 Found 89 hits with Last Name = 'semus' and Initial = 'sf'
Found 89 hits with Last Name = 'semus' and Initial = 'sf' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50005363
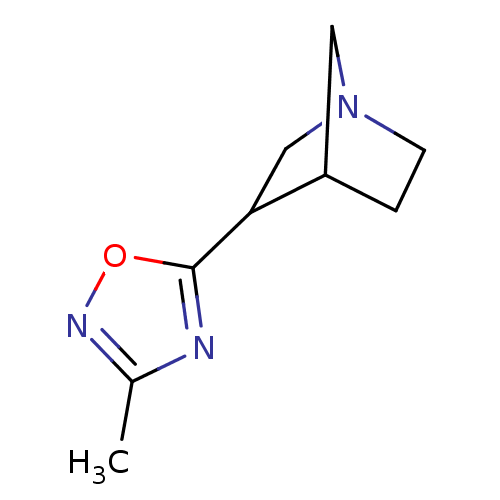
((3R,4R)3-(3-Methyl-[1,2,4]oxadiazol-5-yl)-1-aza-bi...)Show InChI InChI=1S/C9H13N3O/c1-6-10-9(13-11-6)8-5-12-3-2-7(8)4-12/h7-8H,2-5H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Arcus USA
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor using [3H]-OXO-M as the radioligand. |
J Med Chem 41: 4181-5 (1998)
Article DOI: 10.1021/jm980192x
BindingDB Entry DOI: 10.7270/Q2PV6P3B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471859
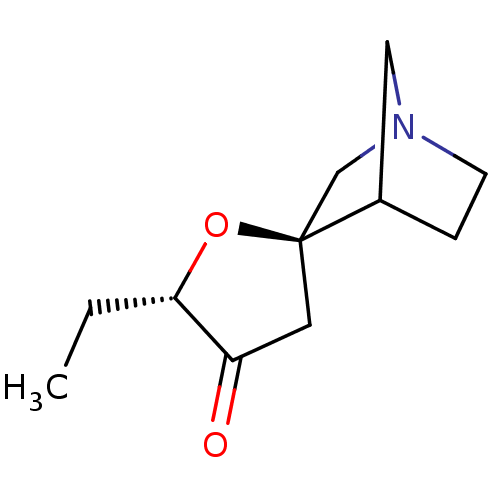
(CHEMBL334387)Show SMILES CC[C@@H]1O[C@]2(CN3CCC2C3)CC1=O |TLB:11:4:10:8.7,THB:3:4:10:8.7| Show InChI InChI=1S/C11H17NO2/c1-2-10-9(13)5-11(14-10)7-12-4-3-8(11)6-12/h8,10H,2-7H2,1H3/t8?,10-,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Arcus USA
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor using [3H]-OXO-M as the radioligand. |
J Med Chem 41: 4181-5 (1998)
Article DOI: 10.1021/jm980192x
BindingDB Entry DOI: 10.7270/Q2PV6P3B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471858
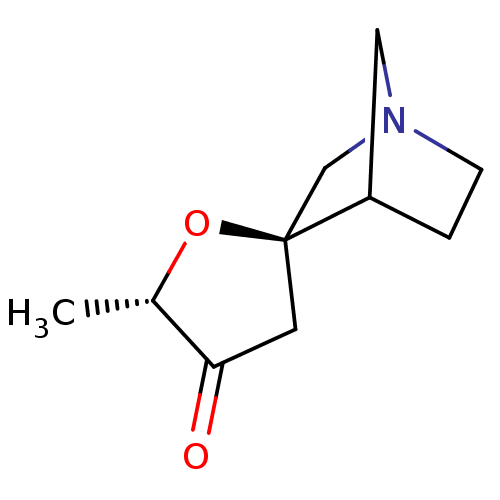
(CHEMBL335503)Show SMILES C[C@@H]1O[C@]2(CN3CCC2C3)CC1=O |TLB:10:3:9:7.6,THB:2:3:9:7.6| Show InChI InChI=1S/C10H15NO2/c1-7-9(12)4-10(13-7)6-11-3-2-8(10)5-11/h7-8H,2-6H2,1H3/t7-,8?,10-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Arcus USA
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor using [3H]-OXO-M as the radioligand. |
J Med Chem 41: 4181-5 (1998)
Article DOI: 10.1021/jm980192x
BindingDB Entry DOI: 10.7270/Q2PV6P3B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471854
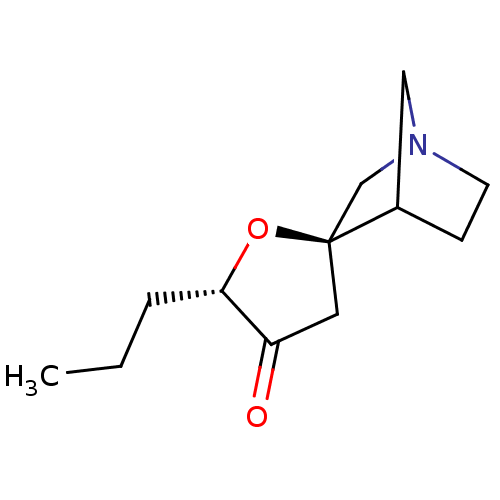
(CHEMBL337509)Show SMILES CCC[C@@H]1O[C@]2(CN3CCC2C3)CC1=O |TLB:12:5:11:9.8,THB:4:5:11:9.8| Show InChI InChI=1S/C12H19NO2/c1-2-3-11-10(14)6-12(15-11)8-13-5-4-9(12)7-13/h9,11H,2-8H2,1H3/t9?,11-,12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Arcus USA
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor using [3H]-OXO-M as the radioligand. |
J Med Chem 41: 4181-5 (1998)
Article DOI: 10.1021/jm980192x
BindingDB Entry DOI: 10.7270/Q2PV6P3B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471855
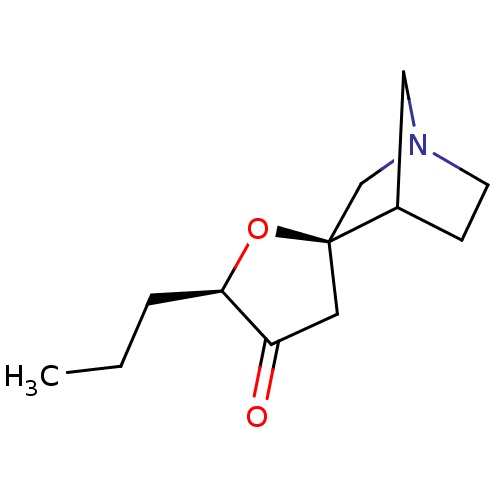
(CHEMBL134860)Show SMILES CCC[C@H]1O[C@]2(CN3CCC2C3)CC1=O |TLB:12:5:11:9.8,THB:4:5:11:9.8| Show InChI InChI=1S/C12H19NO2/c1-2-3-11-10(14)6-12(15-11)8-13-5-4-9(12)7-13/h9,11H,2-8H2,1H3/t9?,11-,12+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Arcus USA
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor using [3H]-OXO-M as the radioligand. |
J Med Chem 41: 4181-5 (1998)
Article DOI: 10.1021/jm980192x
