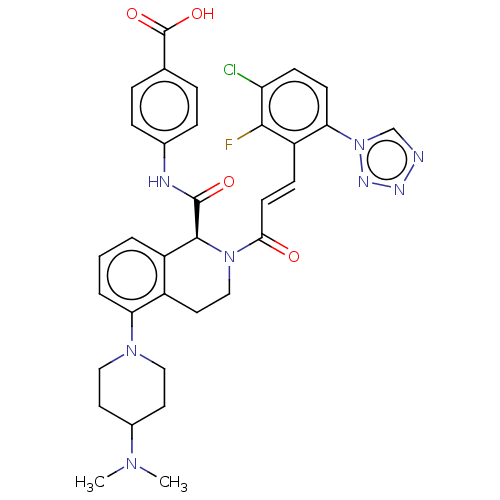
(CHEMBL4097304)Show SMILES CN(C)C1CCN(CC1)c1cccc2[C@H](N(CCc12)C(=O)\C=C\c1c(F)c(Cl)ccc1-n1cnnn1)C(=O)Nc1ccc(cc1)C(O)=O |r| Show InChI InChI=1S/C34H34ClFN8O4/c1-41(2)23-14-17-42(18-15-23)28-5-3-4-25-24(28)16-19-43(32(25)33(46)38-22-8-6-21(7-9-22)34(47)48)30(45)13-10-26-29(44-20-37-39-40-44)12-11-27(35)31(26)36/h3-13,20,23,32H,14-19H2,1-2H3,(H,38,46)(H,47,48)/b13-10+/t32-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0980 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method |
J Med Chem 60: 9703-9723 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01171
BindingDB Entry DOI: 10.7270/Q2K64MHN |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50096792

(CHEMBL3580759)Show SMILES CN1CCN(CC1)C(=O)C[C@H](NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1)c1nc(c(Cl)[nH]1)-c1ccc2[nH]c(=O)cc(O)c2c1 |r| Show InChI InChI=1S/C30H26Cl2N10O4/c1-40-8-10-41(11-9-40)27(46)14-22(35-25(44)7-3-17-12-19(31)4-6-23(17)42-16-33-38-39-42)30-36-28(29(32)37-30)18-2-5-21-20(13-18)24(43)15-26(45)34-21/h2-7,12-13,15-16,22,43H,8-11,14H2,1H3,(H,35,44)/b7-3+,28-18-/t22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis at 37 degC by spectrophotometry |
ACS Med Chem Lett 6: 590-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00066
BindingDB Entry DOI: 10.7270/Q2B27X24 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50269209
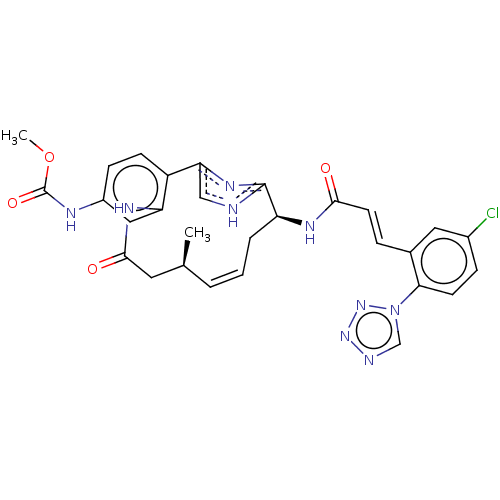
(CHEMBL4063677)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](C\C=C\[C@H](C)CC(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r,t:17| Show InChI InChI=1S/C29H28ClN9O4/c1-17-4-3-5-22(34-26(40)11-6-18-13-19(30)7-10-25(18)39-16-32-37-38-39)28-31-15-24(36-28)21-9-8-20(33-29(42)43-2)14-23(21)35-27(41)12-17/h3-4,6-11,13-17,22H,5,12H2,1-2H3,(H,31,36)(H,33,42)(H,34,40)(H,35,41)/b4-3+,11-6+/t17-,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 27: 3833-3839 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.058
BindingDB Entry DOI: 10.7270/Q2GT5QPS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50269190
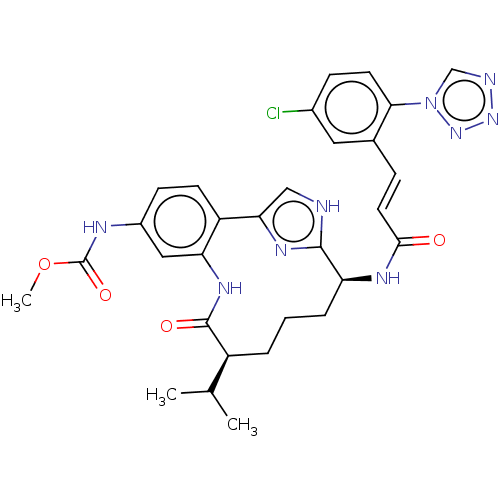
(CHEMBL4103982)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](CCC[C@@H](C(C)C)C(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r| Show InChI InChI=1S/C30H32ClN9O4/c1-17(2)21-5-4-6-23(35-27(41)12-7-18-13-19(31)8-11-26(18)40-16-33-38-39-40)28-32-15-25(36-28)22-10-9-20(34-30(43)44-3)14-24(22)37-29(21)42/h7-17,21,23H,4-6H2,1-3H3,(H,32,36)(H,34,43)(H,35,41)(H,37,42)/b12-7+/t21-,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 27: 3833-3839 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.058
BindingDB Entry DOI: 10.7270/Q2GT5QPS |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50192768
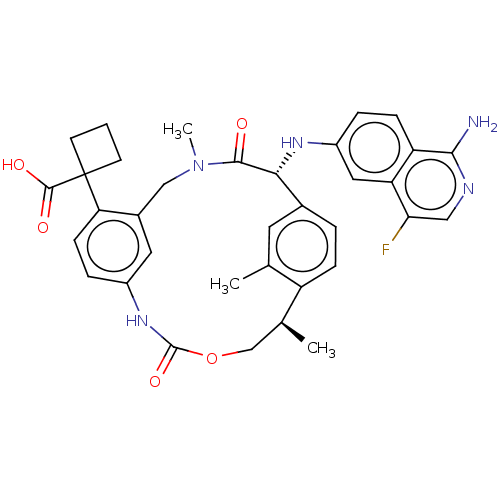
(CHEMBL3900166)Show SMILES C[C@H]1COC(=O)Nc2ccc(c(CN(C)C(=O)[C@H](Nc3ccc4c(N)ncc(F)c4c3)c3ccc1c(C)c3)c2)C1(CCC1)C(O)=O |r| Show InChI InChI=1S/C35H36FN5O5/c1-19-13-21-5-8-25(19)20(2)18-46-34(45)40-23-7-10-28(35(33(43)44)11-4-12-35)22(14-23)17-41(3)32(42)30(21)39-24-6-9-26-27(15-24)29(36)16-38-31(26)37/h5-10,13-16,20,30,39H,4,11-12,17-18H2,1-3H3,(H2,37,38)(H,40,45)(H,43,44)/t20-,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 0.140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TF-factor 7a using factor 10 as substrate at 37 degC |
Bioorg Med Chem Lett 26: 5051-5057 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.088
BindingDB Entry DOI: 10.7270/Q24X59Q6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50230322
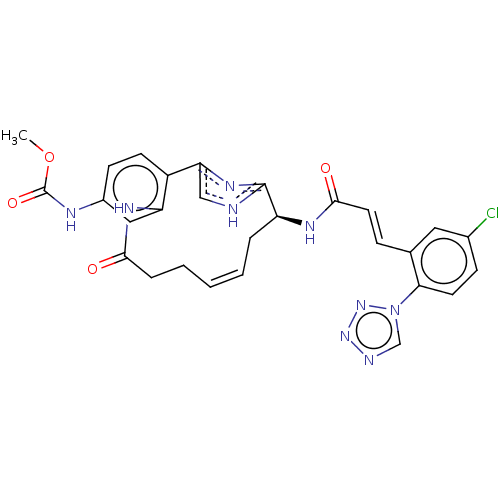
