

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DNA topoisomerase 2-beta (Homo sapiens (Human)) | BDBM50366826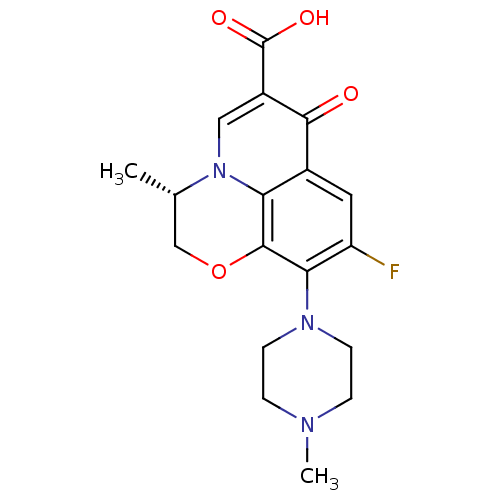 (DR-3355 | Floxacin | Iquix | LEVOFLOXACIN | Levaqu...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | 2.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas University Curated by ChEMBL | Assay Description Binding affinity against sigma receptor | J Med Chem 30: 2283-6 (1987) BindingDB Entry DOI: 10.7270/Q2R78HFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-beta (Homo sapiens (Human)) | BDBM50045000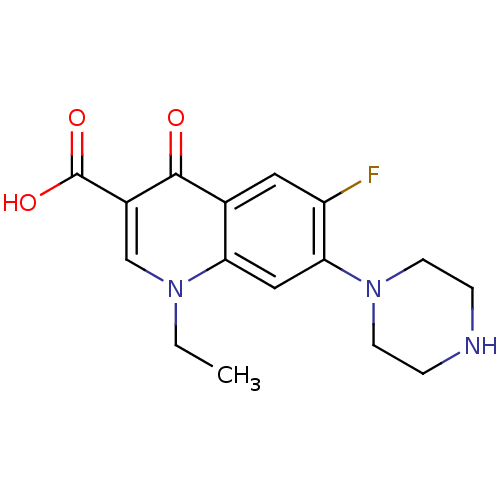 ((NFLX)1-Ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 3.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas University Curated by ChEMBL | Assay Description Binding affinity against sigma receptor | J Med Chem 30: 2283-6 (1987) BindingDB Entry DOI: 10.7270/Q2R78HFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-beta (Homo sapiens (Human)) | BDBM50045004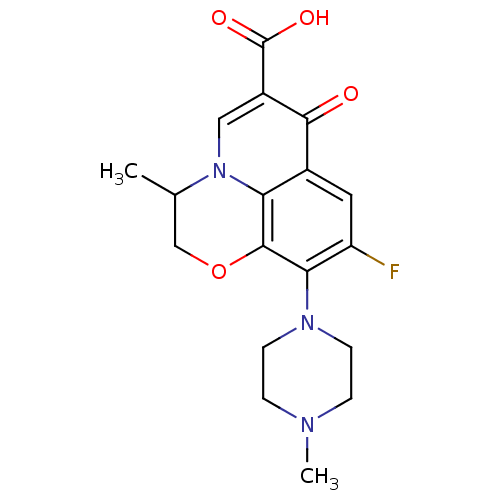 (9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-ox...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 4.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas University Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone | J Med Chem 30: 2283-6 (1987) BindingDB Entry DOI: 10.7270/Q2R78HFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-beta (Homo sapiens (Human)) | BDBM50226409 (DEXTROFLOXACINE) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | 7.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas University Curated by ChEMBL | Assay Description Binding affinity against sigma receptor | J Med Chem 30: 2283-6 (1987) BindingDB Entry DOI: 10.7270/Q2R78HFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-beta (Homo sapiens (Human)) | BDBM50366826 (DR-3355 | Floxacin | Iquix | LEVOFLOXACIN | Levaqu...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | 9.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas University Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone | J Med Chem 30: 2283-6 (1987) BindingDB Entry DOI: 10.7270/Q2R78HFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-beta (Homo sapiens (Human)) | BDBM50045000 ((NFLX)1-Ethyl-6-fluoro-4-oxo-7-piperazin-1-yl-1,4-...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | PubMed | 2.25E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas University Curated by ChEMBL | Assay Description Binding affinity for dopamine receptor D2 was evaluated by the ability to displace [3H]spiperone | J Med Chem 30: 2283-6 (1987) BindingDB Entry DOI: 10.7270/Q2R78HFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-beta (Homo sapiens (Human)) | BDBM50045004 (9-fluoro-3-methyl-10-(4-methylpiperazin-1-yl)-7-ox...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.32E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas University Curated by ChEMBL | Assay Description Binding affinity against sigma receptor | J Med Chem 30: 2283-6 (1987) BindingDB Entry DOI: 10.7270/Q2R78HFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-beta (Homo sapiens (Human)) | BDBM50226409 (DEXTROFLOXACINE) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Similars | PubMed | 2.90E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Kansas University Curated by ChEMBL | Assay Description Binding affinity against sigma receptor | J Med Chem 30: 2283-6 (1987) BindingDB Entry DOI: 10.7270/Q2R78HFX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50349933 (CHEMBL1812144) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.54E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2 alpha-mediated relaxation of supercoiled pBR322 DNA after 30 mins by agarose gel electrophoresis | Eur J Med Chem 46: 3339-47 (2011) Article DOI: 10.1016/j.ejmech.2011.04.059 BindingDB Entry DOI: 10.7270/Q2513ZJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50349934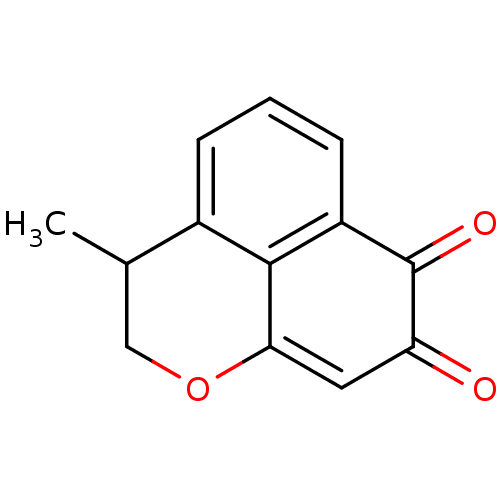 (CHEMBL1812139) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2 alpha-mediated relaxation of supercoiled pBR322 DNA after 30 mins by agarose gel electrophoresis | Eur J Med Chem 46: 3339-47 (2011) Article DOI: 10.1016/j.ejmech.2011.04.059 BindingDB Entry DOI: 10.7270/Q2513ZJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50349932 (CHEMBL1812137) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.88E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2 alpha-mediated relaxation of supercoiled pBR322 DNA after 30 mins by agarose gel electrophoresis | Eur J Med Chem 46: 3339-47 (2011) Article DOI: 10.1016/j.ejmech.2011.04.059 BindingDB Entry DOI: 10.7270/Q2513ZJD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| DNA topoisomerase 2-alpha (Homo sapiens (Human)) | BDBM50127140 ((-)-etoposide | (5S,5aR,8aR,9R)-9-(4-hydroxy-3,5-d...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 6.03E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Sun Yat-sen University Curated by ChEMBL | Assay Description Inhibition of human topoisomerase 2 alpha-mediated relaxation of supercoiled pBR322 DNA after 30 mins by agarose gel electrophoresis | Eur J Med Chem 46: 3339-47 (2011) Article DOI: 10.1016/j.ejmech.2011.04.059 BindingDB Entry DOI: 10.7270/Q2513ZJD | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||