BindingDB Entry DOI: 10.7270/Q2PV6P3B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471860
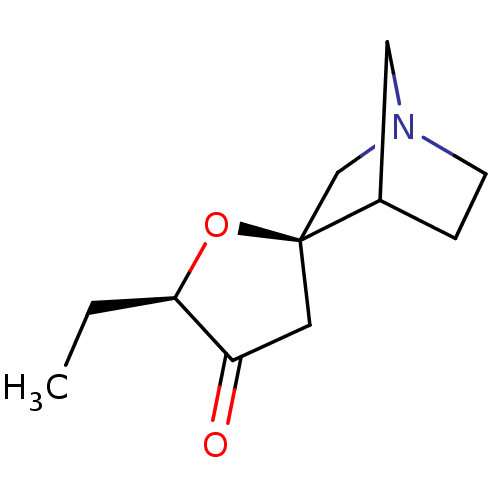
(CHEMBL134937)Show SMILES CC[C@H]1O[C@]2(CN3CCC2C3)CC1=O |TLB:11:4:10:8.7,THB:3:4:10:8.7| Show InChI InChI=1S/C11H17NO2/c1-2-10-9(13)5-11(14-10)7-12-4-3-8(11)6-12/h8,10H,2-7H2,1H3/t8?,10-,11+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Arcus USA
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor using [3H]-OXO-M as the radioligand. |
J Med Chem 41: 4181-5 (1998)
Article DOI: 10.1021/jm980192x
BindingDB Entry DOI: 10.7270/Q2PV6P3B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471857
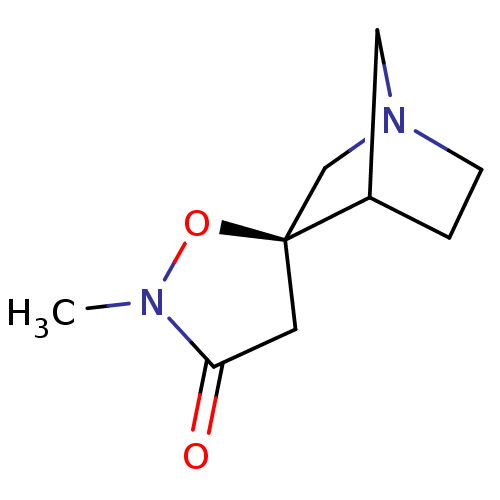
(CHEMBL335496)Show SMILES CN1O[C@]2(CN3CCC2C3)CC1=O |TLB:10:3:9:7.6,THB:2:3:9:7.6| Show InChI InChI=1S/C9H14N2O2/c1-10-8(12)4-9(13-10)6-11-3-2-7(9)5-11/h7H,2-6H2,1H3/t7?,9-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Arcus USA
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor using [3H]-OXO-M as the radioligand. |
J Med Chem 41: 4181-5 (1998)
Article DOI: 10.1021/jm980192x
BindingDB Entry DOI: 10.7270/Q2PV6P3B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50034651
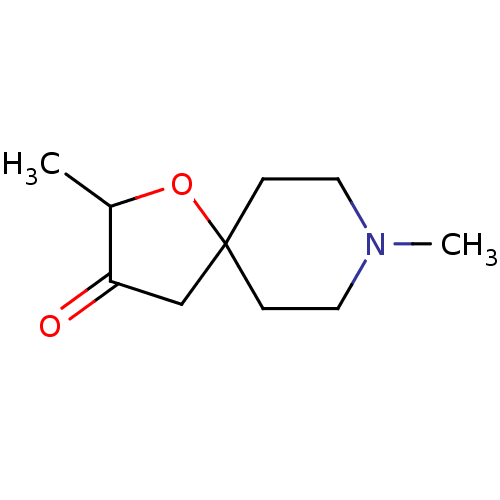
(2,8-Dimethyl-1-oxa-8-aza-spiro[4.5]decan-3-one | C...)Show InChI InChI=1S/C10H17NO2/c1-8-9(12)7-10(13-8)3-5-11(2)6-4-10/h8H,3-7H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Arcus USA
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor using [3H]-OXO-M as the radioligand. |
J Med Chem 41: 4181-5 (1998)
Article DOI: 10.1021/jm980192x
BindingDB Entry DOI: 10.7270/Q2PV6P3B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50004656

((2-Carbamoyloxy-ethyl)-trimethyl-ammonium | (2-Car...)Show InChI InChI=1S/C6H14N2O2/c1-8(2,3)4-5-10-6(7)9/h4-5H2,1-3H3,(H-,7,9)/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Arcus USA
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor using [3H]-OXO-M as the radioligand. |
J Med Chem 41: 4181-5 (1998)
Article DOI: 10.1021/jm980192x
BindingDB Entry DOI: 10.7270/Q2PV6P3B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50067479
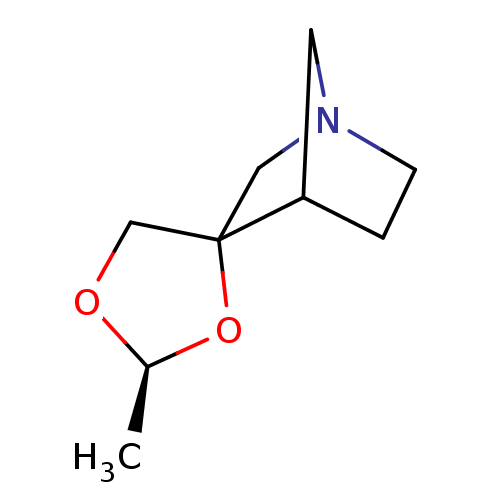
(2'-methyl-(2'S)-spiro[4-azabicyclo[2.2.1]heptane-2...)Show SMILES C[C@H]1OCC2(CN3CCC2C3)O1 |TLB:3:4:10:8.7,THB:11:4:10:8.7| Show InChI InChI=1S/C9H15NO2/c1-7-11-6-9(12-7)5-10-3-2-8(9)4-10/h7-8H,2-6H2,1H3/t7-,8?,9?/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Arcus USA
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor using [3H]-OXO-M as the radioligand. |
J Med Chem 41: 4181-5 (1998)
Article DOI: 10.1021/jm980192x
BindingDB Entry DOI: 10.7270/Q2PV6P3B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(RAT) | BDBM50471856
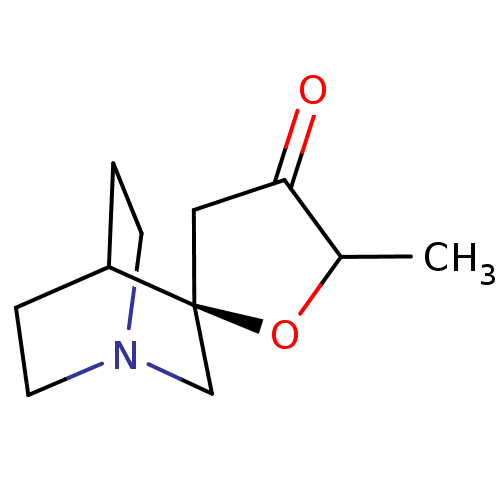