(CHEMBL4071545)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](C\C=C\CCC(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r,t:17| Show InChI InChI=1S/C28H26ClN9O4/c1-42-28(41)32-19-9-10-20-22(14-19)34-25(39)6-4-2-3-5-21(27-30-15-23(20)35-27)33-26(40)12-7-17-13-18(29)8-11-24(17)38-16-31-36-37-38/h2-3,7-16,21H,4-6H2,1H3,(H,30,35)(H,32,41)(H,33,40)(H,34,39)/b3-2+,12-7+/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 27: 3833-3839 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.058
BindingDB Entry DOI: 10.7270/Q2GT5QPS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50230322

(CHEMBL4071545)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](C\C=C\CCC(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r,t:17| Show InChI InChI=1S/C28H26ClN9O4/c1-42-28(41)32-19-9-10-20-22(14-19)34-25(39)6-4-2-3-5-21(27-30-15-23(20)35-27)33-26(40)12-7-17-13-18(29)8-11-24(17)38-16-31-36-37-38/h2-3,7-16,21H,4-6H2,1H3,(H,30,35)(H,32,41)(H,33,40)(H,34,39)/b3-2+,12-7+/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method |
J Med Chem 60: 1060-1075 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01460
BindingDB Entry DOI: 10.7270/Q25D8V31 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM189445
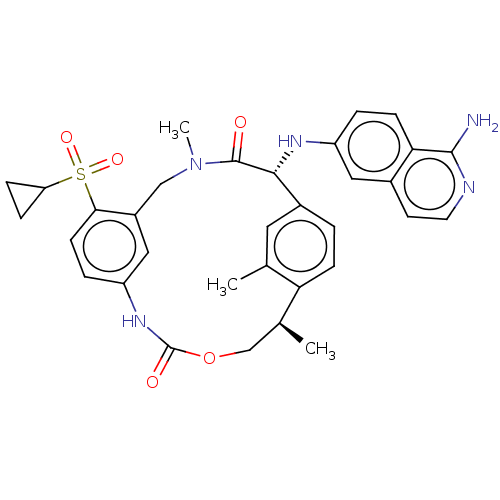
(US9174974, Example 31)Show SMILES C[C@H]1COC(=O)Nc2ccc(c(CN(C)C(=O)[C@H](Nc3ccc4c(N)nccc4c3)c3ccc1c(C)c3)c2)S(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C33H35N5O5S/c1-19-14-22-4-9-27(19)20(2)18-43-33(40)37-25-6-11-29(44(41,42)26-7-8-26)23(16-25)17-38(3)32(39)30(22)36-24-5-10-28-21(15-24)12-13-35-31(28)34/h4-6,9-16,20,26,30,36H,7-8,17-18H2,1-3H3,(H2,34,35)(H,37,40)/t20-,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of human TF-factor 7a (366 to 11 residues) using factor 10 as substrate after 60 mins |
Bioorg Med Chem Lett 26: 5051-5057 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.088
BindingDB Entry DOI: 10.7270/Q24X59Q6 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM189445

(US9174974, Example 31)Show SMILES C[C@H]1COC(=O)Nc2ccc(c(CN(C)C(=O)[C@H](Nc3ccc4c(N)nccc4c3)c3ccc1c(C)c3)c2)S(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C33H35N5O5S/c1-19-14-22-4-9-27(19)20(2)18-43-33(40)37-25-6-11-29(44(41,42)26-7-8-26)23(16-25)17-38(3)32(39)30(22)36-24-5-10-28-21(15-24)12-13-35-31(28)34/h4-6,9-16,20,26,30,36H,7-8,17-18H2,1-3H3,(H2,34,35)(H,37,40)/t20-,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... |
J Med Chem 59: 7125-37 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00469
BindingDB Entry DOI: 10.7270/Q2TM7D37 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50230322

(CHEMBL4071545)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](C\C=C\CCC(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r,t:17| Show InChI InChI=1S/C28H26ClN9O4/c1-42-28(41)32-19-9-10-20-22(14-19)34-25(39)6-4-2-3-5-21(27-30-15-23(20)35-27)33-26(40)12-7-17-13-18(29)8-11-24(17)38-16-31-36-37-38/h2-3,7-16,21H,4-6H2,1H3,(H,30,35)(H,32,41)(H,33,40)(H,34,39)/b3-2+,12-7+/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.08.008
BindingDB Entry DOI: 10.7270/Q23F4T2C |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50230322

(CHEMBL4071545)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](C\C=C\CCC(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r,t:17| Show InChI InChI=1S/C28H26ClN9O4/c1-42-28(41)32-19-9-10-20-22(14-19)34-25(39)6-4-2-3-5-21(27-30-15-23(20)35-27)33-26(40)12-7-17-13-18(29)8-11-24(17)38-16-31-36-37-38/h2-3,7-16,21H,4-6H2,1H3,(H,30,35)(H,32,41)(H,33,40)(H,34,39)/b3-2+,12-7+/t21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126949
BindingDB Entry DOI: 10.7270/Q24Q7ZJH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50269186
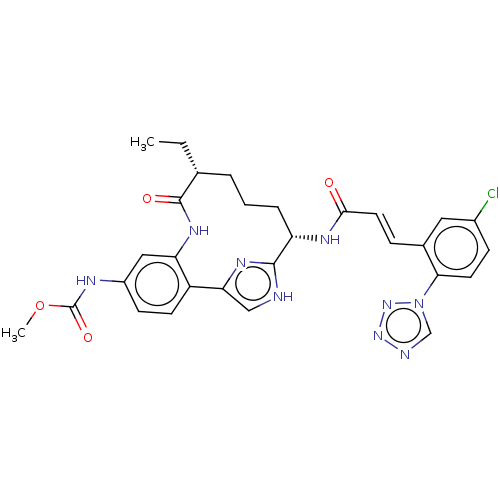