(CHEMBL448736)Show SMILES CC1O[C@@]2(CC1=O)CN1CCC2CC1 |wU:3.2,wD:3.3,TLB:2:3:10.9:12.13,THB:4:3:10.9:12.13,(12.07,-3.46,;10.98,-4.57,;9.44,-4.31,;8.74,-5.69,;9.82,-6.79,;11.2,-6.09,;12.58,-6.81,;8.23,-6.76,;6.96,-6.13,;6.96,-4.58,;7.29,-3.34,;7.37,-4.89,;6.27,-5.67,;5.64,-6.81,)| Show InChI InChI=1S/C11H17NO2/c1-8-10(13)6-11(14-8)7-12-4-2-9(11)3-5-12/h8-9H,2-7H2,1H3/t8?,11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Astra Arcus USA
Curated by ChEMBL
| Assay Description
Binding affinity against Muscarinic acetylcholine receptor using [3H]-OXO-M as the radioligand. |
J Med Chem 41: 4181-5 (1998)
Article DOI: 10.1021/jm980192x
BindingDB Entry DOI: 10.7270/Q2PV6P3B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50034651

(2,8-Dimethyl-1-oxa-8-aza-spiro[4.5]decan-3-one | C...)Show InChI InChI=1S/C10H17NO2/c1-8-9(12)7-10(13-8)3-5-11(2)6-4-10/h8H,3-7H2,1-2H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288143
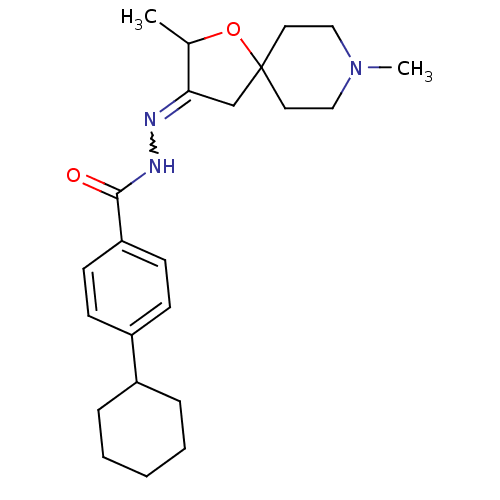
(4-Cyclohexyl-benzoic acid [2,8-dimethyl-1-oxa-8-az...)Show SMILES CC1OC2(CC1=NNC(=O)c1ccc(cc1)C1CCCCC1)CCN(C)CC2 |w:6.7| Show InChI InChI=1S/C23H33N3O2/c1-17-21(16-23(28-17)12-14-26(2)15-13-23)24-25-22(27)20-10-8-19(9-11-20)18-6-4-3-5-7-18/h8-11,17-18H,3-7,12-16H2,1-2H3,(H,25,27) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288159
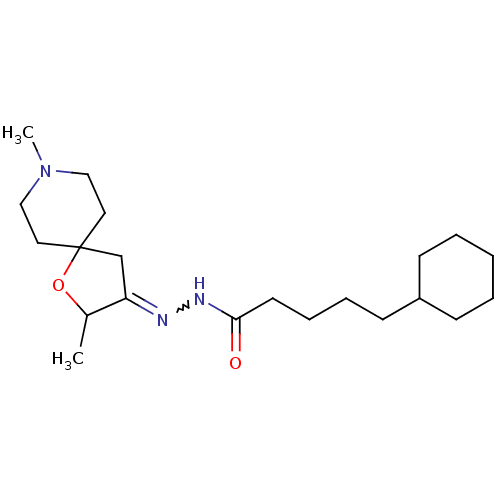
(5-Cyclohexyl-pentanoic acid [2,8-dimethyl-1-oxa-8-...)Show SMILES CC1OC2(CC1=NNC(=O)CCCCC1CCCCC1)CCN(C)CC2 |w:6.7| Show InChI InChI=1S/C21H37N3O2/c1-17-19(16-21(26-17)12-14-24(2)15-13-21)22-23-20(25)11-7-6-10-18-8-4-3-5-9-18/h17-18H,3-16H2,1-2H3,(H,23,25) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288156

(CHEMBL81954 | Octanoic acid N'-[2,8-dimethyl-1-oxa...)Show SMILES CCCCCCCC(=O)N(C)N=C1CC2(CCN(C)CC2)OC1C |w:11.10| Show InChI InChI=1S/C19H35N3O2/c1-5-6-7-8-9-10-18(23)22(4)20-17-15-19(24-16(17)2)11-13-21(3)14-12-19/h16H,5-15H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288153

(CHEMBL420596 | Nonanoic acid [2,8-dimethyl-1-oxa-8...)Show SMILES CCCCCCCCC(=O)NN=C1CC2(CCN(C)CC2)OC1C |w:11.10| Show InChI InChI=1S/C19H35N3O2/c1-4-5-6-7-8-9-10-18(23)21-20-17-15-19(24-16(17)2)11-13-22(3)14-12-19/h16H,4-15H2,1-3H3,(H,21,23) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288167

(CHEMBL84985 | Octanoic acid [2,8-dimethyl-1-oxa-8-...)Show SMILES CCCCCCCC(=O)NN=C1CC2(CCN(C)CC2)OC1C |w:10.9| Show InChI InChI=1S/C18H33N3O2/c1-4-5-6-7-8-9-17(22)20-19-16-14-18(23-15(16)2)10-12-21(3)13-11-18/h15H,4-14H2,1-3H3,(H,20,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
| 9.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288163
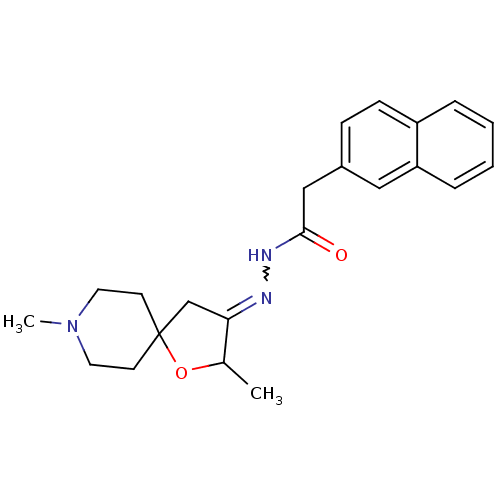
(CHEMBL84109 | Naphthalen-2-yl-acetic acid [2,8-dim...)Show SMILES CC1OC2(CC1=NNC(=O)Cc1ccc3ccccc3c1)CCN(C)CC2 |w:6.7| Show InChI InChI=1S/C22H27N3O2/c1-16-20(15-22(27-16)9-11-25(2)12-10-22)23-24-21(26)14-17-7-8-18-5-3-4-6-19(18)13-17/h3-8,13,16H,9-12,14-15H2,1-2H3,(H,24,26) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288144
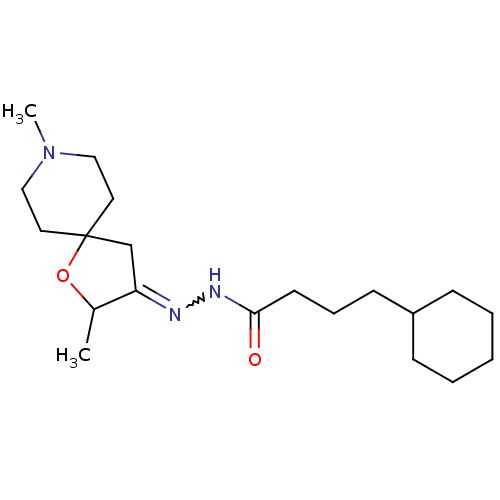
(4-Cyclohexyl-butyric acid [2,8-dimethyl-1-oxa-8-az...)Show SMILES CC1OC2(CC1=NNC(=O)CCCC1CCCCC1)CCN(C)CC2 |w:6.7| Show InChI InChI=1S/C20H35N3O2/c1-16-18(15-20(25-16)11-13-23(2)14-12-20)21-22-19(24)10-6-9-17-7-4-3-5-8-17/h16-17H,3-15H2,1-2H3,(H,22,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288147
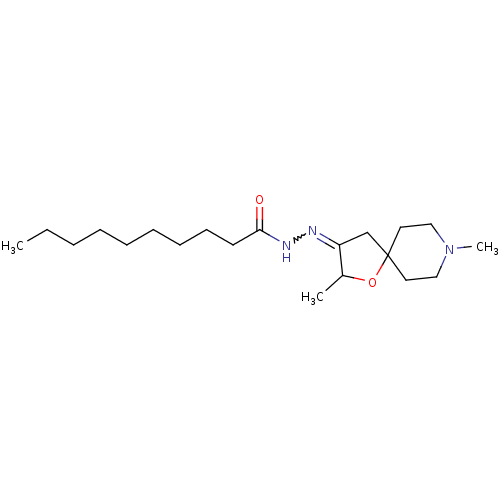
(CHEMBL314796 | Decanoic acid [2,8-dimethyl-1-oxa-8...)Show SMILES CCCCCCCCCC(=O)NN=C1CC2(CCN(C)CC2)OC1C |w:12.11| Show InChI InChI=1S/C20H37N3O2/c1-4-5-6-7-8-9-10-11-19(24)22-21-18-16-20(25-17(18)2)12-14-23(3)15-13-20/h17H,4-16H2,1-3H3,(H,22,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288158
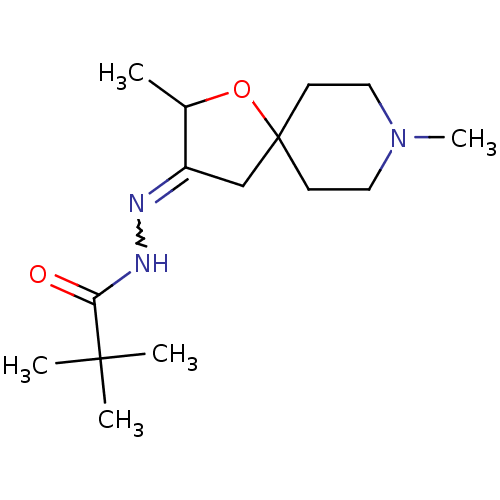
(2,2-Dimethyl-propionic acid [2,8-dimethyl-1-oxa-8-...)Show SMILES CC1OC2(CC1=NNC(=O)C(C)(C)C)CCN(C)CC2 |w:6.7| Show InChI InChI=1S/C15H27N3O2/c1-11-12(16-17-13(19)14(2,3)4)10-15(20-11)6-8-18(5)9-7-15/h11H,6-10H2,1-5H3,(H,17,19) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288161
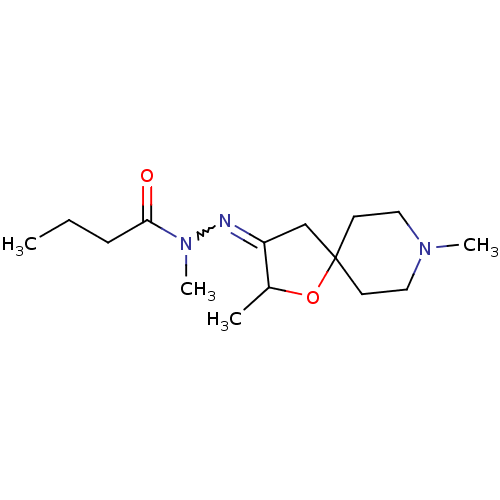
(Butyric acid N'-[2,8-dimethyl-1-oxa-8-aza-spiro[4....)Show InChI InChI=1S/C15H27N3O2/c1-5-6-14(19)18(4)16-13-11-15(20-12(13)2)7-9-17(3)10-8-15/h12H,5-11H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288152
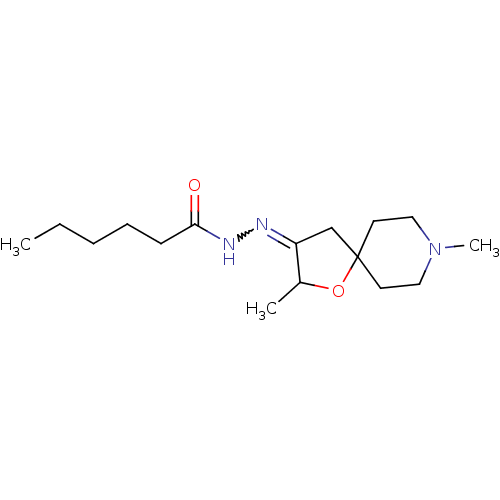
(CHEMBL418933 | Hexanoic acid [2,8-dimethyl-1-oxa-8...)Show InChI InChI=1S/C16H29N3O2/c1-4-5-6-7-15(20)18-17-14-12-16(21-13(14)2)8-10-19(3)11-9-16/h13H,4-12H2,1-3H3,(H,18,20) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288157
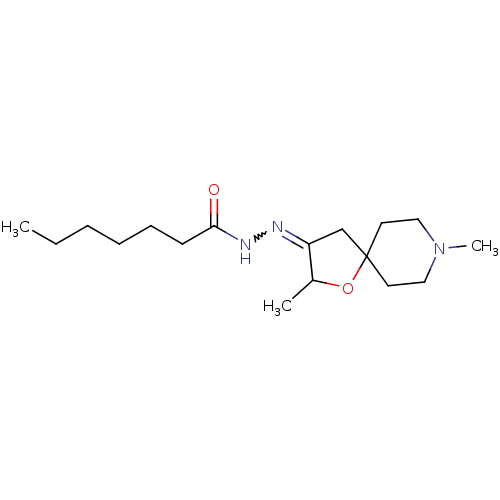
(CHEMBL314421 | Heptanoic acid [2,8-dimethyl-1-oxa-...)Show InChI InChI=1S/C17H31N3O2/c1-4-5-6-7-8-16(21)19-18-15-13-17(22-14(15)2)9-11-20(3)12-10-17/h14H,4-13H2,1-3H3,(H,19,21) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288169
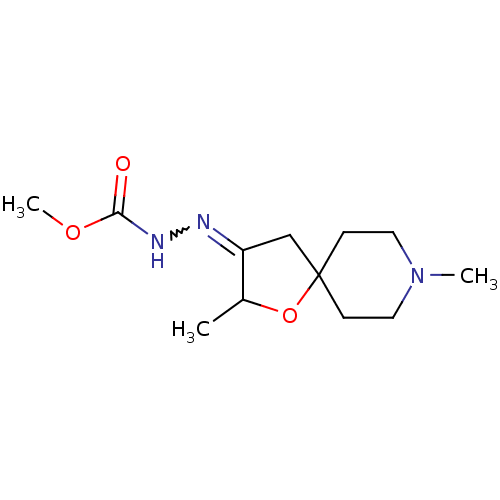
(CHEMBL83410 | N'-[2,8-Dimethyl-1-oxa-8-aza-spiro[4...)Show InChI InChI=1S/C12H21N3O3/c1-9-10(13-14-11(16)17-3)8-12(18-9)4-6-15(2)7-5-12/h9H,4-8H2,1-3H3,(H,14,16) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288154
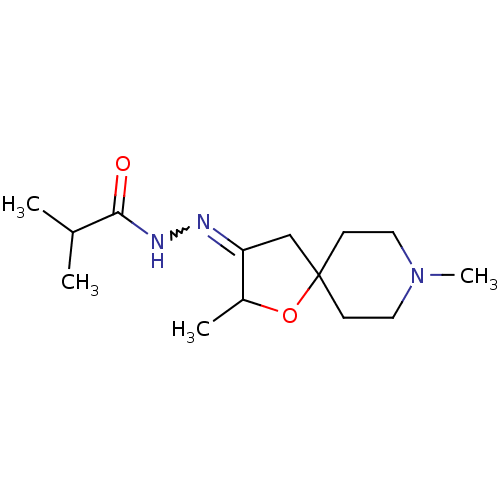
(CHEMBL85729 | Isobutyric acid [2,8-dimethyl-1-oxa-...)Show InChI InChI=1S/C14H25N3O2/c1-10(2)13(18)16-15-12-9-14(19-11(12)3)5-7-17(4)8-6-14/h10-11H,5-9H2,1-4H3,(H,16,18) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288150
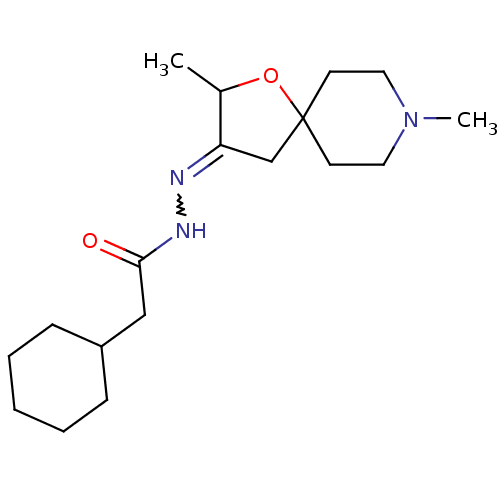
(CHEMBL85953 | Cyclohexyl-acetic acid [2,8-dimethyl...)Show SMILES CC1OC2(CC1=NNC(=O)CC1CCCCC1)CCN(C)CC2 |w:6.7| Show InChI InChI=1S/C18H31N3O2/c1-14-16(13-18(23-14)8-10-21(2)11-9-18)19-20-17(22)12-15-6-4-3-5-7-15/h14-15H,3-13H2,1-2H3,(H,20,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288148