(CHEMBL4089185)Show SMILES CC[C@@H]1CCC[C@H](NC(=O)\C=C\c2cc(Cl)ccc2-n2cnnn2)c2nc(c[nH]2)-c2ccc(NC(=O)OC)cc2NC1=O |r| Show InChI InChI=1S/C29H30ClN9O4/c1-3-17-5-4-6-22(34-26(40)12-7-18-13-19(30)8-11-25(18)39-16-32-37-38-39)27-31-15-24(35-27)21-10-9-20(33-29(42)43-2)14-23(21)36-28(17)41/h7-17,22H,3-6H2,1-2H3,(H,31,35)(H,33,42)(H,34,40)(H,36,41)/b12-7+/t17-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50096843
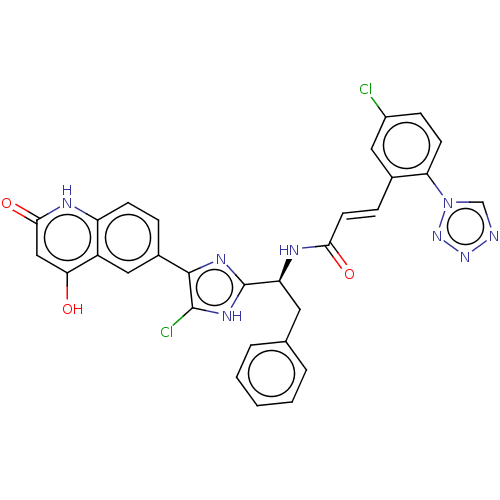
(CHEMBL3580754)Show SMILES Oc1cc(=O)[nH]c2ccc(cc12)-c1nc([nH]c1Cl)[C@H](Cc1ccccc1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r| Show InChI InChI=1S/C30H20Cl2N8O3/c31-20-8-10-24(40-16-33-38-39-40)18(13-20)7-11-26(42)35-23(12-17-4-2-1-3-5-17)30-36-28(29(32)37-30)19-6-9-22-21(14-19)25(41)15-27(43)34-22/h1-11,13-16,23,41H,12H2,(H,35,42)/b11-7+,28-19-/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometry |
ACS Med Chem Lett 6: 590-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00066
BindingDB Entry DOI: 10.7270/Q2B27X24 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50269186

(CHEMBL4089185)Show SMILES CC[C@@H]1CCC[C@H](NC(=O)\C=C\c2cc(Cl)ccc2-n2cnnn2)c2nc(c[nH]2)-c2ccc(NC(=O)OC)cc2NC1=O |r| Show InChI InChI=1S/C29H30ClN9O4/c1-3-17-5-4-6-22(34-26(40)12-7-18-13-19(30)8-11-25(18)39-16-32-37-38-39)27-31-15-24(35-27)21-10-9-20(33-29(42)43-2)14-23(21)36-28(17)41/h7-17,22H,3-6H2,1-2H3,(H,31,35)(H,33,42)(H,34,40)(H,36,41)/b12-7+/t17-,22+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 27: 3833-3839 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.058
BindingDB Entry DOI: 10.7270/Q2GT5QPS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50514438
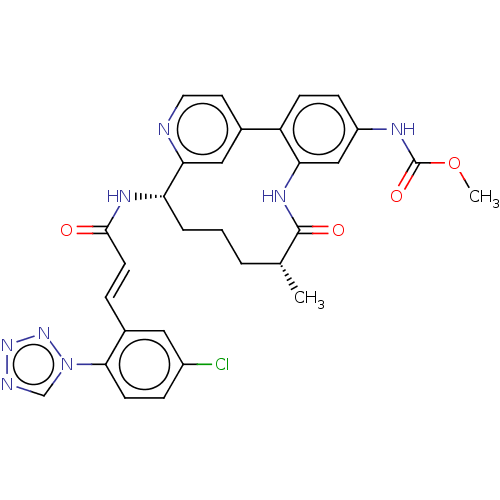
(CHEMBL4439729)Show SMILES OC(=O)C(F)(F)F.COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](NC(=O)\C=C\c3cc(Cl)ccc3-n3cnnn3)c3cc-2ccn3)c1 |r| Show InChI InChI=1S/C30H29ClN8O4.C2HF3O2/c1-18-4-3-5-24(35-28(40)11-6-20-14-21(31)7-10-27(20)39-17-33-37-38-39)26-15-19(12-13-32-26)23-9-8-22(34-30(42)43-2)16-25(23)36-29(18)41;3-2(4,5)1(6)7/h6-18,24H,3-5H2,1-2H3,(H,34,42)(H,35,40)(H,36,41);(H,6,7)/b11-6+;/t18-,24+;/m1./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.180 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50448581
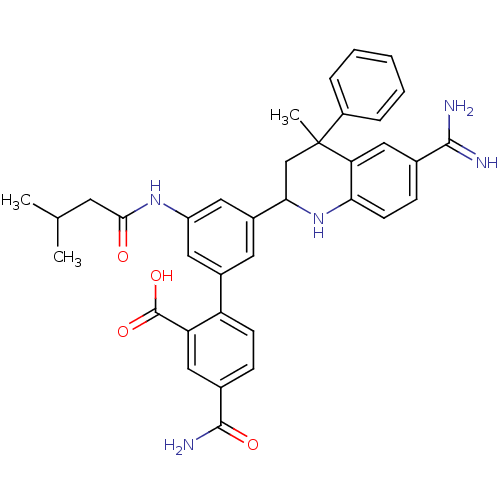
(CHEMBL3127463)Show SMILES CC(C)CC(=O)Nc1cc(cc(c1)-c1ccc(cc1C(O)=O)C(N)=O)C1CC(C)(c2ccccc2)c2cc(ccc2N1)C(N)=N Show InChI InChI=1S/C36H37N5O4/c1-20(2)13-32(42)40-26-15-23(27-11-9-22(34(39)43)17-28(27)35(44)45)14-24(16-26)31-19-36(3,25-7-5-4-6-8-25)29-18-21(33(37)38)10-12-30(29)41-31/h4-12,14-18,20,31,41H,13,19H2,1-3H3,(H3,37,38)(H2,39,43)(H,40,42)(H,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human factor 11a assessed as release of p-nitroaniline after 10 to 120 mins by spectrophotometric analysis |
J Med Chem 57: 955-69 (2014)
Article DOI: 10.1021/jm401670x
BindingDB Entry DOI: 10.7270/Q2G44RTQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50448583
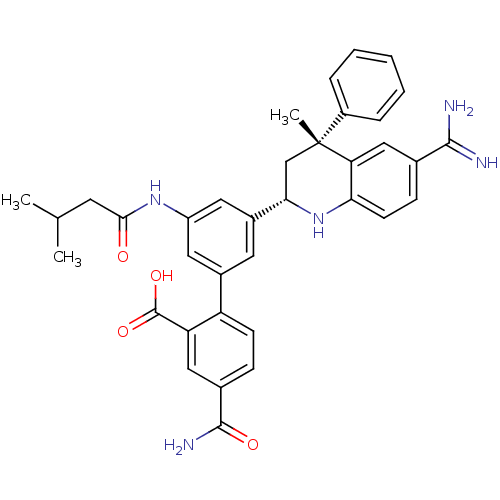
(CHEMBL3127491)Show SMILES CC(C)CC(=O)Nc1cc(cc(c1)-c1ccc(cc1C(O)=O)C(N)=O)[C@@H]1C[C@](C)(c2ccccc2)c2cc(ccc2N1)C(N)=N |r| Show InChI InChI=1S/C36H37N5O4/c1-20(2)13-32(42)40-26-15-23(27-11-9-22(34(39)43)17-28(27)35(44)45)14-24(16-26)31-19-36(3,25-7-5-4-6-8-25)29-18-21(33(37)38)10-12-30(29)41-31/h4-12,14-18,20,31,41H,13,19H2,1-3H3,(H3,37,38)(H2,39,43)(H,40,42)(H,44,45)/t31-,36+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50448581

(CHEMBL3127463)Show SMILES CC(C)CC(=O)Nc1cc(cc(c1)-c1ccc(cc1C(O)=O)C(N)=O)C1CC(C)(c2ccccc2)c2cc(ccc2N1)C(N)=N Show InChI InChI=1S/C36H37N5O4/c1-20(2)13-32(42)40-26-15-23(27-11-9-22(34(39)43)17-28(27)35(44)45)14-24(16-26)31-19-36(3,25-7-5-4-6-8-25)29-18-21(33(37)38)10-12-30(29)41-31/h4-12,14-18,20,31,41H,13,19H2,1-3H3,(H3,37,38)(H2,39,43)(H,40,42)(H,44,45) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human factor 11a assessed as release of p-nitroaniline after 10 to 120 mins by spectrophotometric analysis |