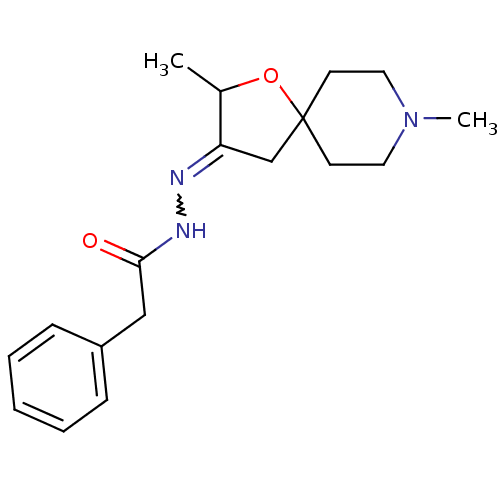
(CHEMBL85999 | Phenyl-acetic acid [2,8-dimethyl-1-o...)Show SMILES CC1OC2(CC1=NNC(=O)Cc1ccccc1)CCN(C)CC2 |w:6.7| Show InChI InChI=1S/C18H25N3O2/c1-14-16(13-18(23-14)8-10-21(2)11-9-18)19-20-17(22)12-15-6-4-3-5-7-15/h3-7,14H,8-13H2,1-2H3,(H,20,22) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288155
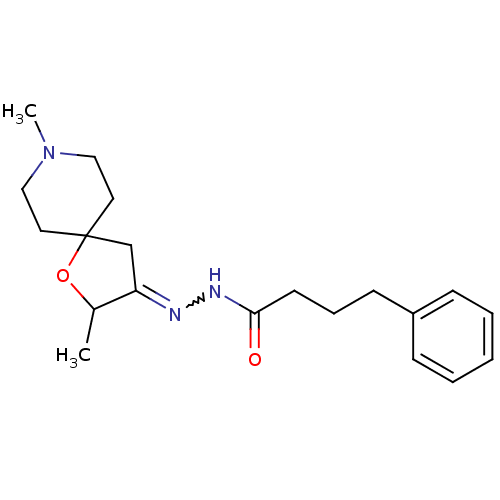
(4-Phenyl-butyric acid [2,8-dimethyl-1-oxa-8-aza-sp...)Show SMILES CC1OC2(CC1=NNC(=O)CCCc1ccccc1)CCN(C)CC2 |w:6.7| Show InChI InChI=1S/C20H29N3O2/c1-16-18(15-20(25-16)11-13-23(2)14-12-20)21-22-19(24)10-6-9-17-7-4-3-5-8-17/h3-5,7-8,16H,6,9-15H2,1-2H3,(H,22,24) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288168
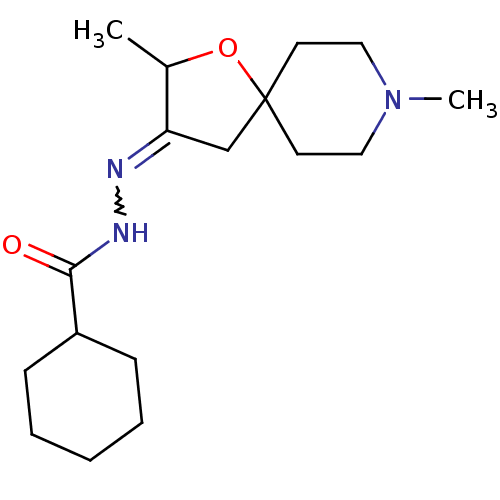
(CHEMBL84222 | Cyclohexanecarboxylic acid [2,8-dime...)Show SMILES CC1OC2(CC1=NNC(=O)C1CCCCC1)CCN(C)CC2 |w:6.7| Show InChI InChI=1S/C17H29N3O2/c1-13-15(12-17(22-13)8-10-20(2)11-9-17)18-19-16(21)14-6-4-3-5-7-14/h13-14H,3-12H2,1-2H3,(H,19,21) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 3.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288165
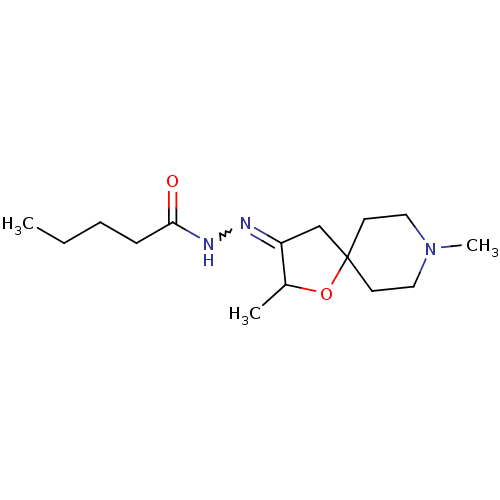
(CHEMBL432186 | Pentanoic acid [2,8-dimethyl-1-oxa-...)Show InChI InChI=1S/C15H27N3O2/c1-4-5-6-14(19)17-16-13-11-15(20-12(13)2)7-9-18(3)10-8-15/h12H,4-11H2,1-3H3,(H,17,19) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 4.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288151
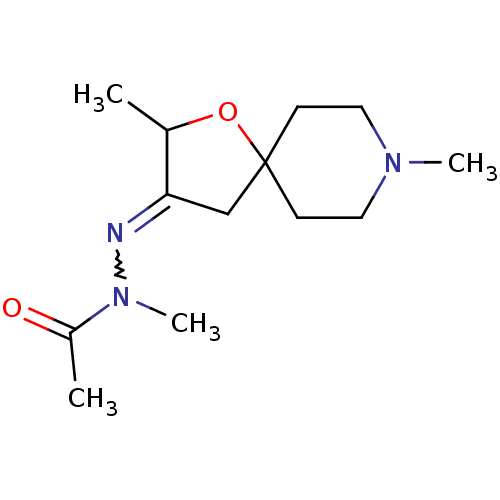
(Acetic acid N'-[2,8-dimethyl-1-oxa-8-aza-spiro[4.5...)Show InChI InChI=1S/C13H23N3O2/c1-10-12(14-16(4)11(2)17)9-13(18-10)5-7-15(3)8-6-13/h10H,5-9H2,1-4H3 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288164
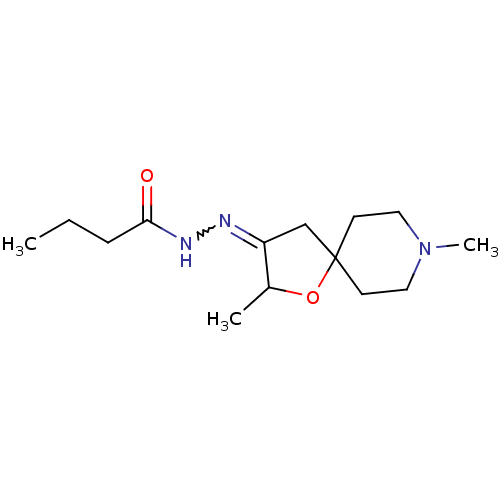
(Butyric acid [2,8-dimethyl-1-oxa-8-aza-spiro[4.5]d...)Show InChI InChI=1S/C14H25N3O2/c1-4-5-13(18)16-15-12-10-14(19-11(12)2)6-8-17(3)9-7-14/h11H,4-10H2,1-3H3,(H,16,18) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288146
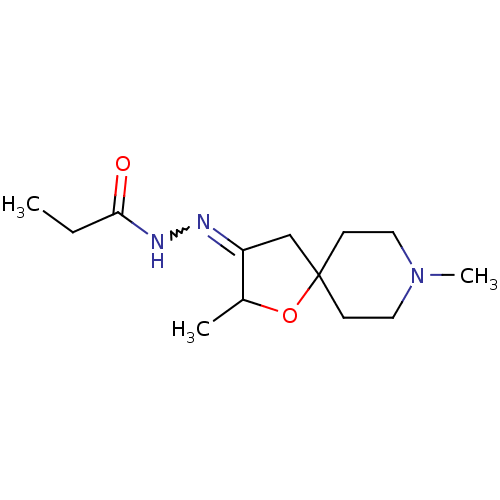
(CHEMBL84825 | Propionic acid [2,8-dimethyl-1-oxa-8...)Show InChI InChI=1S/C13H23N3O2/c1-4-12(17)15-14-11-9-13(18-10(11)2)5-7-16(3)8-6-13/h10H,4-9H2,1-3H3,(H,15,17) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.60E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288149
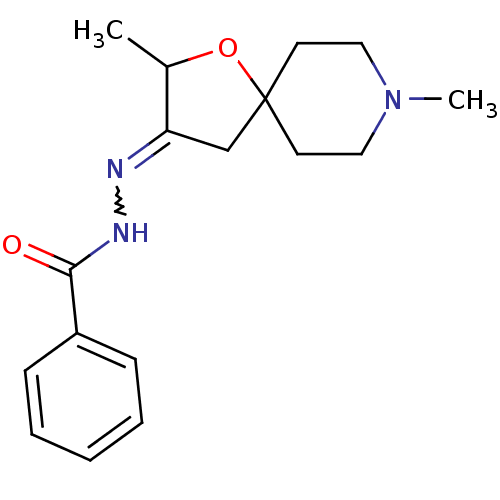
(Benzoic acid [2,8-dimethyl-1-oxa-8-aza-spiro[4.5]d...)Show SMILES CC1OC2(CC1=NNC(=O)c1ccccc1)CCN(C)CC2 |w:6.7| Show InChI InChI=1S/C17H23N3O2/c1-13-15(12-17(22-13)8-10-20(2)11-9-17)18-19-16(21)14-6-4-3-5-7-14/h3-7,13H,8-12H2,1-2H3,(H,19,21) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 7.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288145
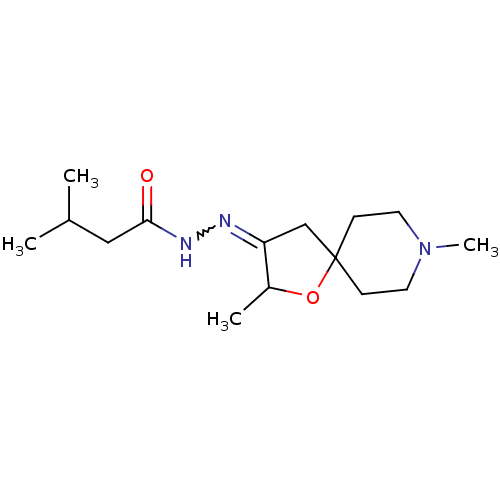
(3-Methyl-butyric acid [2,8-dimethyl-1-oxa-8-aza-sp...)Show InChI InChI=1S/C15H27N3O2/c1-11(2)9-14(19)17-16-13-10-15(20-12(13)3)5-7-18(4)8-6-15/h11-12H,5-10H2,1-4H3,(H,17,19) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288166
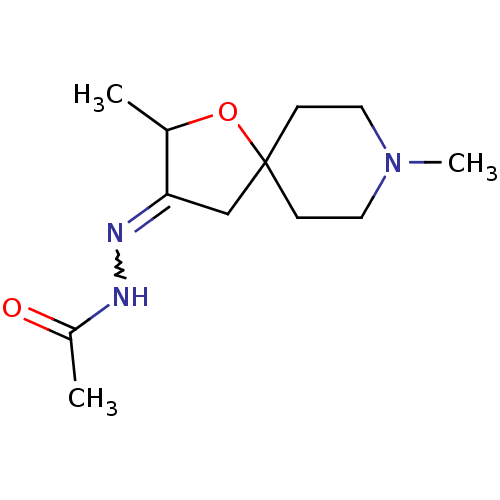
(Acetic acid [2,8-dimethyl-1-oxa-8-aza-spiro[4.5]de...)Show InChI InChI=1S/C12H21N3O2/c1-9-11(14-13-10(2)16)8-12(17-9)4-6-15(3)7-5-12/h9H,4-8H2,1-3H3,(H,13,16) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288162
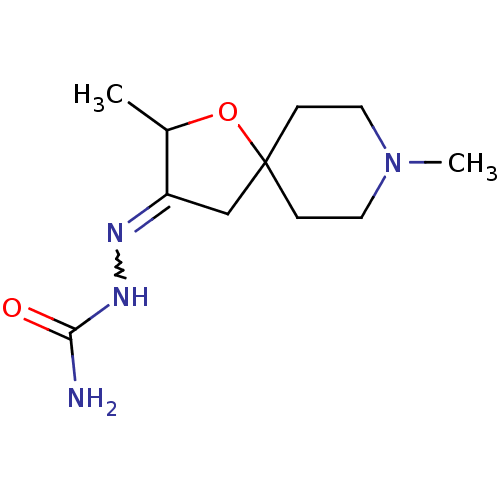
((3E)-2,8-dimethyl-1-oxa-8-azaspiro[4.5]decan-3-one...)Show InChI InChI=1S/C11H20N4O2/c1-8-9(13-14-10(12)16)7-11(17-8)3-5-15(2)6-4-11/h8H,3-7H2,1-2H3,(H3,12,14,16) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1
(RAT) | BDBM50288160
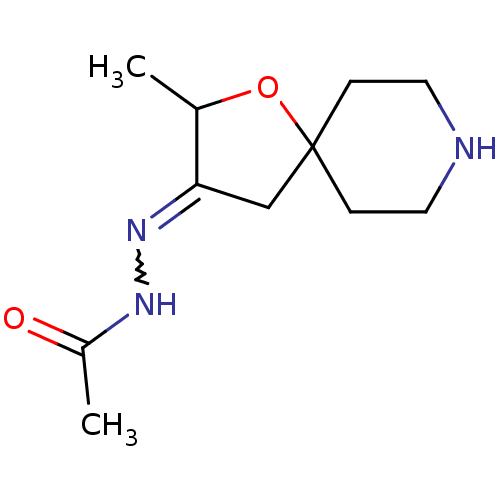
(Acetic acid [2-methyl-1-oxa-8-aza-spiro[4.5]dec-(3...)Show InChI InChI=1S/C11H19N3O2/c1-8-10(14-13-9(2)15)7-11(16-8)3-5-12-6-4-11/h8,12H,3-7H2,1-2H3,(H,13,15) | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| 1.80E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to displace [3H]- pirenzepine from M1 receptor in rat hippocampal membranes |
Bioorg Med Chem Lett 6: 2525-2530 (1996)
Article DOI: 10.1016/0960-894X(96)00471-4
BindingDB Entry DOI: 10.7270/Q2NK3F1B |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM14044

(Aminofurazanyl-azabenzimidazole 6k | N-(3-{[2-(4-a...)Show SMILES CCn1c(nc2cnc(Oc3cccc(NC(=O)c4ccc(CN(C)C)cc4)c3)cc12)-c1nonc1N Show InChI InChI=1S/C26H26N8O3/c1-4-34-21-13-22(28-14-20(21)30-25(34)23-24(27)32-37-31-23)36-19-7-5-6-18(12-19)29-26(35)17-10-8-16(9-11-17)15-33(2)3/h5-14H,4,15H2,1-3H3,(H2,27,32)(H,29,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... |
J Med Chem 50: 2-5 (2007)
Article DOI: 10.1021/jm060873p
BindingDB Entry DOI: 10.7270/Q2F18WZB |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM14042
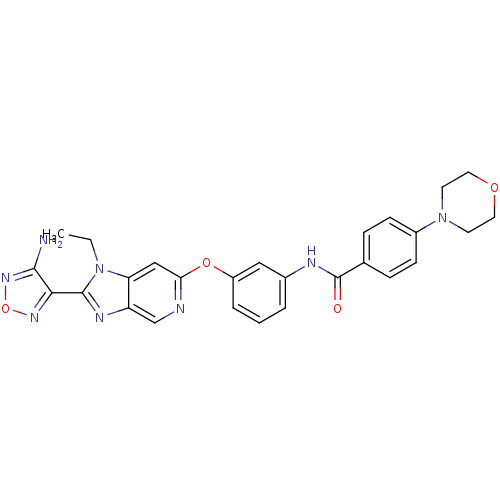
(Aminofurazanyl-azabenzimidazole 6i | N-(3-{[2-(4-a...)Show SMILES CCn1c(nc2cnc(Oc3cccc(NC(=O)c4ccc(cc4)N4CCOCC4)c3)cc12)-c1nonc1N Show InChI InChI=1S/C27H26N8O4/c1-2-35-22-15-23(29-16-21(22)31-26(35)24-25(28)33-39-32-24)38-20-5-3-4-18(14-20)30-27(36)17-6-8-19(9-7-17)34-10-12-37-13-11-34/h3-9,14-16H,2,10-13H2,1H3,(H2,28,33)(H,30,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... |
J Med Chem 50: 2-5 (2007)
Article DOI: 10.1021/jm060873p
BindingDB Entry DOI: 10.7270/Q2F18WZB |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM14043
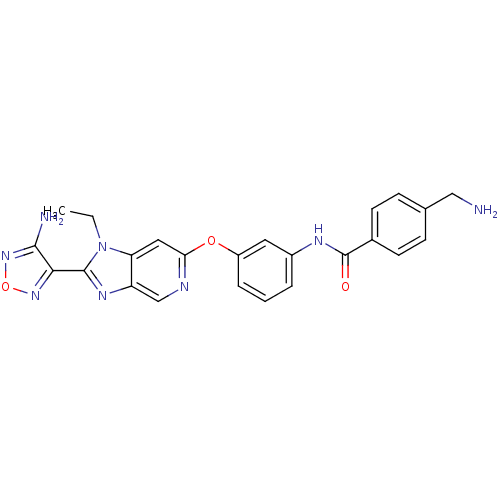
(Aminofurazanyl-azabenzimidazole 6j | N-(3-{[2-(4-a...)Show SMILES CCn1c(nc2cnc(Oc3cccc(NC(=O)c4ccc(CN)cc4)c3)cc12)-c1nonc1N Show InChI InChI=1S/C24H22N8O3/c1-2-32-19-11-20(27-13-18(19)29-23(32)21-22(26)31-35-30-21)34-17-5-3-4-16(10-17)28-24(33)15-8-6-14(12-25)7-9-15/h3-11,13H,2,12,25H2,1H3,(H2,26,31)(H,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... |