J Med Chem 57: 955-69 (2014)
Article DOI: 10.1021/jm401670x
BindingDB Entry DOI: 10.7270/Q2G44RTQ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50514437
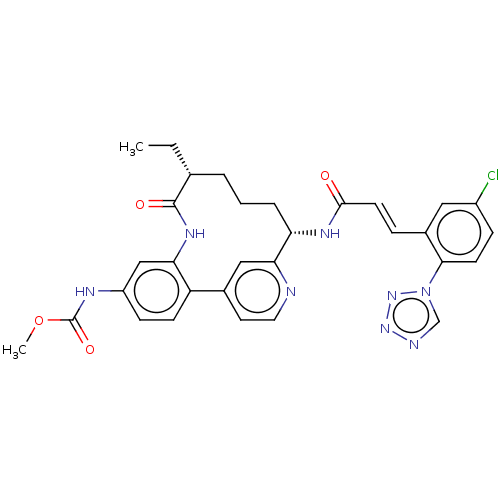
(CHEMBL4444690)Show SMILES CC[C@@H]1CCC[C@H](NC(=O)\C=C\c2cc(Cl)ccc2-n2cnnn2)c2cc(ccn2)-c2ccc(NC(=O)OC)cc2NC1=O |r| Show InChI InChI=1S/C31H31ClN8O4/c1-3-19-5-4-6-25(36-29(41)12-7-21-15-22(32)8-11-28(21)40-18-34-38-39-40)27-16-20(13-14-33-27)24-10-9-23(35-31(43)44-2)17-26(24)37-30(19)42/h7-19,25H,3-6H2,1-2H3,(H,35,43)(H,36,41)(H,37,42)/b12-7+/t19-,25+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50192767
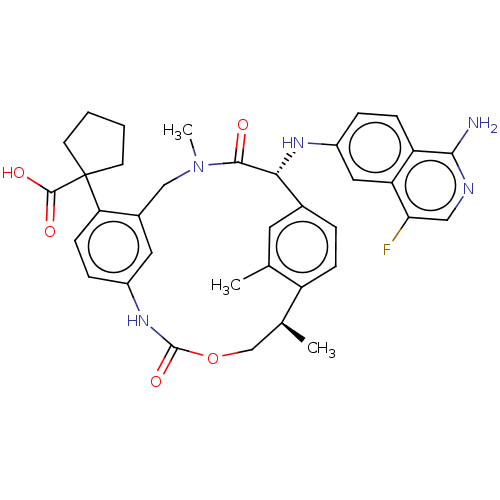
(CHEMBL3984725)Show SMILES C[C@H]1COC(=O)Nc2ccc(c(CN(C)C(=O)[C@H](Nc3ccc4c(N)ncc(F)c4c3)c3ccc1c(C)c3)c2)C1(CCCC1)C(O)=O |r| Show InChI InChI=1S/C36H38FN5O5/c1-20-14-22-6-9-26(20)21(2)19-47-35(46)41-24-8-11-29(36(34(44)45)12-4-5-13-36)23(15-24)18-42(3)33(43)31(22)40-25-7-10-27-28(16-25)30(37)17-39-32(27)38/h6-11,14-17,21,31,40H,4-5,12-13,18-19H2,1-3H3,(H2,38,39)(H,41,46)(H,44,45)/t21-,31+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research& Development
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human TF-factor 7a using factor 10 as substrate at 37 degC |
Bioorg Med Chem Lett 26: 5051-5057 (2016)
Article DOI: 10.1016/j.bmcl.2016.08.088
BindingDB Entry DOI: 10.7270/Q24X59Q6 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM142872
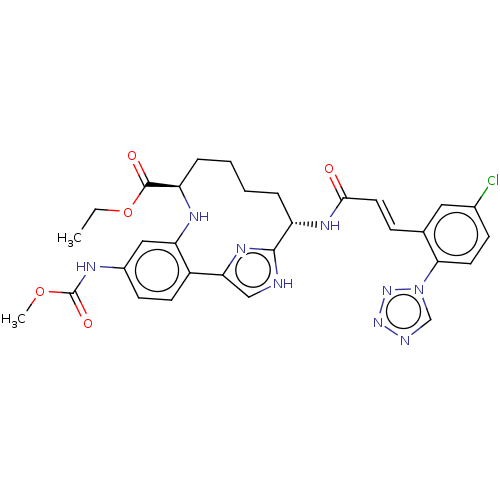
(US8940720, I-67)Show SMILES CCOC(=O)[C@H]1CCCC[C@H](NC(=O)\C=C\c2cc(Cl)ccc2-n2cnnn2)c2nc(c[nH]2)-c2ccc(NC(=O)OC)cc2N1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126949
BindingDB Entry DOI: 10.7270/Q24Q7ZJH |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM189441
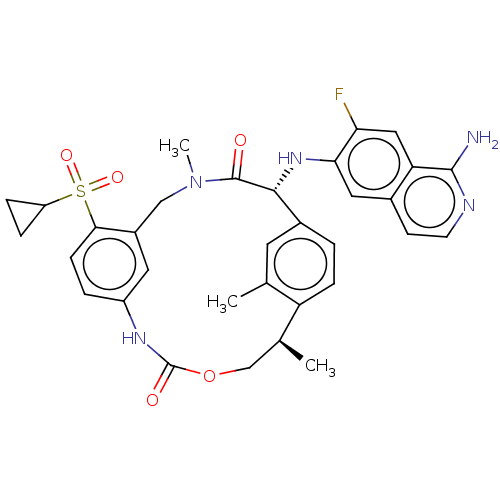
(US9174974, Example 26)Show SMILES C[C@H]1COC(=O)Nc2ccc(c(CN(C)C(=O)[C@H](Nc3cc4ccnc(N)c4cc3F)c3ccc1c(C)c3)c2)S(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C33H34FN5O5S/c1-18-12-21-4-8-25(18)19(2)17-44-33(41)37-23-5-9-29(45(42,43)24-6-7-24)22(13-23)16-39(3)32(40)30(21)38-28-14-20-10-11-36-31(35)26(20)15-27(28)34/h4-5,8-15,19,24,30,38H,6-7,16-17H2,1-3H3,(H2,35,36)(H,37,41)/t19-,30+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... |
J Med Chem 59: 7125-37 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00469
BindingDB Entry DOI: 10.7270/Q2TM7D37 |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50205841
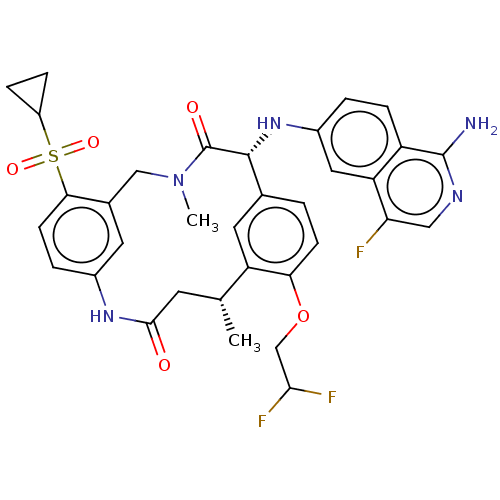
(CHEMBL3898956)Show SMILES C[C@@H]1CC(=O)Nc2ccc(c(CN(C)C(=O)[C@H](Nc3ccc4c(N)ncc(F)c4c3)c3ccc(OCC(F)F)c1c3)c2)S(=O)(=O)C1CC1 |r| Show InChI InChI=1S/C34H34F3N5O5S/c1-18-11-31(43)40-21-5-10-29(48(45,46)23-6-7-23)20(12-21)16-42(2)34(44)32(19-3-9-28(25(18)13-19)47-17-30(36)37)41-22-4-8-24-26(14-22)27(35)15-39-33(24)38/h3-5,8-10,12-15,18,23,30,32,41H,6-7,11,16-17H2,1-2H3,(H2,38,39)(H,40,43)/t18-,32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human factor-7a/TF using S2288 as substrate measured after 60 mins at 37 degC |
ACS Med Chem Lett 8: 67-72 (2017)
Article DOI: 10.1021/acsmedchemlett.6b00375
BindingDB Entry DOI: 10.7270/Q20P120Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50541586
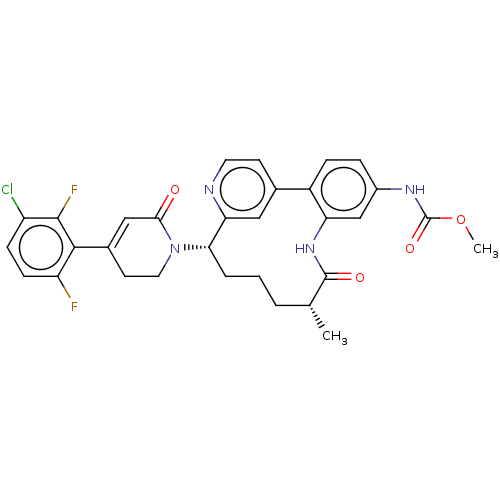
(CHEMBL4638245)Show SMILES COC(=O)Nc1ccc-2c(NC(=O)[C@H](C)CCC[C@H](N3CCC(=CC3=O)c3c(F)ccc(Cl)c3F)c3cc-2ccn3)c1 |r,c:22| Show InChI InChI=1S/C31H29ClF2N4O4/c1-17-4-3-5-26(38-13-11-19(15-27(38)39)28-23(33)9-8-22(32)29(28)34)25-14-18(10-12-35-25)21-7-6-20(36-31(41)42-2)16-24(21)37-30(17)40/h6-10,12,14-17,26H,3-5,11,13H2,1-2H3,(H,36,41)(H,37,40)/t17-,26+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor11a using pyro-Glu-Pro-Arg-pNA(para-nitroaniline) substrate by spectrophotometry |
J Med Chem 63: 7226-7242 (2020)
Article DOI: 10.1021/acs.jmedchem.0c00464
BindingDB Entry DOI: 10.7270/Q2J67MHG |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50525762
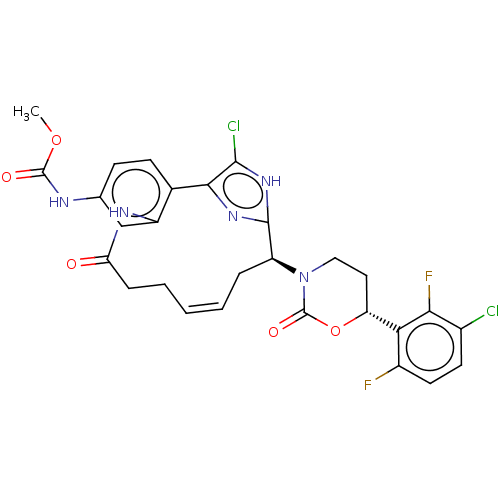
(CHEMBL4467360)Show SMILES COC(=O)Nc1ccc2-c3nc([nH]c3Cl)[C@H](C\C=C\CCC(=O)Nc2c1)N1CC[C@@H](OC1=O)c1c(F)ccc(Cl)c1F |r,t:18| Show InChI InChI=1S/C28H25Cl2F2N5O5/c1-41-27(39)33-14-7-8-15-18(13-14)34-21(38)6-4-2-3-5-19(26-35-24(15)25(30)36-26)37-12-11-20(42-28(37)40)22-17(31)10-9-16(29)23(22)32/h2-3,7-10,13,19-20H,4-6,11-12H2,1H3,(H,33,39)(H,34,38)(H,35,36)/b3-2+/t19-,20+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of activated human coagulation factor 11 using pyroGlu-Pro-Arg-pNA as substrate incubated for 10 to 120 mins by spectrofluorometric method |
Bioorg Med Chem Lett 29: (2019)
Article DOI: 10.1016/j.bmcl.2019.08.008
BindingDB Entry DOI: 10.7270/Q23F4T2C |
More data for this
Ligand-Target Pair | |
Coagulation factor VII/Tissue factor
(Homo sapiens (Human)) | BDBM50191346
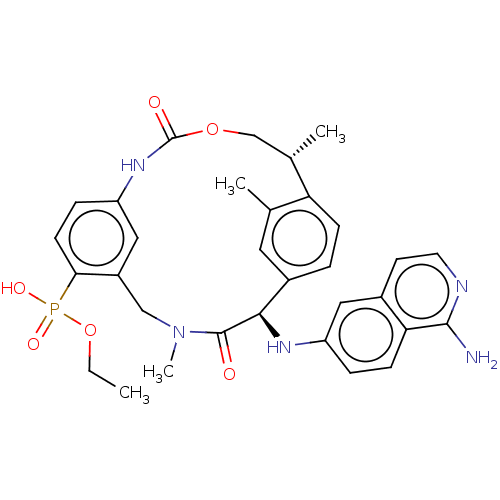
(CHEMBL3978562)Show SMILES CCOP(O)(=O)c1ccc2NC(=O)OC[C@H](C)c3ccc(cc3C)[C@@H](Nc3ccc4c(N)nccc4c3)C(=O)N(C)Cc1c2 |r| Show InChI InChI=1S/C32H36N5O6P/c1-5-43-44(40,41)28-11-8-25-16-23(28)17-37(4)31(38)29(35-24-7-10-27-21(15-24)12-13-34-30(27)33)22-6-9-26(19(2)14-22)20(3)18-42-32(39)36-25/h6-16,20,29,35H,5,17-18H2,1-4H3,(H2,33,34)(H,36,39)(H,40,41)/t20-,29+/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.280 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb R&D
Curated by ChEMBL
| Assay Description
Inhibition of full-length human TF/recombinant human factor 7a assessed as decrease in conversion of factor 10 to factor 10a by measuring S2765 hydro... |
J Med Chem 59: 7125-37 (2016)
Article DOI: 10.1021/acs.jmedchem.6b00469
BindingDB Entry DOI: 10.7270/Q2TM7D37 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50458535
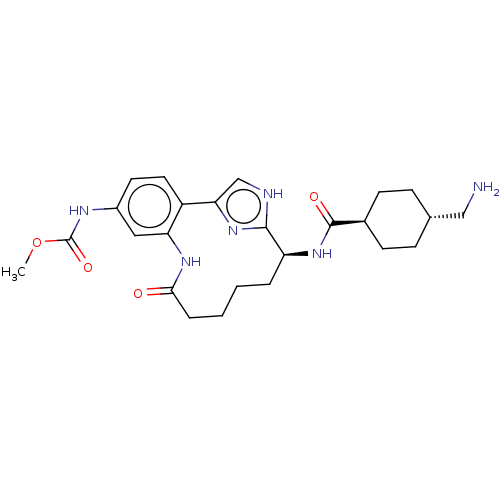
(CHEMBL4203066)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](CCCCC(=O)Nc2c1)NC(=O)[C@H]1CC[C@H](CN)CC1 |r,wU:30.33,14.26,wD:27.29,(54.73,-42.06,;53.82,-40.81,;52.29,-40.97,;51.66,-42.37,;51.39,-39.72,;49.86,-39.88,;49.23,-41.29,;47.69,-41.44,;46.79,-40.19,;45.27,-40.35,;44.49,-41.68,;42.99,-41.36,;42.83,-39.83,;44.24,-39.2,;41.5,-39.06,;41.5,-37.52,;42.84,-36.75,;42.84,-35.21,;44.33,-34.81,;45.42,-35.89,;46.91,-35.48,;45.03,-37.38,;47.42,-38.79,;48.95,-38.63,;40.16,-39.82,;38.83,-39.05,;38.84,-37.51,;37.5,-39.82,;36.17,-39.04,;34.84,-39.82,;34.84,-41.36,;33.5,-42.13,;32.17,-41.36,;36.17,-42.12,;37.5,-41.36,)| Show InChI InChI=1S/C25H34N6O4/c1-35-25(34)28-17-10-11-18-20(12-17)29-22(32)5-3-2-4-19(23-27-14-21(18)30-23)31-24(33)16-8-6-15(13-26)7-9-16/h10-12,14-16,19H,2-9,13,26H2,1H3,(H,27,30)(H,28,34)(H,29,32)(H,31,33)/t15-,16-,19-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a incubated for 60 mins by Cheng-Prusoff equation analysis |
J Med Chem 61: 7425-7447 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00173
BindingDB Entry DOI: 10.7270/Q2QJ7KW0 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032873
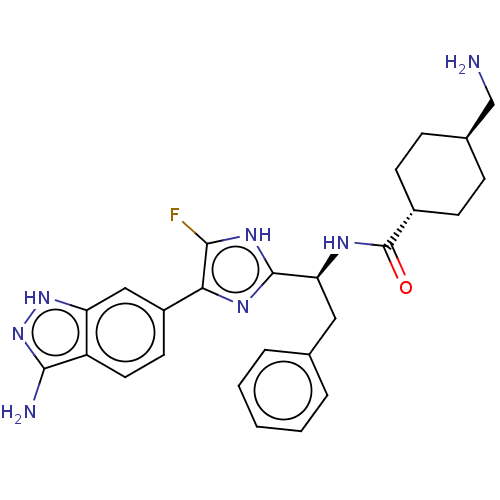
(CHEMBL3355684)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(F)[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:12.14,wD:9.7,18.17,(38,-31.47,;39.32,-32.23,;39.32,-33.76,;40.64,-31.46,;41.96,-32.23,;40.64,-29.94,;41.95,-30.68,;15.71,-45.09,;16.47,-43.75,;18.01,-43.75,;18.79,-45.09,;20.33,-45.09,;21.09,-43.75,;20.33,-42.42,;18.79,-42.42,;22.63,-43.75,;23.41,-45.09,;23.41,-42.42,;24.95,-42.42,;25.71,-43.75,;27.25,-43.75,;28.03,-42.42,;29.57,-42.42,;30.33,-43.75,;29.57,-45.09,;28.03,-45.09,;25.71,-41.09,;25.09,-39.68,;26.23,-38.65,;27.56,-39.42,;28.97,-38.79,;27.24,-40.93,;26.07,-37.12,;27.32,-36.22,;27.16,-34.69,;25.75,-34.05,;25.27,-32.59,;26.18,-31.34,;23.73,-32.59,;23.25,-34.05,;24.5,-34.96,;24.66,-36.49,)| Show InChI InChI=1S/C26H30FN7O.C2HF3O2/c27-23-22(18-10-11-19-20(13-18)33-34-24(19)29)31-25(32-23)21(12-15-4-2-1-3-5-15)30-26(35)17-8-6-16(14-28)7-9-17;3-2(4,5)1(6)7/h1-5,10-11,13,16-17,21H,6-9,12,14,28H2,(H,30,35)(H,31,32)(H3,29,33,34);(H,6,7)/t16-,17-,21-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50448585