J Med Chem 50: 2-5 (2007)
Article DOI: 10.1021/jm060873p
BindingDB Entry DOI: 10.7270/Q2F18WZB |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM14045
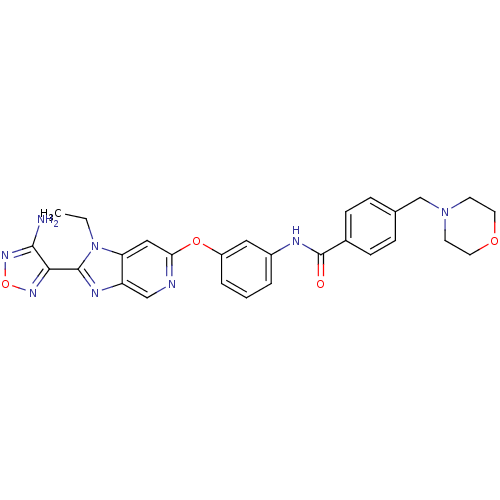
(Aminofurazanyl-azabenzimidazole 6l | N-(3-{[2-(4-a...)Show SMILES CCn1c(nc2cnc(Oc3cccc(NC(=O)c4ccc(CN5CCOCC5)cc4)c3)cc12)-c1nonc1N Show InChI InChI=1S/C28H28N8O4/c1-2-36-23-15-24(30-16-22(23)32-27(36)25-26(29)34-40-33-25)39-21-5-3-4-20(14-21)31-28(37)19-8-6-18(7-9-19)17-35-10-12-38-13-11-35/h3-9,14-16H,2,10-13,17H2,1H3,(H2,29,34)(H,31,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... |
J Med Chem 50: 2-5 (2007)
Article DOI: 10.1021/jm060873p
BindingDB Entry DOI: 10.7270/Q2F18WZB |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM14046
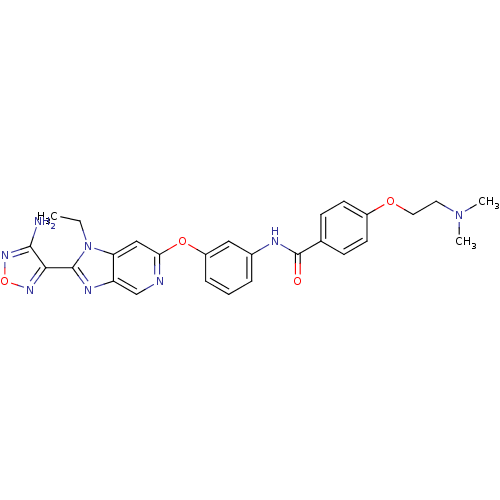
(Aminofurazanyl-azabenzimidazole 6m | N-(3-{[2-(4-a...)Show SMILES CCn1c(nc2cnc(Oc3cccc(NC(=O)c4ccc(OCCN(C)C)cc4)c3)cc12)-c1nonc1N Show InChI InChI=1S/C27H28N8O4/c1-4-35-22-15-23(29-16-21(22)31-26(35)24-25(28)33-39-32-24)38-20-7-5-6-18(14-20)30-27(36)17-8-10-19(11-9-17)37-13-12-34(2)3/h5-11,14-16H,4,12-13H2,1-3H3,(H2,28,33)(H,30,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... |
J Med Chem 50: 2-5 (2007)
Article DOI: 10.1021/jm060873p
BindingDB Entry DOI: 10.7270/Q2F18WZB |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM14035
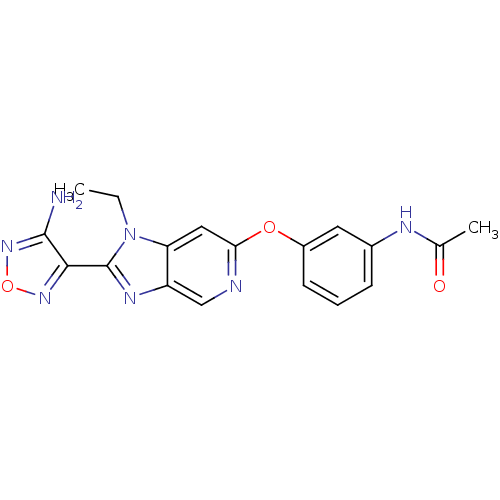
(Aminofurazanyl-azabenzimidazole 6c | N-(3-{[2-(4-a...)Show SMILES CCn1c(nc2cnc(Oc3cccc(NC(C)=O)c3)cc12)-c1nonc1N Show InChI InChI=1S/C18H17N7O3/c1-3-25-14-8-15(27-12-6-4-5-11(7-12)21-10(2)26)20-9-13(14)22-18(25)16-17(19)24-28-23-16/h4-9H,3H2,1-2H3,(H2,19,24)(H,21,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... |
J Med Chem 50: 2-5 (2007)
Article DOI: 10.1021/jm060873p
BindingDB Entry DOI: 10.7270/Q2F18WZB |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM14047
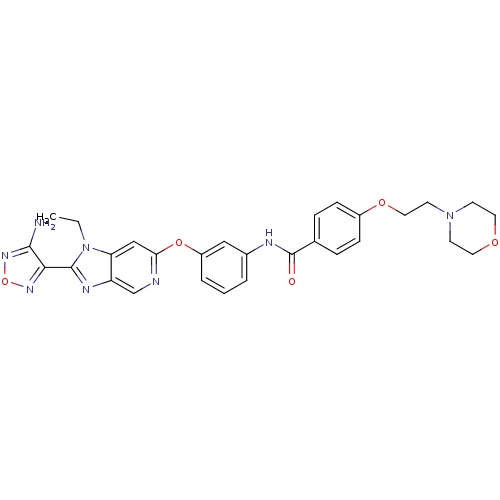
(Aminofurazanyl-azabenzimidazole 6n | N-(3-{[2-(4-a...)Show SMILES CCn1c(nc2cnc(Oc3cccc(NC(=O)c4ccc(OCCN5CCOCC5)cc4)c3)cc12)-c1nonc1N Show InChI InChI=1S/C29H30N8O5/c1-2-37-24-17-25(31-18-23(24)33-28(37)26-27(30)35-42-34-26)41-22-5-3-4-20(16-22)32-29(38)19-6-8-21(9-7-19)40-15-12-36-10-13-39-14-11-36/h3-9,16-18H,2,10-15H2,1H3,(H2,30,35)(H,32,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... |
J Med Chem 50: 2-5 (2007)
Article DOI: 10.1021/jm060873p
BindingDB Entry DOI: 10.7270/Q2F18WZB |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM25494
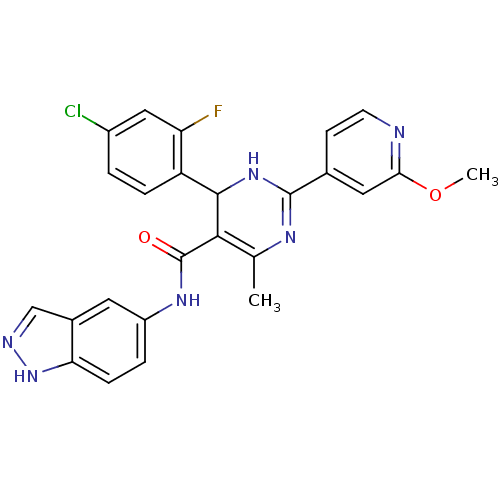
(4-(4-chloro-2-fluorophenyl)-N-(1H-indazol-5-yl)-2-...)Show SMILES COc1cc(ccn1)C1=NC(C)=C(C(N1)c1ccc(Cl)cc1F)C(=O)Nc1ccc2[nH]ncc2c1 |c:12,t:9| Show InChI InChI=1S/C25H20ClFN6O2/c1-13-22(25(34)31-17-4-6-20-15(9-17)12-29-33-20)23(18-5-3-16(26)11-19(18)27)32-24(30-13)14-7-8-28-21(10-14)35-2/h3-12,23H,1-2H3,(H,29,33)(H,30,32)(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK