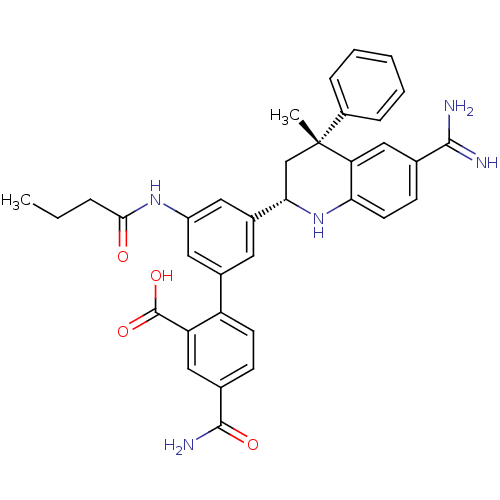
(CHEMBL3127489)Show SMILES CCCC(=O)Nc1cc(cc(c1)-c1ccc(cc1C(O)=O)C(N)=O)[C@@H]1C[C@](C)(c2ccccc2)c2cc(ccc2N1)C(N)=N |r| Show InChI InChI=1S/C35H35N5O4/c1-3-7-31(41)39-25-15-22(26-12-10-21(33(38)42)17-27(26)34(43)44)14-23(16-25)30-19-35(2,24-8-5-4-6-9-24)28-18-20(32(36)37)11-13-29(28)40-30/h4-6,8-18,30,40H,3,7,19H2,1-2H3,(H3,36,37)(H2,38,42)(H,39,41)(H,43,44)/t30-,35+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Binding affinity to human factor 11a assessed as release of p-nitroaniline after 10 to 120 mins by spectrophotometric analysis |
J Med Chem 57: 955-69 (2014)
Article DOI: 10.1021/jm401670x
BindingDB Entry DOI: 10.7270/Q2G44RTQ |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50096838
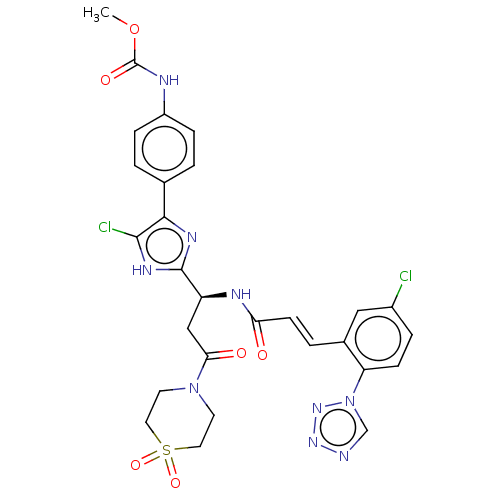
(CHEMBL3580758)Show SMILES COC(=O)Nc1ccc(cc1)-c1nc([nH]c1Cl)[C@H](CC(=O)N1CCS(=O)(=O)CC1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r| | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometry |
ACS Med Chem Lett 6: 590-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00066
BindingDB Entry DOI: 10.7270/Q2B27X24 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50032874
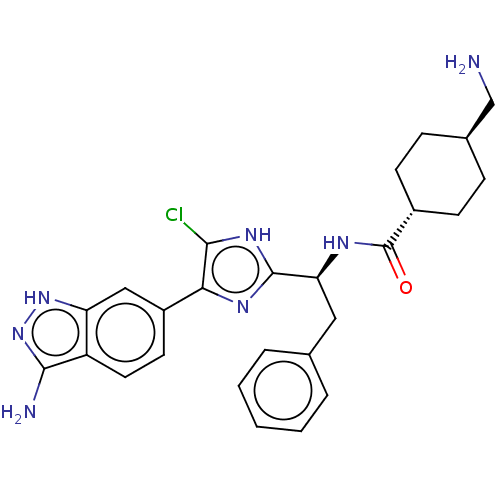
(CHEMBL3355683)Show SMILES OC(=O)C(F)(F)F.NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Cl)[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:12.14,wD:9.7,18.17,(34.53,-30.97,;35.85,-31.73,;35.85,-33.26,;37.17,-30.97,;38.49,-31.73,;37.17,-29.44,;38.49,-30.19,;15.69,-45.08,;16.46,-43.74,;18,-43.74,;18.77,-45.08,;20.31,-45.08,;21.08,-43.74,;20.31,-42.42,;18.77,-42.42,;22.62,-43.74,;23.39,-45.08,;23.39,-42.42,;24.93,-42.42,;25.7,-43.74,;27.24,-43.74,;28.01,-42.42,;29.55,-42.42,;30.32,-43.74,;29.55,-45.08,;28.01,-45.08,;25.7,-41.08,;25.07,-39.67,;26.21,-38.64,;27.55,-39.42,;28.96,-38.78,;27.23,-40.92,;26.05,-37.11,;27.3,-36.21,;27.14,-34.68,;25.73,-34.05,;25.26,-32.58,;26.16,-31.33,;23.72,-32.58,;23.24,-34.05,;24.49,-34.96,;24.65,-36.49,)| Show InChI InChI=1S/C26H30ClN7O.C2HF3O2/c27-23-22(18-10-11-19-20(13-18)33-34-24(19)29)31-25(32-23)21(12-15-4-2-1-3-5-15)30-26(35)17-8-6-16(14-28)7-9-17;3-2(4,5)1(6)7/h1-5,10-11,13,16-17,21H,6-9,12,14,28H2,(H,30,35)(H,31,32)(H3,29,33,34);(H,6,7)/t16-,17-,21-;/m0./s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometrically |
J Med Chem 57: 9915-32 (2014)
Article DOI: 10.1021/jm5010607
BindingDB Entry DOI: 10.7270/Q2RV0Q9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50063581
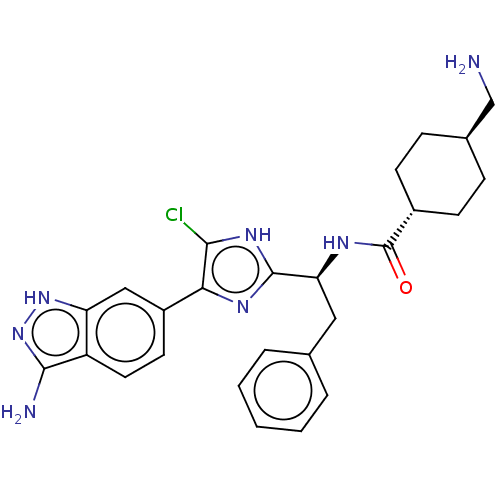
(CHEMBL3398612)Show SMILES NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Cl)[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:5.8,wD:2.1,11.11,(25.62,-40.59,;26.39,-39.26,;27.93,-39.26,;28.7,-40.59,;30.24,-40.59,;31.01,-39.26,;30.24,-37.93,;28.7,-37.93,;32.55,-39.26,;33.32,-40.59,;33.32,-37.93,;34.86,-37.93,;35.63,-39.26,;37.17,-39.26,;37.94,-37.93,;39.48,-37.93,;40.25,-39.26,;39.48,-40.59,;37.94,-40.59,;35.63,-36.59,;35.01,-35.19,;36.14,-34.15,;37.48,-34.93,;38.89,-34.3,;37.16,-36.43,;35.98,-32.62,;37.23,-31.72,;37.07,-30.19,;35.66,-29.56,;35.19,-28.09,;36.09,-26.85,;33.65,-28.09,;33.17,-29.56,;34.42,-30.47,;34.58,-32,)| Show InChI InChI=1S/C26H28ClN7O/c27-23-22(18-10-11-19-20(13-18)33-34-24(19)29)31-25(32-23)21(12-15-4-2-1-3-5-15)30-26(35)17-8-6-16(14-28)7-9-17/h1-5,10-11,13,16-17,21H,6-9,12,14,28-29H2,(H,30,35)/b22-18-/t16-,17-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a at 25 degC |