| Assay Description
The assay of Rock-1 activity involved incubation with peptide substrate and ATP33, and the incorporation of P33 into the peptide was quantified by Sc... |
J Med Chem 51: 6631-4 (2008)
Article DOI: 10.1021/jm8005096
BindingDB Entry DOI: 10.7270/Q2F47MFZ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM14041
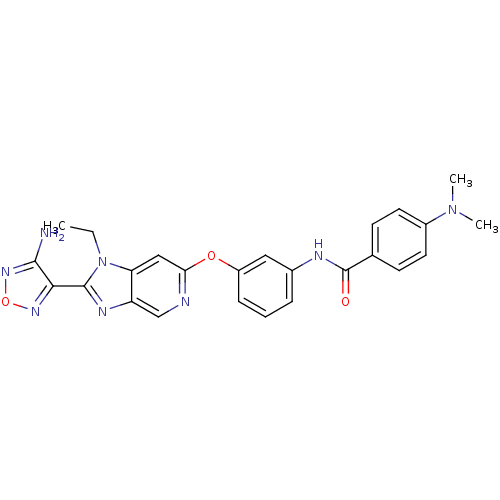
(Aminofurazanyl-azabenzimidazole 6h | N-(3-{[2-(4-a...)Show SMILES CCn1c(nc2cnc(Oc3cccc(NC(=O)c4ccc(cc4)N(C)C)c3)cc12)-c1nonc1N Show InChI InChI=1S/C25H24N8O3/c1-4-33-20-13-21(27-14-19(20)29-24(33)22-23(26)31-36-30-22)35-18-7-5-6-16(12-18)28-25(34)15-8-10-17(11-9-15)32(2)3/h5-14H,4H2,1-3H3,(H2,26,31)(H,28,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GlaxoSmithKline
| Assay Description
The assay of Rock-1 activity involved incubation with peptide substrate and ATP/[gamma-33P] ATP, the subsequent incorporation of 33P into the peptid... |
J Med Chem 50: 2-5 (2007)
Article DOI: 10.1021/jm060873p
BindingDB Entry DOI: 10.7270/Q2F18WZB |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM25495

(4-(4-chloro-2-fluorophenyl)-N-(1H-indazol-5-yl)-2-...)Show SMILES COc1ccc(cn1)C1=NC(C)=C(C(N1)c1ccc(Cl)cc1F)C(=O)Nc1ccc2[nH]ncc2c1 |c:12,t:9| Show InChI InChI=1S/C25H20ClFN6O2/c1-13-22(25(34)31-17-5-7-20-15(9-17)12-29-33-20)23(18-6-4-16(26)10-19(18)27)32-24(30-13)14-3-8-21(35-2)28-11-14/h3-12,23H,1-2H3,(H,29,33)(H,30,32)(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK
| Assay Description
The assay of Rock-1 activity involved incubation with peptide substrate and ATP33, and the incorporation of P33 into the peptide was quantified by Sc... |
J Med Chem 51: 6631-4 (2008)
Article DOI: 10.1021/jm8005096
BindingDB Entry DOI: 10.7270/Q2F47MFZ |
More data for this
Ligand-Target Pair | |
Rho-associated protein kinase 1
(Homo sapiens (Human)) | BDBM25493
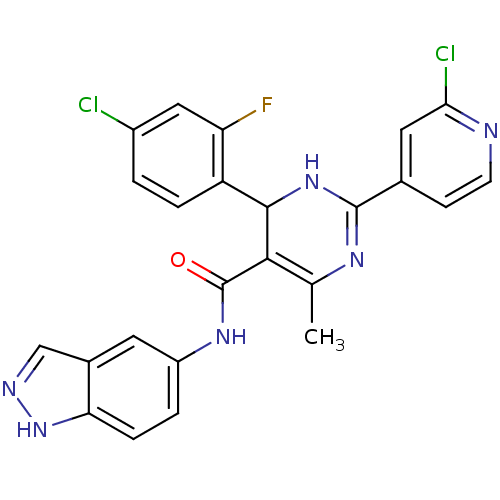
(4-(4-chloro-2-fluorophenyl)-2-(2-chloropyridin-4-y...)Show SMILES CC1=C(C(NC(=N1)c1ccnc(Cl)c1)c1ccc(Cl)cc1F)C(=O)Nc1ccc2[nH]ncc2c1 |c:5,t:1| Show InChI InChI=1S/C24H17Cl2FN6O/c1-12-21(24(34)31-16-3-5-19-14(8-16)11-29-33-19)22(17-4-2-15(25)10-18(17)27)32-23(30-12)13-6-7-28-20(26)9-13/h2-11,22H,1H3,(H,29,33)(H,30,32)(H,31,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | 7.4 | 22 |
GSK
| Assay Description
The assay of Rock-1 activity involved incubation with peptide substrate and ATP33, and the incorporation of P33 into the peptide was quantified by Sc... |
J Med Chem 51: 6631-4 (2008)
Article DOI: 10.1021/jm8005096
BindingDB Entry DOI: 10.7270/Q2F47MFZ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data