Bioorg Med Chem Lett 25: 1635-42 (2015)
Article DOI: 10.1016/j.bmcl.2015.01.028
BindingDB Entry DOI: 10.7270/Q2319XJN |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50539655
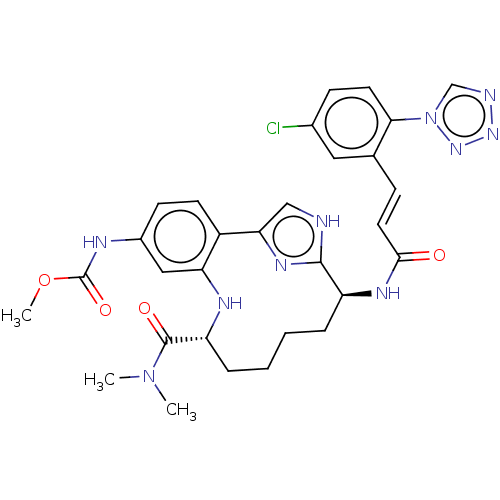
(CHEMBL4632633)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](CCCC[C@@H](Nc2c1)C(=O)N(C)C)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r| Show InChI InChI=1S/C30H33ClN10O4/c1-40(2)29(43)23-7-5-4-6-22(36-27(42)13-8-18-14-19(31)9-12-26(18)41-17-33-38-39-41)28-32-16-25(37-28)21-11-10-20(15-24(21)35-23)34-30(44)45-3/h8-17,22-23,35H,4-7H2,1-3H3,(H,32,37)(H,34,44)(H,36,42)/b13-8+/t22-,23+/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human activated coagulation factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
Bioorg Med Chem Lett 30: (2020)
Article DOI: 10.1016/j.bmcl.2020.126949
BindingDB Entry DOI: 10.7270/Q24Q7ZJH |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50063581

(CHEMBL3398612)Show SMILES NC[C@H]1CC[C@@H](CC1)C(=O)N[C@@H](Cc1ccccc1)c1nc(c(Cl)[nH]1)-c1ccc2c(N)n[nH]c2c1 |r,wU:5.8,wD:2.1,11.11,(25.62,-40.59,;26.39,-39.26,;27.93,-39.26,;28.7,-40.59,;30.24,-40.59,;31.01,-39.26,;30.24,-37.93,;28.7,-37.93,;32.55,-39.26,;33.32,-40.59,;33.32,-37.93,;34.86,-37.93,;35.63,-39.26,;37.17,-39.26,;37.94,-37.93,;39.48,-37.93,;40.25,-39.26,;39.48,-40.59,;37.94,-40.59,;35.63,-36.59,;35.01,-35.19,;36.14,-34.15,;37.48,-34.93,;38.89,-34.3,;37.16,-36.43,;35.98,-32.62,;37.23,-31.72,;37.07,-30.19,;35.66,-29.56,;35.19,-28.09,;36.09,-26.85,;33.65,-28.09,;33.17,-29.56,;34.42,-30.47,;34.58,-32,)| Show InChI InChI=1S/C26H28ClN7O/c27-23-22(18-10-11-19-20(13-18)33-34-24(19)29)31-25(32-23)21(12-15-4-2-1-3-5-15)30-26(35)17-8-6-16(14-28)7-9-17/h1-5,10-11,13,16-17,21H,6-9,12,14,28-29H2,(H,30,35)/b22-18-/t16-,17-,21-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.310 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Competitive inhibition of human coagulation factor 11a assessed as reduction in release of p-nitroaniline after 10 to 120 mins by Michaelis-Menten eq... |
Bioorg Med Chem 24: 2257-72 (2016)
Article DOI: 10.1016/j.bmc.2016.03.062
BindingDB Entry DOI: 10.7270/Q2DN46X0 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
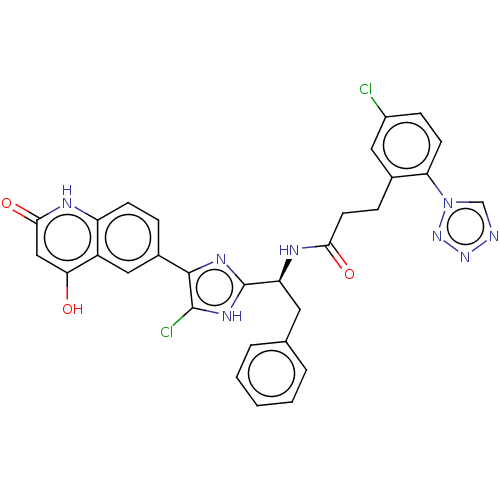
(Homo sapiens (Human)) | BDBM50096844
(CHEMBL3580753)Show SMILES Oc1cc(=O)[nH]c2ccc(cc12)-c1nc([nH]c1Cl)[C@H](Cc1ccccc1)NC(=O)CCc1cc(Cl)ccc1-n1cnnn1 |r| Show InChI InChI=1S/C30H22Cl2N8O3/c31-20-8-10-24(40-16-33-38-39-40)18(13-20)7-11-26(42)35-23(12-17-4-2-1-3-5-17)30-36-28(29(32)37-30)19-6-9-22-21(14-19)25(41)15-27(43)34-22/h1-6,8-10,13-16,23,41H,7,11-12H2,(H,35,42)/b28-19-/t23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a using p-nitroaniline as substrate assessed as substrate hydrolysis by spectrophotometry |
ACS Med Chem Lett 6: 590-5 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00066
BindingDB Entry DOI: 10.7270/Q2B27X24 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50260647
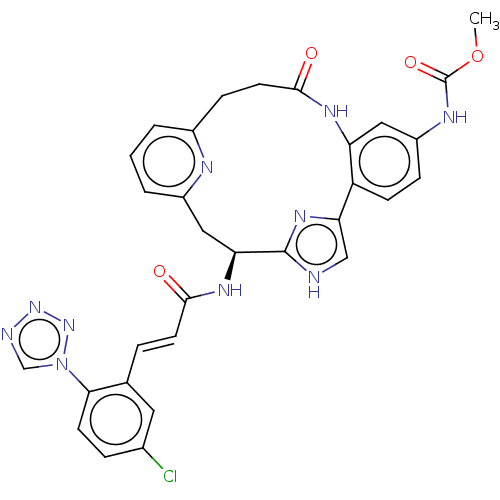
(CHEMBL4067274)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](Cc3cccc(CCC(=O)Nc2c1)n3)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r| Show InChI InChI=1S/C31H27ClN10O4/c1-46-31(45)36-22-7-9-23-24(14-22)37-29(44)12-8-20-3-2-4-21(35-20)15-25(30-33-16-26(23)39-30)38-28(43)11-5-18-13-19(32)6-10-27(18)42-17-34-40-41-42/h2-7,9-11,13-14,16-17,25H,8,12,15H2,1H3,(H,33,39)(H,36,45)(H,37,44)(H,38,43)/b11-5+/t25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company, Research and Development, 350 Carter Road, Hopewell, NJ 08540 United States.
Curated by ChEMBL
| Assay Description
Inhibition of human F11a using peptide substrate by spectrophotometry |
Bioorg Med Chem Lett 27: 4056-4060 (2017)
Article DOI: 10.1016/j.bmcl.2017.07.048
BindingDB Entry DOI: 10.7270/Q2TB19B3 |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50269191
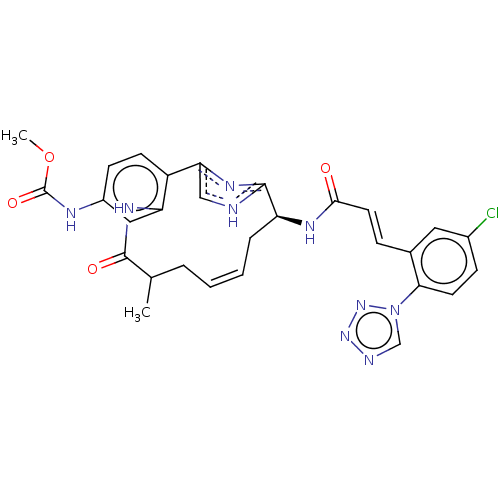
(CHEMBL4063746)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](C\C=C\CC(C)C(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r,t:17| Show InChI InChI=1S/C29H28ClN9O4/c1-17-5-3-4-6-22(34-26(40)12-7-18-13-19(30)8-11-25(18)39-16-32-37-38-39)27-31-15-24(35-27)21-10-9-20(33-29(42)43-2)14-23(21)36-28(17)41/h3-4,7-17,22H,5-6H2,1-2H3,(H,31,35)(H,33,42)(H,34,40)(H,36,41)/b4-3+,12-7+/t17?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 27: 3833-3839 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.058
BindingDB Entry DOI: 10.7270/Q2GT5QPS |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50269191

(CHEMBL4063746)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](C\C=C\CC(C)C(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r,t:17| Show InChI InChI=1S/C29H28ClN9O4/c1-17-5-3-4-6-22(34-26(40)12-7-18-13-19(30)8-11-25(18)39-16-32-37-38-39)27-31-15-24(35-27)21-10-9-20(33-29(42)43-2)14-23(21)36-28(17)41/h3-4,7-17,22H,5-6H2,1-2H3,(H,31,35)(H,33,42)(H,34,40)(H,36,41)/b4-3+,12-7+/t17?,22-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 27: 3833-3839 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.058
BindingDB Entry DOI: 10.7270/Q2GT5QPS |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50136575
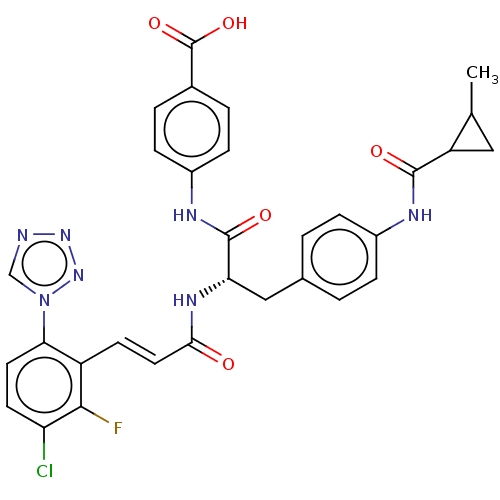
(CHEMBL3752610)Show SMILES CC1CC1C(=O)Nc1ccc(C[C@H](NC(=O)\C=C\c2c(F)c(Cl)ccc2-n2cnnn2)C(=O)Nc2ccc(cc2)C(O)=O)cc1 |r| Show InChI InChI=1S/C31H27ClFN7O5/c1-17-14-23(17)29(42)35-20-6-2-18(3-7-20)15-25(30(43)36-21-8-4-19(5-9-21)31(44)45)37-27(41)13-10-22-26(40-16-34-38-39-40)12-11-24(32)28(22)33/h2-13,16-17,23,25H,14-15H2,1H3,(H,35,42)(H,36,43)(H,37,41)(H,44,45)/b13-10+/t17?,23?,25-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human coagulation factor 11a assessed as substrate hydrolysis to p-nitroaniline incubated for 10 to 120 mins by spectrophotometry analy... |
Bioorg Med Chem Lett 26: 472-8 (2016)
Article DOI: 10.1016/j.bmcl.2015.11.089
BindingDB Entry DOI: 10.7270/Q27H1MFM |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50250501

(CHEMBL4078562)Show SMILES OC(=O)c1ccc(NC(=O)[C@H]2N(CCc3c2cccc3N2CCOCC2)C(=O)\C=C\c2c(F)c(Cl)ccc2-n2cnnn2)cc1 |r| Show InChI InChI=1S/C31H27ClFN7O5/c32-24-9-10-26(40-18-34-36-37-40)23(28(24)33)8-11-27(41)39-13-12-21-22(2-1-3-25(21)38-14-16-45-17-15-38)29(39)30(42)35-20-6-4-19(5-7-20)31(43)44/h1-11,18,29H,12-17H2,(H,35,42)(H,43,44)/b11-8+/t29-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human factor 11a using pyro-Glu-Pro-Arg-pNA as substrate at 37 degC after 10 to 120 mins by spectrophotometric method |
J Med Chem 60: 9703-9723 (2017)
Article DOI: 10.1021/acs.jmedchem.7b01171
BindingDB Entry DOI: 10.7270/Q2K64MHN |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50269195
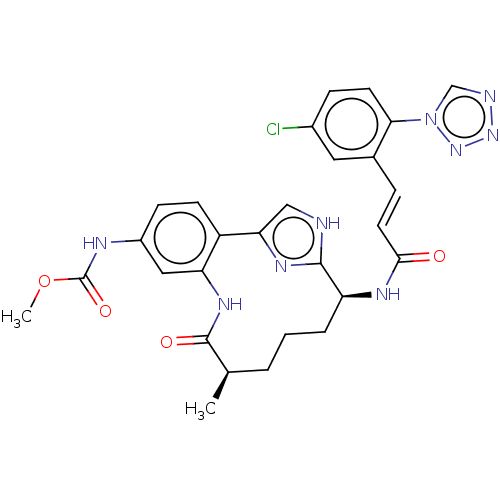
(CHEMBL4101766)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](CCC[C@@H](C)C(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r| Show InChI InChI=1S/C28H28ClN9O4/c1-16-4-3-5-21(33-25(39)11-6-17-12-18(29)7-10-24(17)38-15-31-36-37-38)26-30-14-23(34-26)20-9-8-19(32-28(41)42-2)13-22(20)35-27(16)40/h6-16,21H,3-5H2,1-2H3,(H,30,34)(H,32,41)(H,33,39)(H,35,40)/b11-6+/t16-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human activated factor XI using pyro-Glu-Pro-Arg-pNA as substrate by spectrophotometry |
J Med Chem 63: 784-803 (2020)
Article DOI: 10.1021/acs.jmedchem.9b01768
BindingDB Entry DOI: 10.7270/Q2J67M9S |
More data for this
Ligand-Target Pair | |
Coagulation factor XI
(Homo sapiens (Human)) | BDBM50269195

(CHEMBL4101766)Show SMILES COC(=O)Nc1ccc2-c3c[nH]c(n3)[C@H](CCC[C@@H](C)C(=O)Nc2c1)NC(=O)\C=C\c1cc(Cl)ccc1-n1cnnn1 |r| Show InChI InChI=1S/C28H28ClN9O4/c1-16-4-3-5-21(33-25(39)11-6-17-12-18(29)7-10-24(17)38-15-31-36-37-38)26-30-14-23(34-26)20-9-8-19(32-28(41)42-2)13-22(20)35-27(16)40/h6-16,21H,3-5H2,1-2H3,(H,30,34)(H,32,41)(H,33,39)(H,35,40)/b11-6+/t16-,21+/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.390 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of human Factor XIa using S-2366 as chromogenic substrate after 60 mins by Lineweaver-Burk plot analysis |
Bioorg Med Chem Lett 27: 3833-3839 (2017)
Article DOI: 10.1016/j.bmcl.2017.06.058
BindingDB Entry DOI: 10.7270/Q2GT5QPS |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data